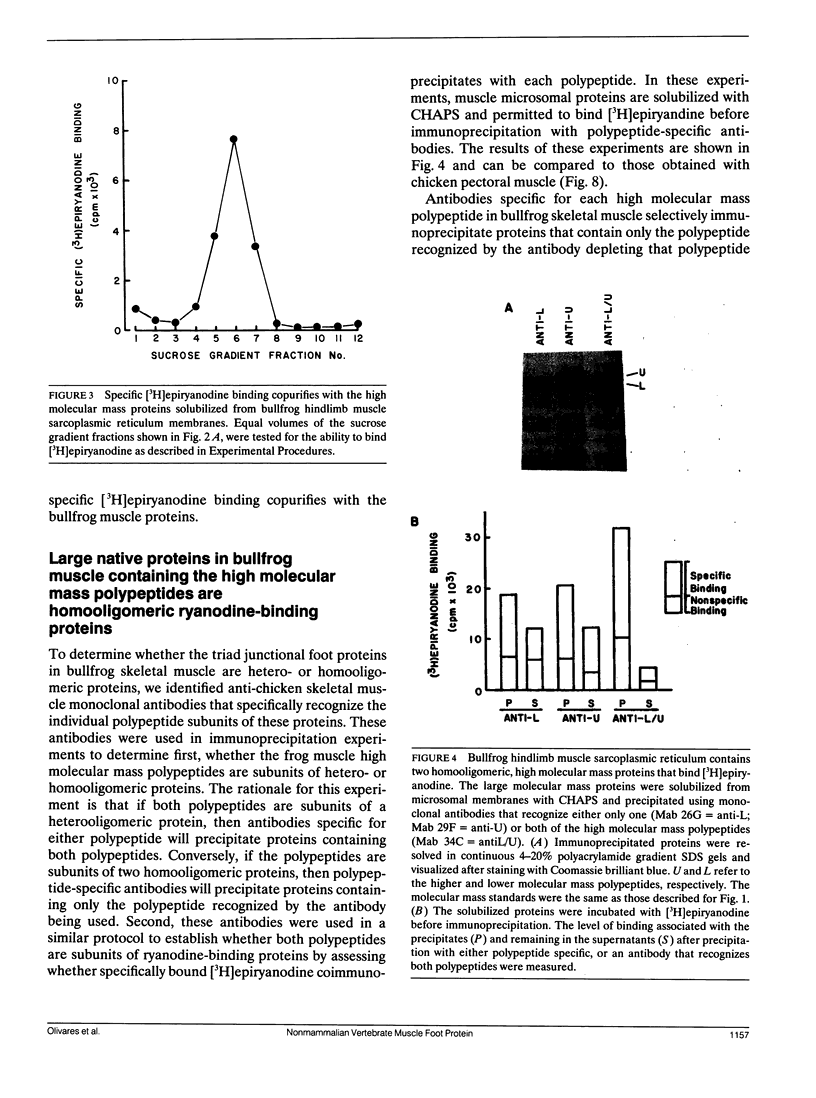
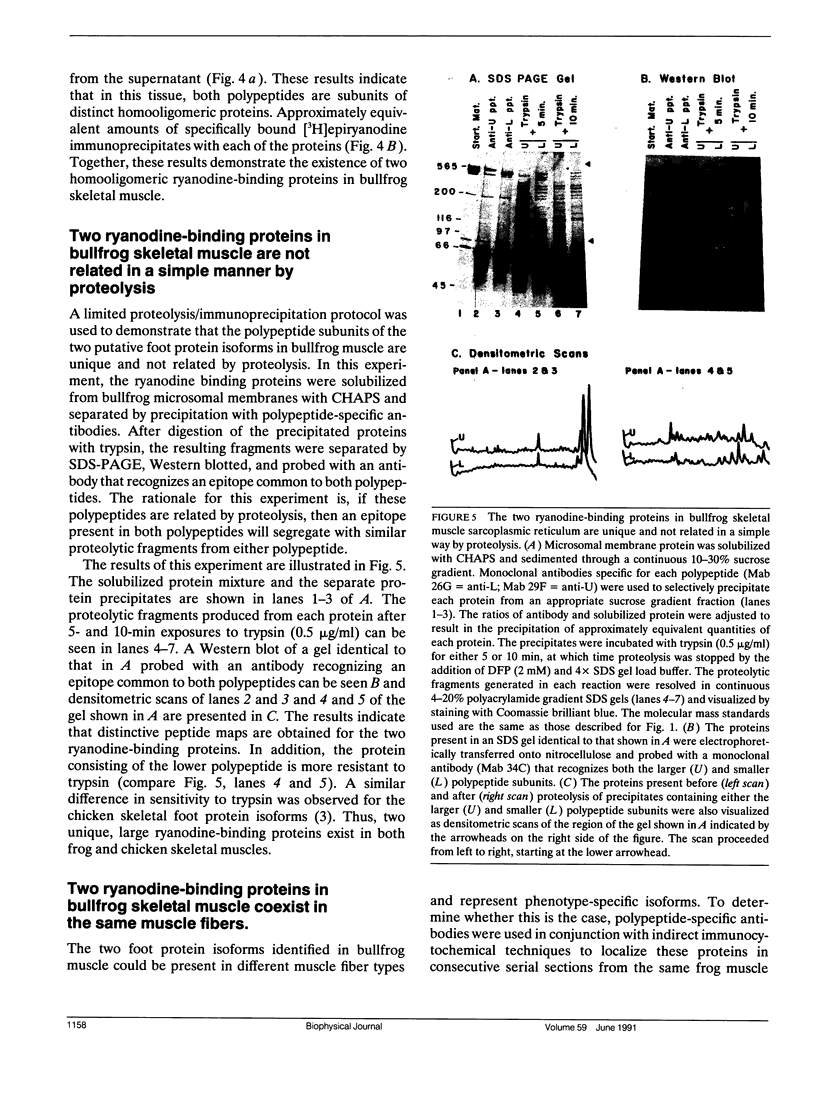
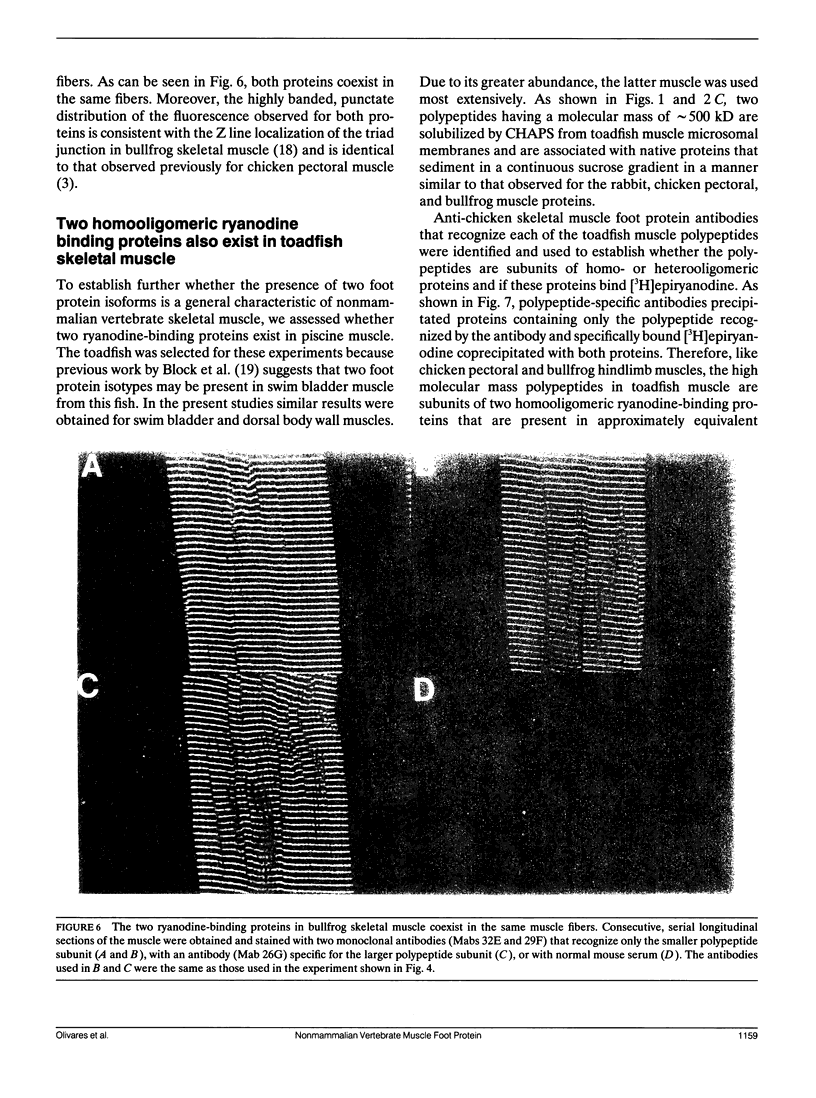
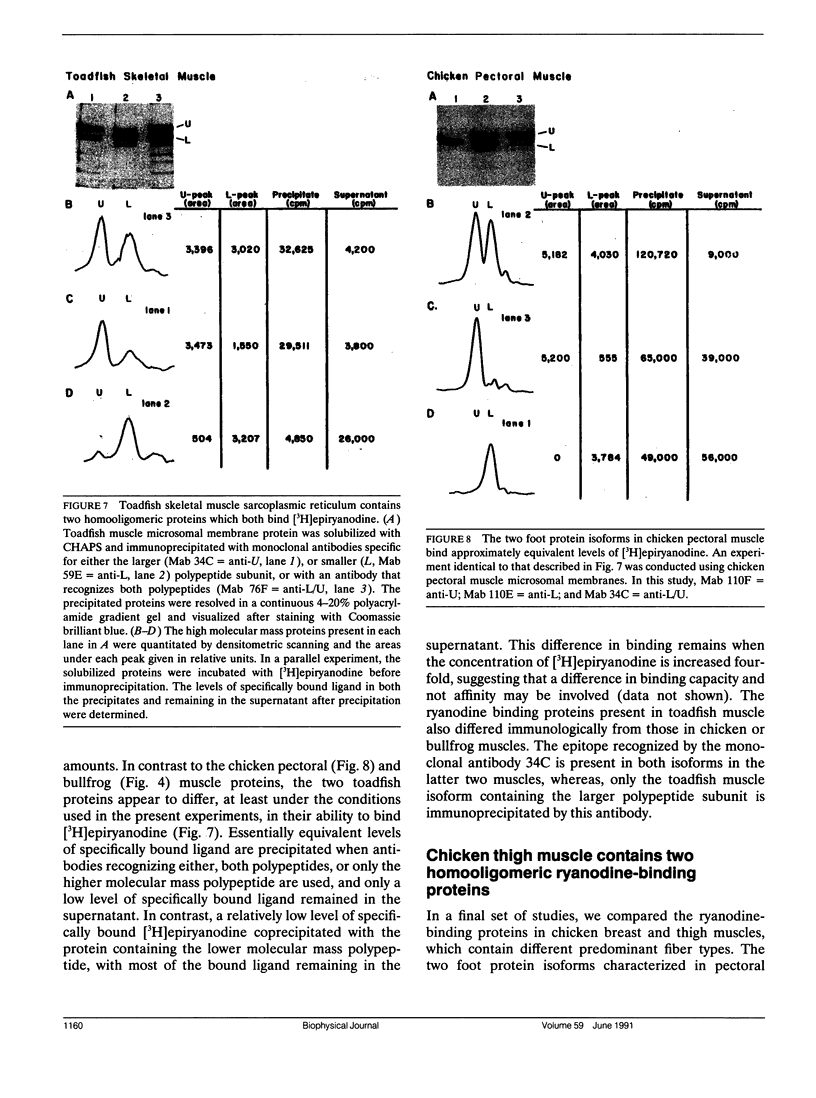
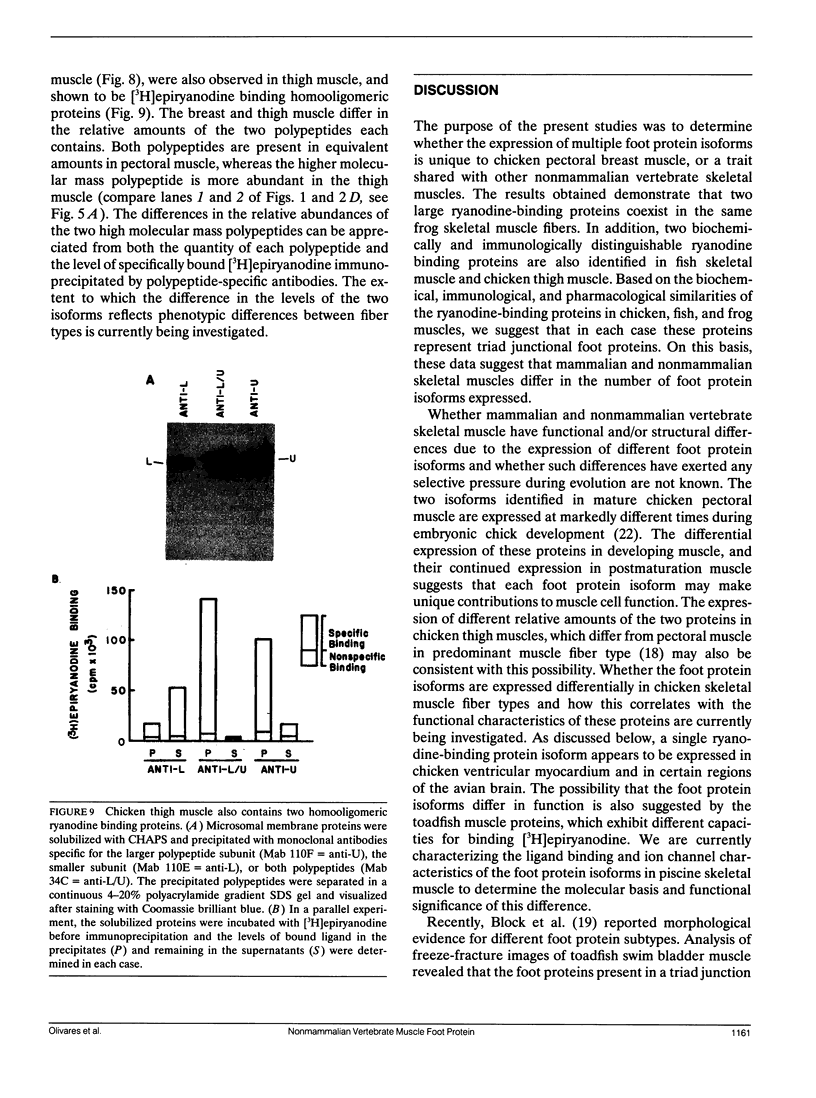
Abstract
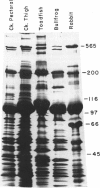
