Abstract
A major question in the study of leishmaniasis is what dictates clinical disease expression produced by different Leishmania species, i.e., cutaneous versus systemic and healing versus nonhealing. Animal models using a Leishmania species associated with self-limiting cutaneous disease (L. major) have revealed that protective immunity requires CD40/CD40 ligand (CD40L)-dependent, interleukin-12 (IL-12)-driven Th1 responses. We recently showed that L. major can prime human dendritic cells (DCs) for CD40L-triggered IL-12p70 secretion and that these cells can drive a Th1 response in autologous T cells from sensitized individuals. Here we show that in contrast to L. major, Leishmania species responsible for visceral disease (L. donovani), as well as species associated with persistent, cutaneous lesions and occasional systemic disease (L. tropica), did not induce CD40L-dependent IL-12p70 production, despite comparable levels of uptake by DCs. Up-regulated surface expression of CD40 did not correlate with IL-12p70 production, and appreciable CD40L-induced IL-12p40 secretion was observed in uninfected as well as infected DCs, regardless of species. Reverse transcription-PCR analysis confirmed that the production of heterodimeric IL-12 was limited by expression of IL-12p35 mRNA, which was dependent on both a microbial priming signal and CD40 engagement for its high-level induction. The intrinsic differences in the ability of Leishmania species to prime DCs for CD40L-dependent IL-12p70 secretion may account, at least in part, for the evolution of healing and nonhealing forms of leishmanial disease.
Leishmaniasis is a vector-borne parasitic disease which, depending mainly upon the species of Leishmania, can display a spectrum of clinical manifestations ranging from localized cutaneous lesions that heal spontaneously to generalized systemic disease with fatal outcome. Regardless of species and clinical outcome, all Leishmania infections are initiated by infectious-stage metacyclic promastigotes which are deposited in the skin following a sand fly bite. These forms are taken up by macrophages and replicate as intracellular amastigotes. The pathology that is typical of localized cutaneous disease is immune mediated and is accompanied by the control of infection in the skin. In contrast, the absence of strong acquired immunity, while moderating the development of dermal pathology, results in parasite dissemination and uncontained parasite growth in liver, spleen, and bone marrow. The control of leishmanial infection requires the induction of immune responses capable of activating infected macrophages to a microbicidal state. The most potent cytokine for the induction of leishmanicidal activity in macrophages is gamma interferon (IFN-γ), which is dependent on the proinflammatory cytokine interleukin-12 (IL-12) for its high-level production by NK cells and Th1 cells (28, 45). IL-12 is composed of two subunits, p35 and p40, encoded by different genes, each of which must be expressed to generate the bioactive IL-12p70 heterodimer (53). While in many cells IL-12 synthesis is regulated at the level of p40 gene expression, in human monocytes and monocyte-derived dendritic cells (DCs), the expression of both subunits has been shown to be tightly controlled (16, 29, 47). The importance of IL-12 in immunity to experimental Leishmania major infection is clear: animals lacking the IL-12 genes, or genetically resistant mice treated with anti-IL-12 antibodies, are unable to heal, and treatment of susceptible BALB/c mice with recombinant IL-12 renders these animals resistant (3, 19, 52).
Given its central role in conditioning the adaptive immune response, the source of IL-12 and the stimuli necessary for its high-level induction have remained important issues to address. Although macrophages efficiently ingest Leishmania, they are not activated and their ability to produce IL-12 in response to strong proinflammatory stimuli is selectively impaired (7, 39, 42). In contrast, murine DCs, including epidermal Langerhans cells, take up L. major parasites, acquire a mature phenotype, and release IL-12p40 (5, 26, 55). Their role in promoting the development of Leishmania-specific Th1 immunity in vivo has also been shown (34, 56). In humans, infection of myeloid DCs with L. major up-regulates major histocompatibility complex class II and costimulatory molecules, and in conjunction with CD40 ligand (CD40L), these cells will produce large amounts of biologically active IL-12p70 (31). These data suggest that Leishmania-infected DCs may initiate a protective response following interaction with antigen-specific, CD40L-bearing T cells in lymphoid tissue. That CD40/CD40L interactions provide a necessary costimulus for IL-12 production in vivo is supported by outcomes in CD40L knockout mice and in mice treated with neutralizing anti-CD40L antibody, which in each case made reduced amounts of IL-12 and lost their ability to control L. major infection (3, 6, 18).
To the extent that development of cell-mediated immunity in human leishmaniasis also involves parasite- and CD40L-driven IL-12 production by DCs, it is possible that nonhealing, disseminating forms of leishmanial disease might be associated with strains having intrinsic defects in their ability to elicit an appropriate response in these cells. In contrast to L. major, which is invariably associated with localized, cutaneous ulcers that permanently heal within 2 to 6 months, cutaneous lesions due to Leishmania tropica tend to form dry ulcers which require a long time to heal, typically 1 to 2 years, and which can recur at the periphery of the original lesion, termed recidivans type (35, 36). In addition to cutaneous lesions, L. tropica has now been shown to cause a chronic debilitating systemic illness, termed viscerotropic leishmaniasis, observed in veterans of Operation Desert Storm (30). Lastly, L. tropica has been found in Middle Eastern, Kenyan. and Indian patients with classic kala-azar (visceral leishmaniasis) (33, 41, 43). The usual agent of visceral leishmaniasis in the Old World is Leishmania donovani, which typically produces little or no pathology at the site of inoculation but disseminates from the skin to produce uncontrolled, systemic disease. The present studies compare the infectious-stage metacyclic promastigotes of L. major, L. tropica, and L. donovani for their ability to activate human DCs, either alone or in conjunction with CD40L, for production of IL-12. While the uptakes of metacyclic promastigotes of these different species were comparable, IL-12p70 production was confined to DCs infected with certain strains of L. major, which provided an essential priming signal for transcriptional up-regulation of IL-12p35.
MATERIALS AND METHODS
DC generation.
CD14+ monocytes were obtained by elutriation from normal volunteer blood donors at the National Institutes of Health Clinical Center Department of Transfusion Medicine. Cells were frozen at 5 × 107cells/ml until use. Generation of DCs was as previously described (32). Briefly, monocytes were thawed, and 5 × 106 cells were cultured in six-well tissue culture plates in a volume of 2 ml. All cells were cultured in RPMI 1640 (source) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml at 37°C under 5% CO2. IL-4 (activity, ≥5 × 106 U/mg; PeptroTech EC Ltd., Rocky Hill, N.J.) and granulocyte-macrophage colony-stimulating factor (activity, ≥5 × 107 U/mg; PeptroTech EC Ltd.) were added to cultures on days 0, 3, and 6 at 50 ng/ml. Cells were harvested on days 7 to 10 of culture with 0.5 mM EDTA and washed twice with Mg- and Ca-free phosphate-buffered saline. Cell number and viability were determined by trypan blue exclusion, and during stimulation cells were cultured overnight without exogenous cytokine. The DCs used in this study were routinely CD14low, CD1a+, HLA-DR+, CD86+, and CD40+, as determined by flow cytometry.
