Abstract
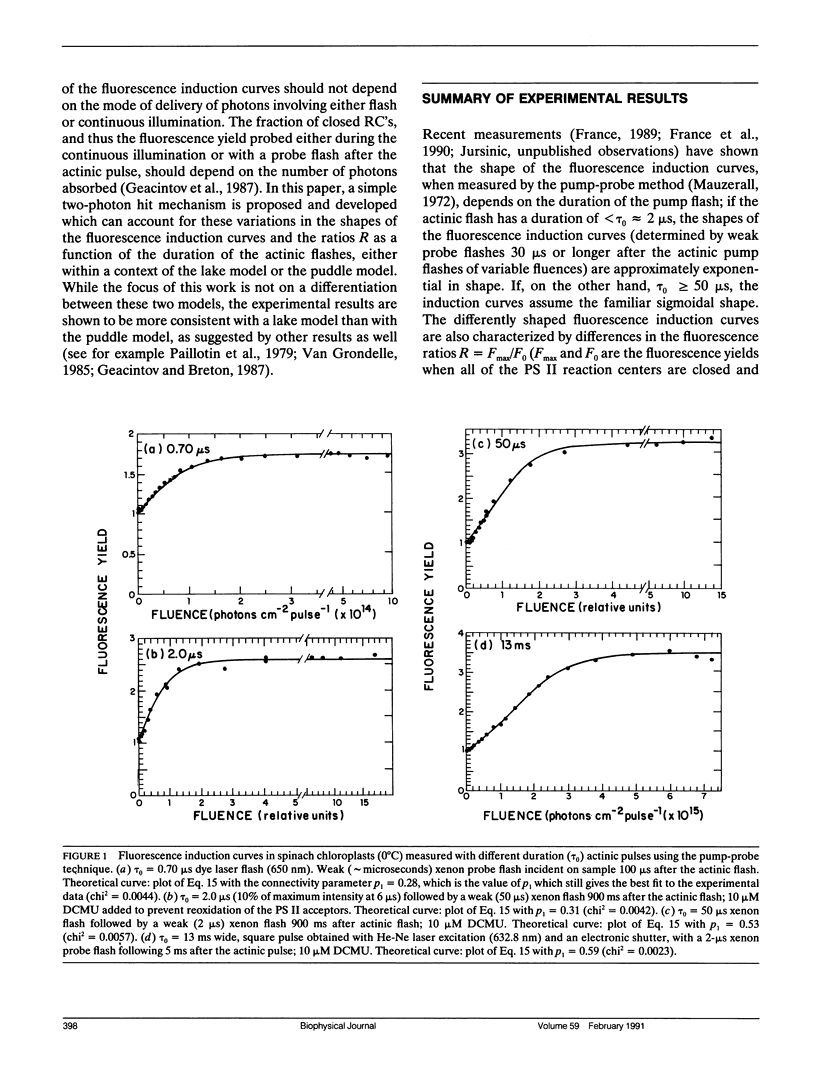
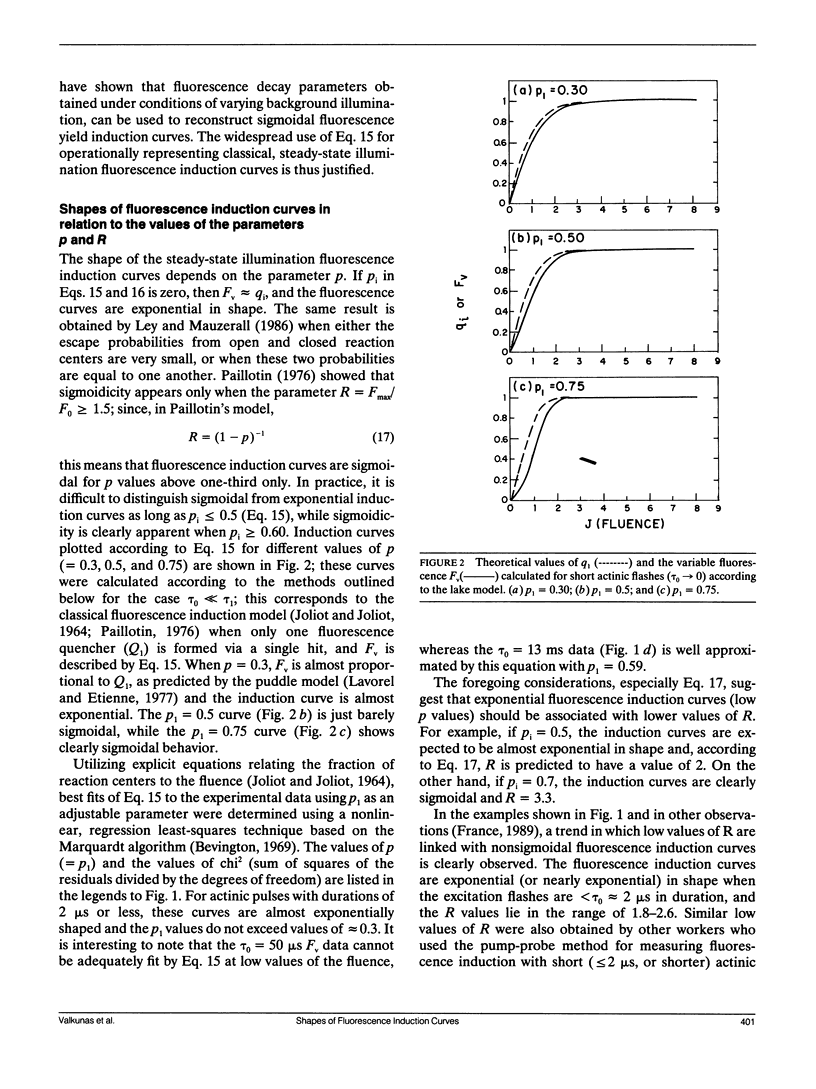
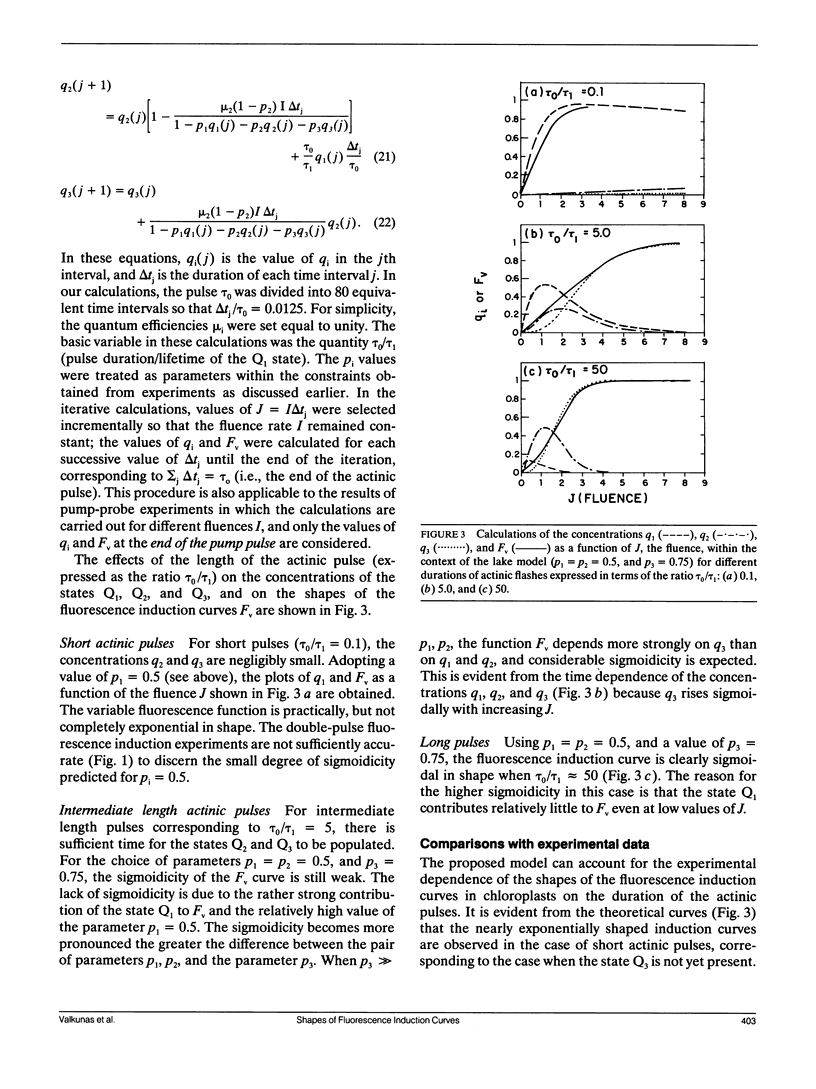
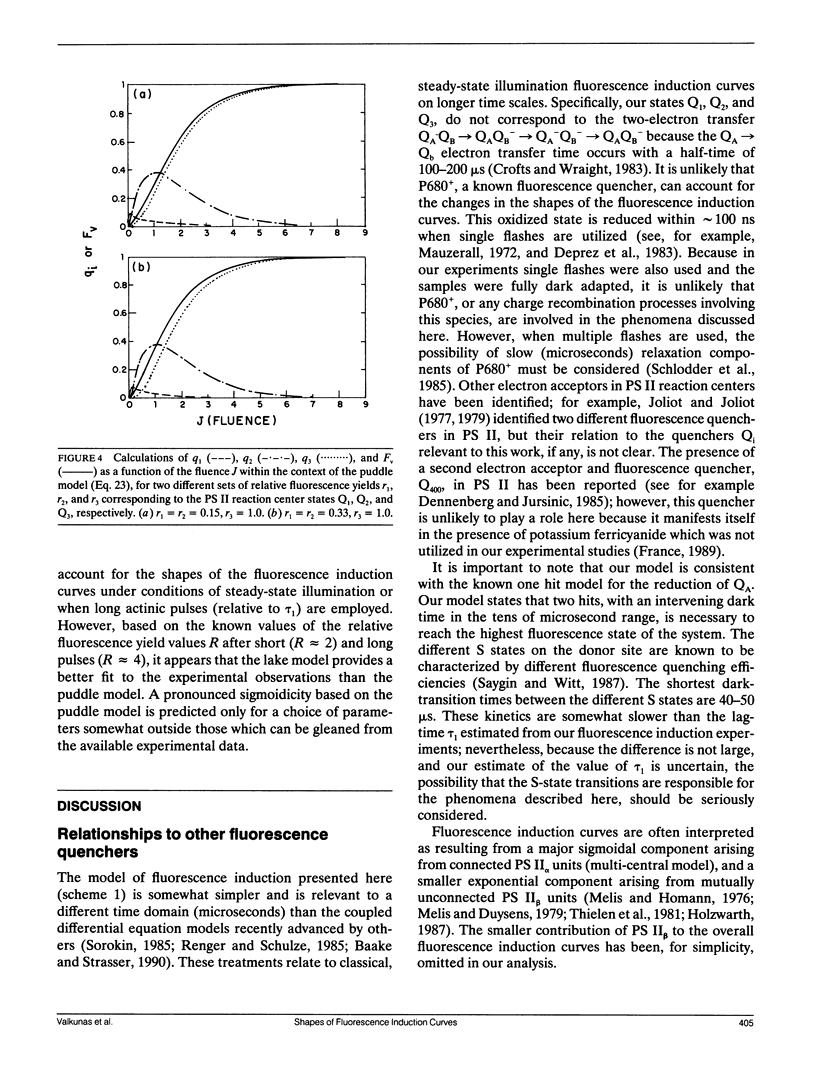
The shapes of fluorescence induction curves in spinach chloroplasts, measured using double-flash pump-probe techniques, are shown to depend on the duration of the actinic flashes. For flash durations τ0 ≤ 2 μs, the variable fluorescence Fν grows exponentially (or nearly so) with increasing fluence J of the actinic pulses and the fluorescence induction ratio R = Fmax/F0 is ≤2.6. When τo ≥ 50 μs, the shapes of the Fν vs. J curves are sigmoidal, and R > 3.2. Overall, the experimentally observed trends suggest that, as the duration τ0 of the actinic pulses is increased, the degree of sigmoidicity, the deduced values of the interunit excitation transfer parameter p, and the fluorescence induction ratios R, also tend to increase. These results can be accounted for in terms of a simple double-photon hit model in which a dark lag time τ1 = 0.4-10 μs between the two hits is necessary for the observance of sigmoidal fluorescence induction curves and relatively high R ratios. It is shown that, in principle, such a model can account for the exponential