Abstract
The noncolligative peptide and glycopeptide antifreezes found in some cold-water fish act by binding to the ice surface and preventing crystal growth, not by altering the equilibrium freezing point of the water. A simple crystal growth and etching technique allows determination of the crystallographic planes where the binding occurs. In the case of elongated molecules, such as the alpha-helical peptides in this report, it also allows a deduction of the molecular alignment on the ice surface. The structurally similar antifreeze peptides from winter flounder (Pseudopleuronectes americanus) and Alaskan plaice (Pleuronectes quadritaberulatus) adsorb onto the (2021) pyramidal planes of ice, whereas the sculpin (Myoxocephalus scorpius) peptide adsorbs on (2110), the secondary prism planes. All three are probably aligned along (0112). These antifreeze peptides have 11-amino acid sequence repeats ending with a polar residue, and each repeat constitutes a distance of 16.5 A along the helix, which nearly matches the 16.7 A repeat spacing along (0112) in ice. This structural match is undoubtedly important, but the mechanism of binding is not yet clear. The suggested mechanism of growth inhibition operates through the influence of local surface curvature upon melting point and results in complete inhibition of the crystal growth even though individual antifreeze molecules bind at only one interface orientation.
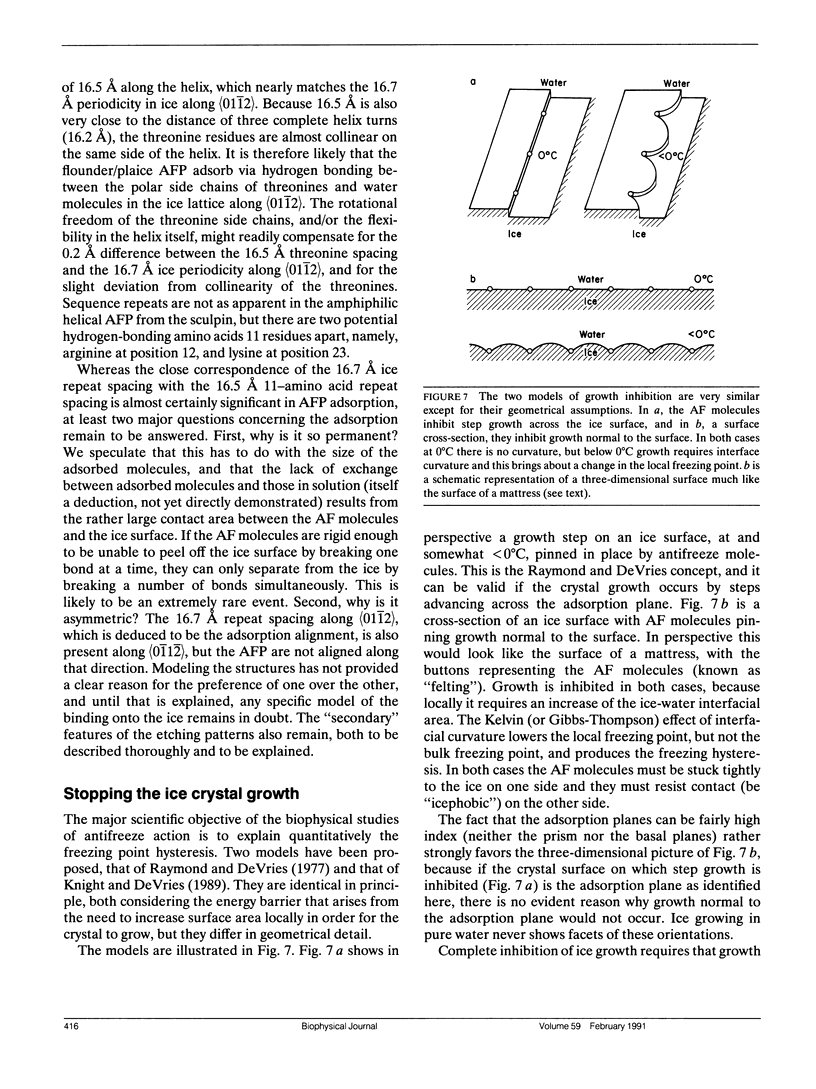
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng C. H., DeVries A. L. Structures of antifreeze peptides from the antarctic eel pout, Austrolycicthys brachycephalus. Biochim Biophys Acta. 1989 Jul 27;997(1-2):55–64. doi: 10.1016/0167-4838(89)90135-0. [DOI] [PubMed] [Google Scholar]
- Harrison K., Hallett J., Burcham T. S., Feeney R. E., Kerr W. L., Yeh Y. Ice growth in supercooled solutions of antifreeze glycoprotein. Nature. 1987 Jul 16;328(6127):241–243. doi: 10.1038/328241a0. [DOI] [PubMed] [Google Scholar]
- Hew C. L., Joshi S., Wang N. C., Kao M. H., Ananthanarayanan V. S. Structures of shorthorn sculpin antifreeze polypeptides. Eur J Biochem. 1985 Aug 15;151(1):167–172. doi: 10.1111/j.1432-1033.1985.tb09081.x. [DOI] [PubMed] [Google Scholar]
- Knight C. A., DeVries A. L., Oolman L. D. Fish antifreeze protein and the freezing and recrystallization of ice. Nature. 1984 Mar 15;308(5956):295–296. doi: 10.1038/308295a0. [DOI] [PubMed] [Google Scholar]
- Knight C. A., Devries A. L. Melting inhibition and superheating of ice by an antifreeze glycopeptide. Science. 1989 Aug 4;245(4917):505–507. doi: 10.1126/science.245.4917.505. [DOI] [PubMed] [Google Scholar]
- Raymond J. A., DeVries A. L. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2589–2593. doi: 10.1073/pnas.74.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond J. A., Wilson P., DeVries A. L. Inhibition of growth of nonbasal planes in ice by fish antifreezes. Proc Natl Acad Sci U S A. 1989 Feb;86(3):881–885. doi: 10.1073/pnas.86.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G. K., Davies P. L., Shears M. A., Fletcher G. L. Structural variations in the alanine-rich antifreeze proteins of the pleuronectinae. Eur J Biochem. 1987 Nov 2;168(3):629–633. doi: 10.1111/j.1432-1033.1987.tb13462.x. [DOI] [PubMed] [Google Scholar]
- Vaida M., Shimon L. J., Weisinger-Lewin Y., Frolow F., Lahav M., Leiserowitz L., McMullan R. K. The structure and symmetry of crystalline solid solutions: a general revision. Science. 1988 Sep 16;241(4872):1475–1479. doi: 10.1126/science.241.4872.1475. [DOI] [PubMed] [Google Scholar]
- Yang D. S., Sax M., Chakrabartty A., Hew C. L. Crystal structure of an antifreeze polypeptide and its mechanistic implications. Nature. 1988 May 19;333(6170):232–237. doi: 10.1038/333232a0. [DOI] [PubMed] [Google Scholar]