Parasites.
The following Leishmania strains were used in this study: L. major NIH Friedlin V1 strain (MHOM/IL/80/FN), isolated from a patient with localized cutaneous leishmaniasis in Israel; L. major substrains IR173 (MHOM/IR/-173), CC-1 (MHOM/IR/83/LT252), and IR76 (MRHO/IR/76/ER), isolated from patients with localized cutaneous leishmaniasis in Iran; L. major LV39 (MRHO/SU/59/P), isolated from a gerbil reservoir in southern Russia; L. major NIH S strain (MHOM/SN/74/Seidman), isolated from a patient from Senegal, West Africa, with multiple subcutaneous nodules; L. major World Health Organization reference strain 5-ASKH (MHOM/SU/73/5-ASKH), a human cutaneous isolate from Turkmenskaya; L. tropica KK27 (MHOM/AF/88/KK27), isolated from a patient with a cutaneous lesion in Afghanistan; L. tropica NIH RUP (MHOM/AF/87/RUP), isolated from a reactivated lesion of a patient from Afghanistan; L. donovani strain 1S (MHOM/SD/62/1S), isolated from a patient with visceral leishmaniasis in Sudan; and L. donovani strains 9515 (MHOM/IN/95/9515) and 9520 (MHOM/IN/95/9520), isolated from splenic aspirates of patients with visceral leishmaniasis in India. All Leishmania parasites were cultured at 26°C without CO2 in medium 199 (M199) supplemented with 20% heat-inactivated fetal calf serum (HyClone Laboratories Inc., Logan, Utah), 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2 mM l-glutamine, 40 mM HEPES, 0.1 mM adenine (in 50 mM HEPES), 5 mg of hemin (in 50% triethanolamine) per ml, and 1 mg of 6-biotin/ml. Infective-stage metacyclic promastigotes were isolated from stationary cultures (4 to 5 days old) by negative selection using peanut agglutinin (Vector Laboratories Inc., Burlingame, Calif.) for L. major (40) and monoclonal antibody 1H2-A8 for L. tropica or MG-1 for L. donovani (27). For some experiments, metacyclic promastigotes of each strain were purified by using a uniform procedure based on a modification of a recently described method of density gradient purification (50). Briefly, 4 ml of a parasite suspension in Dulbecco modified Eagle medium containing approximately 1010 stationary-phase promastigotes was layered on a step gradient in a 15-ml conical Falcon tube containing 4 ml of 20% Ficoll stock solution made in distilled water and 4 ml of 10% Ficoll diluted in 10× M199 to a final 1× concentration of M199. The gradients were spun for 15 min at 1,300 × g at room temperature, and the parasites in the upper 10% Ficoll were collected and washed by centrifugation at 3,000 × g. Before infection parasites were opsonized with 5% normal human serum by incubation at 37°C for 30 min. Parasites tested negative for mycoplasma (PCR detection method; American Type Culture Collection, Manassas, Va.) and tested below the detection limits for endotoxin (<0.25 U/ml) (Limulus amoebocyte assay; Endosafe, Charleston, N.C.).
Infections.
DCs were cultured at 2 × 105 cells/0.3 ml in 48-well tissue culture plates. Recombinant human IFN-γ (PeptroTech EC Ltd.) and/or opsonized parasites were added at multiple infection ratios (ranging from 2 to 5 per DC). For all conditions, experiments were performed in duplicate. Cells were infected/or primed with IFN-γ (10 ng/ml) for 8 h before the addition of the second stimulus for the final 10 to 12 h. The second stimuli included CD40LT (a generous gift from Immunex Corporation, Seattle, Wash.), Staphylococcus aureus Cowan 1 bacteria (1/10,000 dilution of Sansorbin; Calbiochem, La Jolla, Calif.), and bacterial lipopolysaccharide (LPS) (100 ng/ml; Sigma, St. Louis, Mo.). Treatment of cultures with neutralizing, purified anticytokine antibodies, including monoclonal anti-human IFN-γ (1 μg/ml; PharMingen [San Diego, Calif.] clone B27), monoclonal anti-human IL-10 (100 ng/ml; PharMingen clone JES3-9D7), and polyclonal anti-human transforming growth factor-β1 (TGF-β1) (1 μg/ml; R&D Systems AF-246-NA), was initiated at the start of infection and was maintained throughout subsequent stimulation with CD40LT. Cell supernatants were collected after centrifugation and stored at −70°C until analyzed. Infected cells were harvested at the conclusion of the experiment, cytospins were prepared and Wright-Giemsa stained, and infections were monitored by light microscopy. Only the DCs with similar infection levels were compared.
Flow cytometry.
Cells were harvested with 0.5 mM EDTA, washed two times with PBS, and incubated with 10% normal mouse serum to block nonspecific binding before staining. Staining of cells was performed in 50-μl volumes of blocking buffer with phycoerythrin-conjugated antibodies, and the cells were evaluated using a FACSCalibur and CellQuest Software (Becton Dickinson, San Jose, Calif.). The anti-human monoclonal antibodies and isotype controls used included anti-CD1a clone HI149 (PharMingen), anti-CD14 clone M5E2 (PharMingen and Coulter), anti-HLA-DR clone 12-RD1 (Coulter), anti-CD86 clone BU63 (Caltag), anti-CD40 clone MAB89 (Immunotech), mouse immunoglobulin G1κ1 (IgG1κ) clone MOPC-21 (PharMingen), mouse IgG2ακ clone G155-178 (PharMingen), and mouse IgG2a-RD1 clone 7T4-1F5 (Coulter).
RT-PCR.
Relative levels of IL-12p35 and IL-12p40 mRNA were determined by real-time PCR. mRNA levels were analyzed after 8 h of stimulation. Total RNA was prepared with the SV Total RNA Isolation System (Promega Corp., Madison, Wis.). Five micrograms of total RNA was reverse transcribed using random hexamer primers with Superscript First-Strand Synthesis System for reverse transcription-PCR (RT-PCR) (Invitrogen, Carlsbad, Calif.) according to the manufacturer's instructions. For analysis of IL-12p35 and IL-12p40 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA expression, Taqman predeveloped assay kits were purchased from Applied Biosystems (Foster City, Calif.). Taqman reactions were performed according to the manufacturer's recommendations with an ABI Prism 7700 sequence detection system (Perkin-Elmer). The relative number of mRNA copies for p35 and p40 was determined by the following formula: number of copies = 2−ΔΔct, where ΔΔct = Δct(experimental) − Δct(calibrator), Δct = ct(experimental) − ct(GAPDH), ct = cycle at which there is a statistically significant increase in the emission intensity over the background, and Δct(calibrator) = mean Δct for the uninfected and unstimulated control from each donor.