and sigmoidal shapes of the fluorescence induction curves either within the context of a lake model of the photosynthetic antenna bed (free transfer of excitation between photosynthetic units) or the isolated (puddle) model of photosystem II reaction centers. However, from the known values of the R ratio measured with actinic pulses of different durations, or under continuous illumination, the lake model offers a better description of the experimental phenomena than the puddle model.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breton J., Geacintov N. E., Swenberg C. E. Quenching of fluorescence by triplet excited states in chloroplasts. Biochim Biophys Acta. 1979 Dec 6;548(3):616–635. doi: 10.1016/0005-2728(79)90069-0. [DOI] [PubMed] [Google Scholar]
- JOLIOT A., JOLIOT P. ETUDE CIN'ETIQUE DE LA R'EACTION PHOTOCHIMIQUE LIB'ERANT L'OXYG'ENE AU COURS DE LA PHOTOSYNTH'ESE. C R Hebd Seances Acad Sci. 1964 May 4;258:4622–4625. [PubMed] [Google Scholar]
- Joliot P., Joliot A. Comparative study of the fluorescence yield and of the C550 absorption change at room temperature. Biochim Biophys Acta. 1979 Apr 11;546(1):93–105. doi: 10.1016/0005-2728(79)90173-7. [DOI] [PubMed] [Google Scholar]