Cytokine detection assays.
Secreted cytokines were detected in culture supernatants by using cytokine-specific enzyme-linked immunosorbent assays (ELISAs). Paired antibodies for IL-12p70, IL-10, and tumor necrosis factor alpha were purchased from Endogen (Woburn, Mass.). An IL-12p40 capture antibody from PharMingen was used with the IL-12p70 detecting antibody from Endogen to detect secreted IL-12p40. The sensitivities for the assays were as follows: for IL-12p70, 10 pg/ml; for IL-12p40, 10 pg/ml, for IL-10, 31 pg/ml; and for tumor necrosis factor alpha, 78 pg/ml. Assays were performed according to the Endogen guidelines. Statistical analysis was performed by a paired Student's t test.
RESULTS
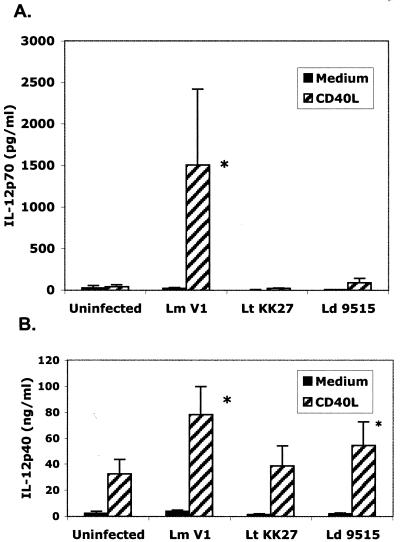
Metacyclic promastigotes of L. major have been previously shown to activate human DCs for CD40L-dependent IL-12p70 production (31). In agreement with these studies, L. major-infected DCs derived from 11 different donors in each case secreted significantly elevated levels of cytokines in response to a concentration of CD40L (0.5 μg/ml) that failed to induce IL-12p70 secretion in uninfected cells (Fig. 1A). In contrast, little or no CD40L-induced IL-12p70 secretion was observed in cells infected with L. tropica or L. donovani metacyclic promastigotes, despite comparable levels of parasite uptake by each of the donor cell preparations (the percentage of cells infected ranged from 50 to 65% for L. major, 33 to 81% for L. tropica, and 37 to 68% for L. donovani; the number of parasites per 100 cells ranged from 86 to 681 for L. major, 86 to 455 for L. tropica, and 59 to 396 for L. donovani). Because different reagents were used to purify metacyclic promastigotes from the different species, it was necessary to rule out any contribution of these lectins or antibodies to the response or lack of response observed. Thus, an alternative, uniform method of metacyclic promastigote purification, based on their differential sedimentation properties, was applied to each of the Old World species. A comparison of CD40L-induced IL-12p70 secretion by DCs infected with metacyclic promastigotes prepared by negative selection versus gradient centrifugation indicated that in each case, only the L. major metacyclic promastigotes activated the DCs for high-level IL-12 production in response to CD40L (data not shown).
FIG. 1.
CD40L-induced IL-12p70 secretion by human DCs is restricted to L. major-infected cells. Human DCs prepared from 11 different donors were infected with metacyclic promastigotes prepared from L. major (Lm) strain V1, L. tropica (Lt) strain KK27, or L. donovani (Ld) strain 9515, followed by stimulation with 0.5 μg of CD40L per ml. Supernatants were analyzed for IL-12p70 or IL-12p40 by ELISA, and the values represent the means ± standard errors of the means. An asterisk indicates a statistically significant difference compared to uninfected controls.
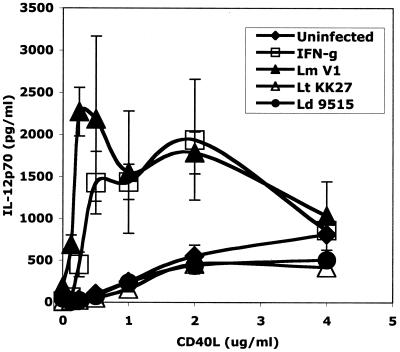
These striking differences in IL-12p70 secretion were not observed when IL-12p40 was assayed. CD40L induced substantial levels of IL-12p40 even in uninfected cells (30 ng/ml), and while the levels in the L. major-infected DCs were enhanced, the IL-12p40 levels produced by the L. tropica- and L. donovani-infected cells were not reduced and did not appear to be limiting with respect to heterodimer synthesis (Fig. 1B). That the L. tropica- and L. donovani-infected cells were capable of making IL-12p70 is indicated by the CD40L titration analysis, in which low levels of the heterodimer could be detected in culture supernatants of DCs stimulated with 1, 2, or 4 μg of CD40L per ml (Fig. 2). These levels appeared to be maximal at approximately 2 μg of CD40L per ml and were not significantly reduced compared to those for uninfected cells. On the other hand, there was no indication that uptake of L. tropica or L. donovani potentiated the response to CD40L at any concentration tested. In contrast, uptake of L. major clearly altered the response profile of the CD40L-stimulated cells, such that at 0.25 to 0.5 μg/ml, the peak levels of IL-12p70 produced by the L. major-infected DCs were four- to fivefold greater than the peak levels produced by the uninfected or L. donovani- or L. tropica-infected cells. The response profiles observed with L. major-infected cells were similar to those obtained with IFN-γ as a priming stimulus, and this is consistent with prior observations indicating that high-level production of IL-12p70 requires two signals (48).
FIG. 2.
CD40L titration in Leishmania-infected human DCs. DCs were infected with L. major (Lm) strain V1, L. tropica (Lt) strain KK27, or L. donovani (Ld) strain 9515 or stimulated with IFN-γ as a positive control. Infected or IFN-γ-treated cells were stimulated with different amounts of CD40L (0 to 4 μg/ml), and IL-12p70 secretion was assessed by ELISA. The values represent means ± standard errors of the means (n = 3 to 9 different donors).
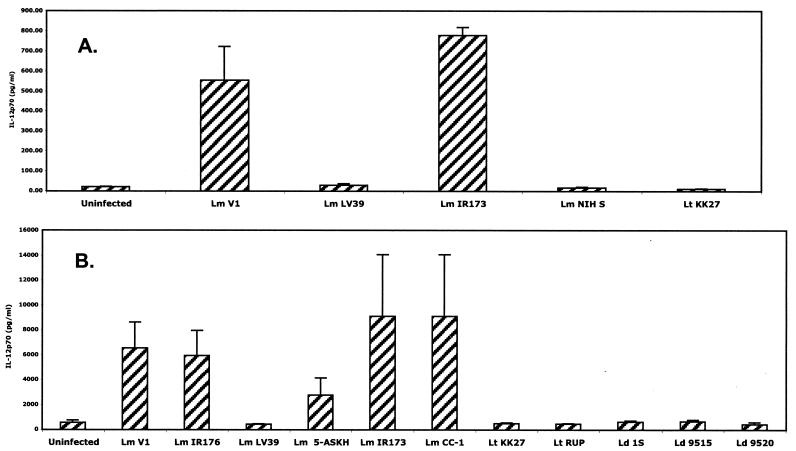
It was important to determine if the species differences in microbial priming for CD40L-dependent IL-12p70 production could be reproduced using other species-defined Leishmania isolates for infection. Figure 3 shows the levels of CD40L-induced IL-12p70 secretion by infected DCs from two different donors with seven distinct isolates of L. major, three of L. donovani, and two of L. tropica. The uptakes of serum-opsonized metacyclic promastigotes were again comparable in each case, in terms of both the percentage of cells infected and the number of parasites per cell (data not shown). Five of the seven L. major strains, including the strain from the Jordan Valley, the strain from Turkmenistan, and the three strains from Iran, effectively primed DCs. Each of these isolates was associated with localized cutaneous ulcers that eventually healed. In contrast, the L. major NIH S strain, which produced multiple subcutaneous nodules in a patient from West Africa, and the L. major LV39 strain, for which no clinical association is known as it was isolated from a gerbil reservoir, failed to provide a priming signal for CD40L-induced IL-12p70 secretion. Each of the L. tropica and L. donovani isolates consistently failed to activate DCs for CD40L-dependent IL-12p70 production.
FIG. 3.
CD40L-dependent IL-12p70 secretion by infected DCs is parasite species and strain dependent. Cells were infected using metacyclic promastigotes of the various strains indicated, followed by stimulation with 0.5 μg/ml CD40L. The supernatants were analyzed for IL-12p70 by ELISA. Results from two representative donors (A and B) are shown. Lm, L. major; Lt, L. tropica; Ld, L. donovani. Error bars indicate standard deviations.
Infection of human DCs with L. major has been shown to up-regulate expression of cell surface major histocompatibility complex class II and CD86 costimulatory molecules, and to slightly up-regulate CD40 (31). In the present studies, CD40 expression was already relatively high on uninfected DCs of all donors studied, but its up-regulation following infection was quite variable depending on the donor (data not shown). Overall, the level of CD40 surface expression on the infected cells did not correlate with the level of CD40L-induced IL-12p70 secretion. A similar variable pattern of HLA-DR up-regulation was observed on the infected DCs, while a small increase in the level of CD86 was a more consistent finding, both in response to the different parasite species and by most of the donor cells examined.
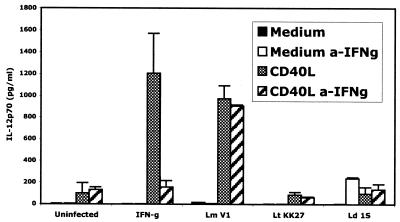
The possibility that uptake of L. major metacyclic promastigotes activated the DCs to produce IFN-γ, providing an autocrine priming stimulus in these assays, was explored by culturing the cells in the presence of neutralizing anti-IFN-γ antibodies (Fig. 4). While the IL-12p70 response of the cells primed with exogenous IFN-γ was inhibited by the anti-IFN-γ antibodies, the antibodies did not affect the response of the L. major-infected cells.
FIG. 4.
CD40L-induced IL-12p70 production by L. major-infected human DCs is not dependent on IFN-γ. Cells were infected with L. major (Lm) strain V1, L. tropica (Lt) strain KK27, and L. donovani (Ld) strain 9515, or stimulated with IFN-γ, in the presence or absence of antibodies against human IFN-γ (a-IFNg). IL-12p70 secretion in response to subsequent stimulation with 0.5 μg of CD40L per ml was determined by ELISA. Results for a single representative donor out of three donors are shown. Error bars indicate standard deviations.
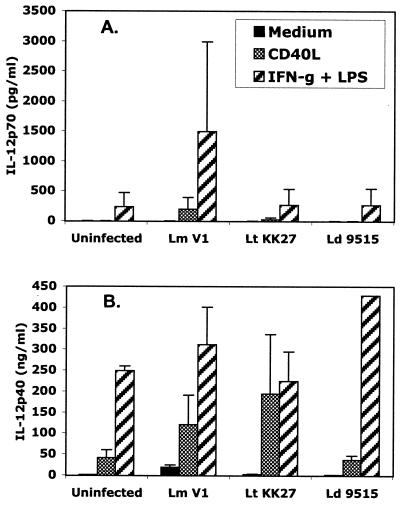
The roles of IL-10 and TGF-β as potential negative regulators of IL-12p70 induction in the L. donovani- and L. tropica-infected cells were addressed by treatment of the infected, CD40L-stimulated cells with anti-IL-10 or anti-TGF-β antibodies starting at the time of infection. The antibodies did not reveal any ability of the L. donovani- or L. tropica-infected cells to release IL-12p70 (data not shown). To more generally address the question of whether L. donovani and L. tropica actively suppress IL-12p70 induction pathways in DCs, the response of infected cells to alternative priming and triggering signals, IFN-γ and LPS, was assessed in a single donor. The CD40L-induced response of these cells was typical, with IL-12p70 secretion confined to the L. major-infected cells (Fig. 5A). In contrast, IFN-γ- and LPS-induced IL-12p70 secretion was observed in both uninfected and infected cells, and neither the L. tropica nor the L. donovani strain inhibited this response. Interestingly, L. major priming further enhanced the IFN-γ- and LPS-induced IL-12p70 response. The high levels of IL-12p40 produced in response to either CD40L or IFN-γ and LPS also were not inhibited, and in some cases were enhanced, in cells infected with either L. tropica or L. donovani (Fig. 5B).
FIG. 5.
IFN-γ- and LPS-induced IL-12p70 (A) and IL-12p40 (B) secretion is not inhibited in L. tropica- or L. donovani-infected human DCs. Cells were infected with L. major (Lm) strain V1, L. tropica (Lt) strain KK27, and L. donovani (Ld) strain 9515, followed by stimulation with either 0.5 μg of CD40L per ml or IFN-γ plus LPS. Supernatants were analyzed for IL-12p70 or IL-12p40 by ELISA. Results from a single donor are shown. Error bars indicate standard deviations.
RT-PCR analysis was performed to determine IL-12p40 and IL-12p35 mRNA levels in the infected and/or CD40L-stimulated cells. In all five donors analyzed (Table 1), CD40L did not elevate p35 message in uninfected cells, whereas the two L. major strains tested induced a slight up-regulation that was markedly increased following further stimulation with CD40L. In contrast, in three of the five donor DCs examined, IL-12p35 message was not elevated in L. tropica- and L. donovani-infected cells even in the presence of CD40L, and in the two DC preparations in which they were elevated, the baseline p35 levels in the uninfected cells were extremely low and the enhancement was 100- to 1,000-fold less than that in the L. major-infected cells. The L. major strains also provided a more powerful stimulus for IL-12p40 transcription in response to the parasites alone and especially in conjunction with CD40L. In this case, however, the L. tropica- or L. donovani-infected, CD40L-stimulated cells expressed elevated p40 message in all of the DC preparations examined.