- Joliot P., Joliot A. Evidence for a double hit process in photosystem II based on fluorescence studies. Biochim Biophys Acta. 1977 Dec 23;462(3):559–574. doi: 10.1016/0005-2728(77)90101-3. [DOI] [PubMed] [Google Scholar]
- Kolber Z., Zehr J., Falkowski P. Effects of Growth Irradiance and Nitrogen Limitation on Photosynthetic Energy Conversion in Photosystem II. Plant Physiol. 1988 Nov;88(3):923–929. doi: 10.1104/pp.88.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavorel J., Joliot P. A connected model of the photosynthetic unit. Biophys J. 1972 Jul;12(7):815–831. doi: 10.1016/S0006-3495(72)86125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauzerall D. Light-induced fluorescence changes in Chlorella, and the primary photoreactions for the production of oxygen. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1358–1362. doi: 10.1073/pnas.69.6.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauzerall D. Multiple excitations in photosynthetic systems. Biophys J. 1976 Jan;16(1):87–91. doi: 10.1016/S0006-3495(76)85665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A., Homann P. H. Heterogeneity of the photochemical centers in system II of chloroplasts. Photochem Photobiol. 1976 May;23(5):343–350. doi: 10.1111/j.1751-1097.1976.tb07259.x. [DOI] [PubMed] [Google Scholar]
- Paillotin G., Geacintov N. E., Breton J. A master equation theory of fluorescence induction, photochemical yield, and singlet-triplet exciton quenching in photosynthetic systems. Biophys J. 1983 Oct;44(1):65–77. doi: 10.1016/S0006-3495(83)84278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillotin G., Swenberg C. E., Breton J., Geacintov N. E. Analysis of picosecond laser induced fluorescence phenomena in photosynthetic membranes utilizing a master equation approach. Biophys J. 1979 Mar;25(3):513–533. doi: 10.1016/S0006-3495(79)85320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G. H., Brock H., Holzwarth A. R. Picosecond kinetics of fluorescence and absorbance changes in photosystem II particles excited at low photon density. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8414–8418. doi: 10.1073/pnas.84.23.8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielen A. P., van Gorkom H. J., Rijgersberg C. P. Chlorophyll composition of photosystems II alpha, II beta and I in tobacco chloroplasts. Biochim Biophys Acta. 1981 Mar 12;635(1):121–131. doi: 10.1016/0005-2728(81)90013-x. [DOI] [PubMed] [Google Scholar]