TABLE 1.
RT-PCR analysis of IL-12p35 and IL-12p40 mRNA expression in infected and/or CD40L-stimulated DCs
| Donor no. | Infection | p35
|
p40
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Medium
|
CD40L
|
Medium
|
CD40L
|
||||||
| Level | Fold | Level | Fold | Level | Fold | Level | Fold | ||
| 1127 | Uninfected | 1.78 | 1.00 | 1.59 | 0.90 | 1.62 | 1.00 | 6.22 | 3.84 |
| L. major V1 | 7.96 | 4.47 | 274.95 | 154.34 | 2.12 | 1.31 | 37.95 | 23.43 | |
| L. major IR173 | 7.23 | 4.06 | 90.70 | 50.91 | 2.67 | 1.65 | 44.20 | 27.28 | |
| L. tropica KK | 8.65 | 4.86 | NDa | ND | 1.28 | 0.79 | ND | ND | |
| L. donovani 9515 | 1.67 | 0.94 | 2.40 | 1.35 | 0.14 | 0.08 | 25.92 | 16.00 | |
| 1139 | Uninfected | 45.98 | 1.00 | 45.35 | 0.99 | 63.38 | 1.00 | 116.98 | 1.84 |
| L. major V1 | 482.04 | 10.48 | 1,836.83 | 39.95 | 305.71 | 4.82 | 1,156.86 | 18.25 | |
| L. major IR173 | 286.62 | 6.23 | 4,336.56 | 94.35 | 281.31 | 4.44 | 4,347.59 | 68.59 | |
| L. tropica KK | 22.83 | 0.50 | 88.83 | 1.93 | 17.58 | 0.28 | 132.15 | 2.08 | |
| L. donovani 9515 | 31.41 | 0.68 | 39.21 | 0.85 | 106.60 | 1.68 | 258.85 | 4.08 | |
| 1150 | Uninfected | 5.71 | 1.00 | 1.41 | 0.25 | 0.71 | 1.00 | 1.19 | 1.69 |
| L. major V1 | 12.67 | 2.22 | 20.29 | 3.56 | 8.79 | 12.47 | 13.23 | 18.77 | |
| L. major IR173 | 9.94 | 1.74 | 31.41 | 5.50 | 0.71 | 1.00 | 16.87 | 23.92 | |
| L. tropica KK | 7.80 | 1.37 | 4.39 | 0.77 | 0.62 | 0.88 | 4.55 | 6.45 | |
| L. donovani 9515 | 2.68 | 0.47 | 1.40 | 0.24 | 0.74 | 1.04 | 2.56 | 3.63 | |
| 0414 | Uninfected | 0.02 | 1.00 | 0.01 | 0.34 | 1.22 | 1.00 | 0.12 | 0.10 |
| L. major V1 | 60.67 | 3,444.31 | 850.99 | 48,308.85 | 18.52 | 15.14 | 216.43 | 178.53 | |
| L. major IR173 | ND | ND | ND | ND | ND | ND | ND | ND | |
| L. tropica KK | 0.25 | 14.27 | 9.05 | 512.78 | 1.19 | 0.97 | 13.94 | 11.39 | |
| L. donovani 9515 | 0.32 | 17.94 | 4.40 | 249.86 | 1.71 | 1.40 | 17.83 | 14.57 | |
| 0456 | Uninfected | 0.12 | 1.00 | 0.11 | 0.93 | 0.01 | 1.00 | 0.96 | 85.33 |
| L. major V1 | 0.88 | 7.24 | 21.01 | 173.04 | 0.22 | 19.84 | 6.30 | 560.28 | |
| L. major IR173 | 1.10 | 9.03 | 1,182.81 | 9,741.98 | 0.51 | 45.57 | 342.75 | 30,467.85 | |
| L. tropica KK | 0.03 | 0.27 | 1.76 | 14.52 | 0.00 | 0.35 | 1.41 | 125.37 | |
| L. donovani 9515 | 0.06 | 0.47 | 0.30 | 2.44 | 0.00 | 0.26 | 0.58 | 51.98 | |
ND, not determined.
DISCUSSION
Langerhans cells and DCs are antigen-presenting cells that are actively involved in the surveillance of their environment in the skin. Following an encounter with certain microbes or their products, these cells can initiate a maturation process that promotes their migration to lymphoid tissue and their function as potent activators of naive T cells (2). The ability of sand fly-derived metacyclic promastigotes to be taken up by and activate DCs in the skin is therefore a likely early and critical event in the development of antileishmanial immunity. IL-12 production is a crucial component of the DC maturation response for selective activation of T cells along a Th1 pathway. In addition to certain microbial stimuli, CD40 engagement by activated T cells is thought to provide an especially powerful signal for IL-12 production by DCs (9, 10, 25). L. major metacyclic promastigotes and amastigotes have been shown previously to prime human DCs for high-level IL-12p70 production in response to CD40L. This response is consistent with the powerful cell-mediated immunity that develops in human hosts following infection with L. major and the complete, relatively rapid resolution of the cutaneous lesions that typically occurs. In the present studies, we explored the possibility that the nonhealing, systemic diseases which are a hallmark of infections involving L. donovani and L. tropica are related to intrinsic defects in the ability of these strains to up-regulate IL-12 induction pathways in DCs. Using relatively immature DCs derived from human peripheral blood CD14+ cells, metacyclic promastigotes of each of the three Old World species were efficiently taken up by these cells, but only L. major metacyclic promastigotes were able to prime DCs for CD40L-dependent IL-12p70 secretion. In contrast, L. donovani and L. tropica failed to deliver a microbial priming signal required for high-level expression of IL-12p40, and especially IL-12p35, mRNA.
The ability of L. major to activate human DCs for IL-12p70 production in a CD40L-dependent manner is in agreement with prior in vitro studies indicating that two signals are required for high-level production of heterodimeric IL-12p70 by DCs (21, 37, 44, 48). Uptake of the parasites alone provided an insufficient stimulus, even for production of IL-12p40. In murine bone marrow- or skin-derived DCs, even when Leishmania amastigotes or promastigotes induced detectable IL-12p40 secretion by these cells, no heterodimer secretion was detected (4, 26, 55). The ability of Leishmania to prime murine DCs for CD40L-induced IL-12p70 production has not, so far as we are aware, been investigated. In contrast to these results involving microbial priming, there is some evidence with both murine and human DCs that CD40 engagement alone will activate IL-12p70 production by these cells (10, 20, 46). However, as pointed out previously (48), the activated T cells used for CD40 engagement in those studies also secrete IFN-γ. In the present studies, while high concentrations of CD40L were able to induce some IL-12p70 secretion by uninfected cells, these levels remained far lower than the peak levels produced by CD40L-stimulated cells previously exposed to L. major or to IFN-γ. These treatments in each case lowered the concentration of CD40L required for optimal triggering of the IL-12 response. The ability of L. major to so effectively prime DCs for IL-12 production could be attributed to effects on the expression of IL-12p35 and IL-12p40 mRNAs, which were in each case markedly up-regulated following sequential exposure to L. major and CD40L. The expression of the IL-12p35 subunit, which in other cell types can be constitutively expressed (8, 12), appeared to be more tightly regulated than that of the IL-12p40 subunit in these cells, consistent with previous reports (17, 47). The amount of IL-12p40 secreted by uninfected and L. donovani- or L. tropica-infected DCs in response to CD40L was in many instances sufficiently large to argue against IL-12p40 being limiting with respect to heterodimer synthesis. That IL-12p35 was in fact limiting was supported by the RT-PCR results indicating that two signals were required for its expression.
CD40 engagement via activated T cells is thought to provide an especially relevant physiologic signal for antigen-driven IL-12 production by DCs (24, 51). Its role in acquired resistance in leishmaniasis is strongly supported by the ability of CD40L to potentiate vaccine-induced immunity to L. major (11, 15) and by the failure of mice with induced or genetic deficiencies in CD40 engagement to control infection, which in each case was associated with impaired production of IL-12 (3, 6, 18, 49). That CD40 ligation alone is insufficient for IL-12 production in vivo has been demonstrated in mice, which required a microbial exposure using a soluble antigen derived from Toxoplasma gondii to produce high levels of the cytokine following treatment with agonistic anti-CD40 antibodies (44). In those studies, the microbial stimulus was found to induce IL-12p40 and to up-regulate CD40 expression on splenic DCs, which was reasoned to be an essential and sufficient condition for induction of IL-12p35 by CD40L or anti-CD40 antibodies. We do not believe that the main effect of L. major priming on monocyte-derived human DCs in vitro is to augment CD40 expression above a threshold necessary for triggering. As previously reported and confirmed here, the levels of CD40 expression on these cytokine-derived cells are already high (14, 22, 31), and CD40 engagement did induce substantial levels of IL-12p40 secretion by uninfected cells. Furthermore, while CD40 up-regulation following uptake of L. major was in some instances substantially higher than that following uptake of the other strains, with other donor cells the up-regulated expression of CD40 was similar, and still the differences in CD40L-induced IL-12p70 production were observed. These results and others (1, 23) argue that CD40 engagement is not a sufficient condition for high-level IL-12p35 mRNA expression and that microbial priming affects other components of the CD40 signaling pathway. Whether or not these downstream events are identical to those induced by IFN-γ, which alters the initiation site for IL-12p35 gene transcription in human monocytes (16), needs to be determined. The ability of L. major to prime DCs did not, however, appear to involve an autocrine IFN-γ response, since addition of neutralizing anti-IFN-γ antibodies to the cultures did not inhibit the priming effect.
It is interesting that not all of the L. major strains tested provided the priming stimulus required for IL-12p70 secretion. Two of seven strains failed to do so, and it may be relevant that one of the strains (NIH S) differs from the other human L. major isolates in that it produced multiple and persistent subcutaneous nodules that were difficult to treat (38). The clinical association of the LV39 strain is not known, as it was isolated from a natural rodent host. A crucial question raised by the present data is whether these L. major substrains, as well as the L. donovani and L. tropica strains, lack the appropriate ligands or else overcome their priming potential by actively suppressing the signaling pathways involved. The L. tropica and L. donovani strains did not significantly inhibit the CD40L-induced IL-12p40 response of DCs or the IL-12p70 response in cells activated with high concentrations of CD40L. In addition, they were unable to inhibit the IL-12p70 or IL-12p40 secretion seen in response to alternative priming and triggering stimuli (IFN-γ plus LPS). Neutralization of either IL-10 or TGF-β, potential autocrine inhibitors of IL-12p70 production by DCs, did not rescue a response in L. donovani- or L. tropica-infected cells, again consistent with the interpretation that these strains fail to provide an appropriate priming signal rather than induce an inhibitory pathway. It should also be noted that in contrast to its encounter with cultured human myeloid DCs, L. donovani has been shown to induce IL-12p40 by splenic DCs in the mouse following intravenous inoculation (13). In contrast to humans, however, all mouse strains demonstrate at least partial resistance to L. donovani, as high-dose intravenous challenge is required to establish visceral infection, and progressive, fatal disease is never observed. While it is true that many individuals who are exposed to L. donovani also do not develop disease, there is little evidence that this is due to their ability to mount an acquired immune response as opposed to expression of some innate resistance.
The molecules that distinguish Leishmania species and account for the spectrum of clinical outcomes that they typically produce, i.e., cutaneous versus systemic disease and healing versus nonhealing, remain essentially unknown. Of possible relevance is the L. donovani-specific A2 gene, which when introduced into L. major increased its ability to survive in the spleens of BALB/c mice (57). The present studies, while not identifying the virulence factors per se, have identified a possible point of departure for their activities in the host. The finding that Leishmania species can have intrinsic differences in their ability to induce crucial elements of the innate response suggests that they differ with respect to the molecules that can engage the set of pattern recognition receptors through which DC activation signals are known to occur. We are currently investigating the possibility that these interactions are influenced by the inter- and intraspecies polymorphisms displayed by the family of abundant Leishmania cell surface and secreted phosphoglycans (54).
Acknowledgments
We thank Brian Kelsall, Alan Sher, Genevieve Milon, and Mark Udey for critical review of the manuscript and Immunex Corporation, Seattle, Wash., for generous donation of CD40LT.
Editor: J. M. Mansfield
REFERENCES
- 1.Aicher, A., G. L. Shu, D. Magaletti, T. Mulvania, A. Pezzutto, A. Craxton, and E. A. Clark. 1999. Differential role for p38 mitogen-activated protein kinase in regulating CD40-induced gene expression in dendritic cells and B cells. J. Immunol. 163:5786-5795. [PubMed] [Google Scholar]
- 2.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid, Y., S. Mendez, R. Lira, N. Kadambi, G. Milon, and D. Sacks. 2000. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165:969-977. [DOI] [PubMed] [Google Scholar]
- 4.Bennett, C. L., A. Misslitz, L. Colledge, T. Aebischer, and C. C. Blackburn. 2001. Silent infection of bone marrow-derived dendritic cells by Leishmania mexicana amastigotes. Eur. J. Immunol. 31:876-883. [DOI] [PubMed] [Google Scholar]
- 5.Blank, C., H. Fuchs, K. Rappersberger, M. Rollinghoff, and H. Moll. 1993. Parasitism of epidermal Langerhans cells in experimental cutaneous leishmaniasis with Leishmania major. J. Infect. Dis. 167:418-425. [DOI] [PubMed] [Google Scholar]
- 6.Campbell, K. A., P. J. Ovendale, M. K. Kennedy, W. C. Fanslow, S. G. Reed, and C. R. Maliszewski. 1996. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity 4:283-289. [DOI] [PubMed] [Google Scholar]
- 7.Carrera, L., R. T. Gazzinelli, R. Badolato, S. Hieny, W. Muller, R. Kuhn, and D. L. Sacks. 1996. Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J. Exp. Med. 183:515-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassatella, M. A., L. Meda, S. Gasperini, A. D'Andrea, X. Ma, and G. Trinchieri. 1995. Interleukin-12 production by human polymorphonuclear leukocytes. Eur. J. Immunol. 25:1-5. [DOI] [PubMed] [Google Scholar]
- 9.Caux, C., C. Massacrier, B. Vanbervliet, B. Dubois, C. Van Kooten, I. Durand, and J. Banchereau. 1994. Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 180:1263-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cella, M., D. Scheidegger, K. Palmer-Lehmann, P. Lane, A. Lanzavecchia, and G. Alber. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184:747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, G., P. A. Darrah, and D. M. Mosser. 2001. Vaccination against the intracellular pathogens Leishmania major and L. amazonensis by directing CD40 ligand to macrophages. Infect. Immun. 69:3255-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Andrea, A., M. Rengaraju, N. M. Valiante, J. Chehimi, M. Kubin, M. Aste, S. H. Chan, M. Kobayashi, D. Young, E. Nickbarg, et al. 1992. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J. Exp. Med. 176:1387-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorak, P. M., C. R. Engwerda, and P. M. Kaye. 1998. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur. J. Immunol. 28:687-695. [DOI] [PubMed] [Google Scholar]
- 14.Goriely, S., B. Vincart, P. Stordeur, J. Vekemans, F. Willems, M. Goldman, and D. De Wit. 2001. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol. 166:2141-2146. [DOI] [PubMed] [Google Scholar]
- 15.Gurunathan, S., K. R. Irvine, C. Y. Wu, J. I. Cohen, E. Thomas, C. Prussin, N. P. Restifo, and R. A. Seder. 1998. CD40 ligand/trimer DNA enhances both humoral and cellular immune responses and induces protective immunity to infectious and tumor challenge. J. Immunol. 161:4563-4571. [PMC free article] [PubMed] [Google Scholar]
- 16.Hayes, M. P., F. J. Murphy, and P. R. Burd. 1998. Interferon-gamma-dependent inducible expression of the human interleukin-12 p35 gene in monocytes initiates from a TATA-containing promoter distinct from the CpG-rich promoter active in Epstein-Barr virus-transformed lymphoblastoid cells. Blood 91:4645-4651. [PubMed] [Google Scholar]
- 17.Hayes, M. P., J. Wang, and M. A. Norcross. 1995. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-gamma of lipopolysaccharide-inducible p35 and p40 genes. Blood 86:646-650. [PubMed] [Google Scholar]
- 18.Heinzel, F. P., R. M. Rerko, and A. M. Hujer. 1998. Underproduction of interleukin-12 in susceptible mice during progressive leishmaniasis is due to decreased CD40 activity. Cell. Immunol. 184:129-142. [DOI] [PubMed] [Google Scholar]
- 19.Heinzel, F. P., D. S. Schoenhaut, R. M. Rerko, L. E. Rosser, and M. K. Gately. 1993. Recombinant interleukin 12 cures mice infected with Leishmania major. J. Exp. Med. 177:1505-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heufler, C., F. Koch, U. Stanzl, G. Topar, M. Wysocka, G. Trinchieri, A. Enk, R. M. Steinman, N. Romani, and G. Schuler. 1996. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur. J. Immunol. 26:659-668. [DOI] [PubMed] [Google Scholar]
- 21.Hilkens, C. M., P. Kalinski, M. de Boer, and M. L. Kapsenberg. 1997. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood 90:1920-1926. [PubMed] [Google Scholar]
- 22.Kalinski, P., C. M. Hilkens, A. Snijders, F. G. Snijdewint, and M. L. Kapsenberg. 1997. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J. Immunol. 159:28-35. [PubMed] [Google Scholar]
- 23.Kato, T., R. Hakamada, H. Yamane, and H. Nariuchi. 1996. Induction of IL-12 p40 messenger RNA expression and IL-12 production of macrophages via CD40-CD40 ligand interaction. J. Immunol. 156:3932-3938. [PubMed] [Google Scholar]
- 24.Kelsall, B. L., E. Stuber, M. Neurath, and W. Strober. 1996. Interleukin-12 production by dendritic cells. The role of CD40-CD40L interactions in Th1 T-cell responses. Ann. N.Y. Acad. Sci. 795:116-126. [DOI] [PubMed] [Google Scholar]
- 25.Koch, F., U. Stanzl, P. Jennewein, K. Janke, C. Heufler, E. Kampgen, N. Romani, and G. Schuler. 1996. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 184:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konecny, P., A. J. Stagg, H. Jebbari, N. English, R. N. Davidson, and S. C. Knight. 1999. Murine dendritic cells internalize Leishmania major promastigotes, produce IL-12 p40 and stimulate primary T cell proliferation in vitro. Eur. J. Immunol. 29:1803-1811. [DOI] [PubMed] [Google Scholar]
- 27.Lira, R., S. Sundar, A. Makharia, R. Kenney, A. Gam, E. Saraiva, and D. Sacks. 1999. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J. Infect. Dis. 180:564-567. [DOI] [PubMed] [Google Scholar]
- 28.Louis, J., H. Himmelrich, C. Parra-Lopez, F. Tacchini-Cottier, and P. Launois. 1998. Regulation of protective immunity against Leishmania major in mice. Curr. Opin. Immunol. 10:459-464. [DOI] [PubMed] [Google Scholar]
- 29.Ma, X., J. M. Chow, G. Gri, G. Carra, F. Gerosa, S. F. Wolf, R. Dzialo, and G. Trinchieri. 1996. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J. Exp. Med. 183:147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magill, A. J., M. Grogl, R. A. Gasser, Jr., W. Sun, and C. N. Oster. 1993. Visceral infection caused by Leishmania tropica in veterans of Operation Desert Storm. N. Engl. J. Med. 328:1383-1387. [DOI] [PubMed] [Google Scholar]
- 31.Marovich, M. A., M. A. McDowell, E. K. Thomas, and T. B. Nutman. 2000. IL-12p70 production by Leishmania major-harboring human dendritic cells is a CD40/CD40 ligand-dependent process. J. Immunol. 164:5858-5865. [DOI] [PubMed] [Google Scholar]
- 32.McRae, B. L., T. Nagai, R. T. Semnani, J. M. van Seventer, and G. A. van Seventer. 2000. Interferon-alpha and -beta inhibit the in vitro differentiation of immunocompetent human dendritic cells from CD14(+) precursors. Blood 96:210-217. [PubMed] [Google Scholar]
- 33.Mebrahtu, Y., P. Lawyer, J. Githure, J. B. Were, R. Muigai, L. Hendricks, J. Leeuwenburg, D. Koech, and C. Roberts. 1989. Visceral leishmaniasis unresponsive to pentostam caused by Leishmania tropica in Kenya. Am. J. Trop. Med. Hyg. 41:289-294. [DOI] [PubMed] [Google Scholar]
- 34.Moll, H., and S. Flohe. 1997. Dendritic cells induce immunity to cutaneous leishmaniasis in mice. Adv. Exp. Med. Biol. 417:541-545. [DOI] [PubMed] [Google Scholar]
- 35.Momeni, A. Z., and M. Aminjavaheri. 1994. Clinical picture of cutaneous leishmaniasis in Isfahan, Iran. Int. J. Dermatol. 33:260-265. [DOI] [PubMed] [Google Scholar]
- 36.Momeni, A. Z., and M. Aminjavaheri. 1995. Treatment of recurrent cutaneous Leishmaniasis. Int. J. Dermatol. 34:129-133. [DOI] [PubMed] [Google Scholar]
- 37.Mosca, P. J., A. C. Hobeika, T. M. Clay, S. K. Nair, E. K. Thomas, M. A. Morse, and H. K. Lyerly. 2000. A subset of human monocyte-derived dendritic cells expresses high levels of interleukin-12 in response to combined CD40 ligand and interferon-gamma treatment. Blood 96:3499-3504. [PubMed] [Google Scholar]
- 38.Neva, F. A., D. Wyler, and T. Nash. 1979. Cutaneous leishmaniasis—a case with persistent organisms after treatment in presence of normal immune response. Am. J. Trop. Med. Hyg. 28:467-471. [PubMed] [Google Scholar]
- 39.Reiner, S. L., S. Zheng, Z. E. Wang, L. Stowring, and R. M. Locksley. 1994. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J. Exp. Med. 179:447-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sacks, D. L., S. Hieny, and A. Sher. 1985. Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of Leishmania major promastigotes. J. Immunol. 135:564-569. [PubMed] [Google Scholar]
- 41.Sacks, D. L., R. T. Kenney, R. D. Kreutzer, C. L. Jaffe, A. K. Gupta, M. C. Sharma, S. P. Sinha, F. A. Neva, and R. Saran. 1995. Indian kala-azar caused by Leishmania tropica. Lancet 345:959-961. [DOI] [PubMed] [Google Scholar]
- 42.Sartori, A., M. A. Oliveira, P. Scott, and G. Trinchieri. 1997. Metacyclogenesis modulates the ability of Leishmania promastigotes to induce IL-12 production in human mononuclear cells. J. Immunol. 159:2849-2857. [PubMed] [Google Scholar]
- 43.Schnur, L. F., M. L. Chance, F. Ebert, S. C. Thomas, and W. Peters. 1981. The biochemical and serological taxonomy of visceralizing leishmania. Ann. Trop. Med. Parasitol. 75:131-144. [DOI] [PubMed] [Google Scholar]
- 44.Schulz, O., D. A. Edwards, M. Schito, J. Aliberti, S. Manickasingham, A. Sher, and C. Reis e Sousa. 2000. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity 13:453-462. [DOI] [PubMed] [Google Scholar]
- 45.Scott, P., B. Hondowicz, A. Eaton, and T. Scharton-Kersten. 1996. The role of IL-12 in regulation of T helper cell subsets in vivo. Lessons from experimental cutaneous leishmaniasis. Ann. N.Y. Acad. Sci. 795:250-256. [DOI] [PubMed] [Google Scholar]
- 46.Shu, U., M. Kiniwa, C. Y. Wu, C. Maliszewski, N. Vezzio, J. Hakimi, M. Gately, and G. Delespesse. 1995. Activated T cells induce interleukin-12 production by monocytes via CD40-CD40 ligand interaction. Eur. J. Immunol. 25:1125-1128. [DOI] [PubMed] [Google Scholar]
- 47.Snijders, A., C. M. Hilkens, T. C. van der Pouw Kraan, M. Engel, L. A. Aarden, and M. L. Kapsenberg. 1996. Regulation of bioactive IL-12 production in lipopolysaccharide-stimulated human monocytes is determined by the expression of the p35 subunit. J. Immunol. 156:1207-1212. [PubMed] [Google Scholar]
- 48.Snijders, A., P. Kalinski, C. M. Hilkens, and M. L. Kapsenberg. 1998. High-level IL-12 production by human dendritic cells requires two signals. Int. Immunol. 10:1593-1598. [DOI] [PubMed] [Google Scholar]
- 49.Soong, L., J. C. Xu, I. S. Grewal, P. Kima, J. Sun, B. J. Longley, Jr., N. H. Ruddle, D. McMahon-Pratt, and R. A. Flavell. 1996. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity 4:263-273. [DOI] [PubMed] [Google Scholar]
- 50.Spath, G. F., and S. M. Beverley. 2001. An LPG-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp. Parasitol. 99:97-103. [DOI] [PubMed] [Google Scholar]
- 51.Stuber, E., W. Strober, and M. Neurath. 1996. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J. Exp. Med. 183:693-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sypek, J. P., C. L. Chung, S. E. Mayor, J. M. Subramanyam, S. J. Goldman, D. S. Sieburth, S. F. Wolf, and R. G. Schaub. 1993. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J. Exp. Med. 177:1797-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trinchieri, G. 1998. Immunobiology of interleukin-12. Immunol. Res. 17:269-278. [DOI] [PubMed] [Google Scholar]
- 54.Turco, S. J., G. F. Spath, and S. M. Beverley. 2001. Is lipophosphoglycan a virulence factor? A surprising diversity between Leishmania species. Trends Parasitol. 17:223-226. [DOI] [PubMed] [Google Scholar]
- 55.von Stebut, E., Y. Belkaid, T. Jakob, D. L. Sacks, and M. C. Udey. 1998. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J. Exp. Med. 188:1547-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von Stebut, E., Y. Belkaid, B. V. Nguyen, M. Cushing, D. L. Sacks, and M. C. Udey. 2000. Leishmania major-infected murine langerhans cell-like dendritic cells from susceptible mice release IL-12 after infection and vaccinate against experimental cutaneous Leishmaniasis. Eur. J. Immunol. 30:3498-3506. [DOI] [PubMed] [Google Scholar]
- 57.Zhang, W. W., and G. Matlashewski. 2001. Characterization of the A2-A2rel gene cluster in Leishmania donovani: involvement of A2 in visceralization during infection. Mol. Microbiol. 39:935-948. [DOI] [PubMed] [Google Scholar]