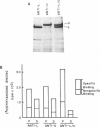
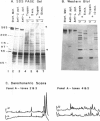
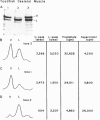
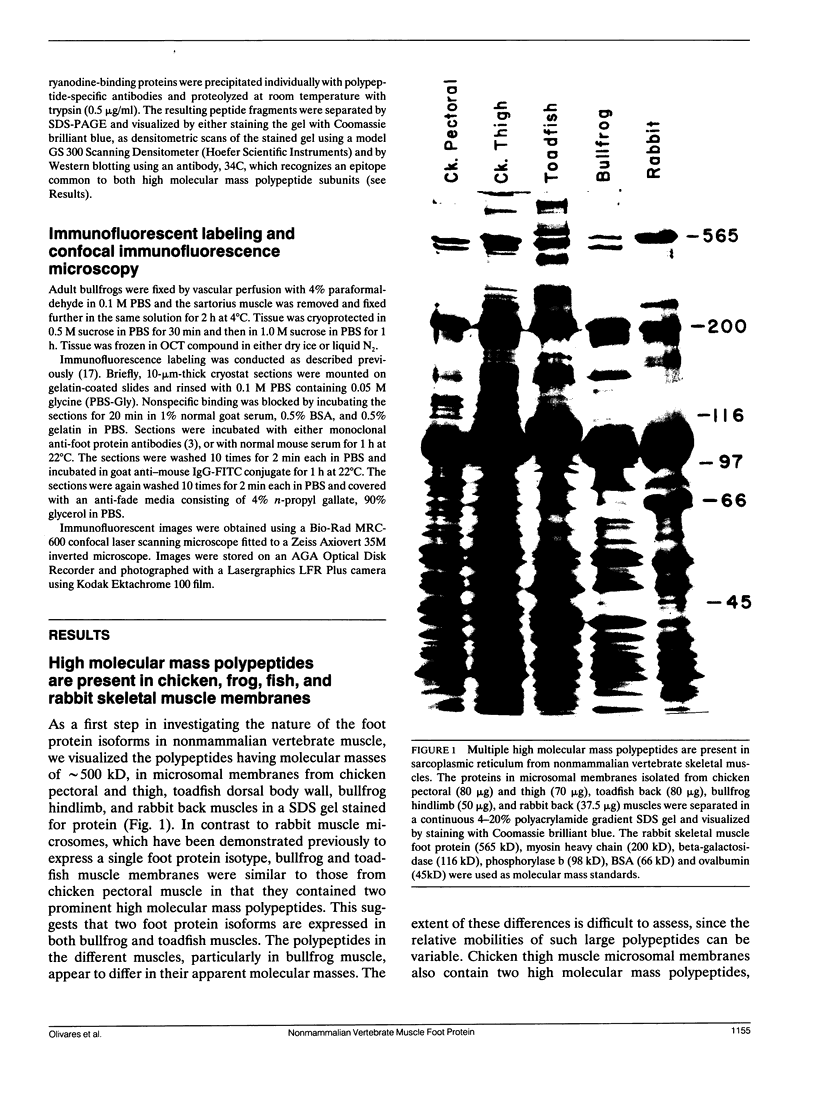
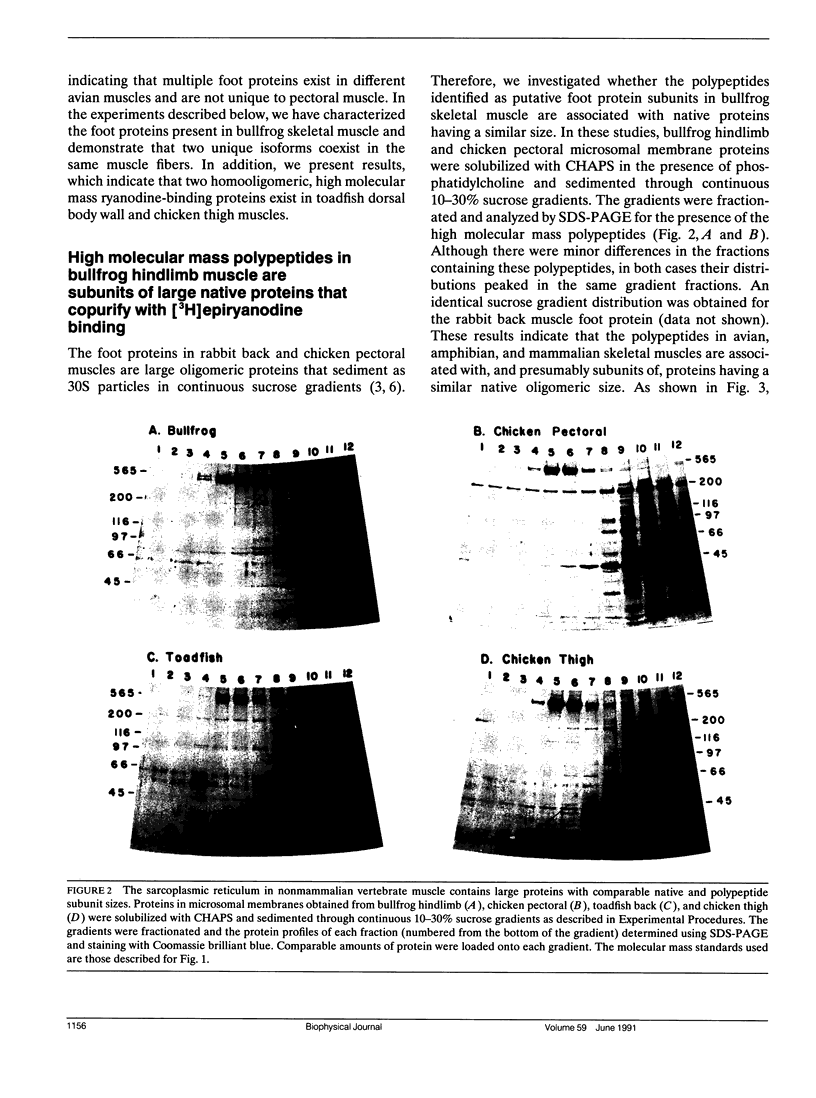
Mammalian skeletal muscles express a single triad junctional foot protein, whereas avian muscles have two isoforms of this protein. We investigated whether either case is representative of muscles from other vertebrate classes. We identified two foot proteins in bullfrog and toadfish muscles on the basis of (a) copurification with [3H]epiryanodine binding; (b) similarity to avian muscle foot proteins in native and subunit molecular weights; (c) recognition by anti-foot protein antibodies. The bullfrog and toadfish proteins exist as homooligomers. The subunits of the bullfrog muscle foot protein isoforms are shown to be unique by peptide mapping. In addition, immunocytochemical localization established that the bullfrog muscle isoforms coexist in the same muscle cells. The isoforms in either bullfrog and chicken muscles have comparable [3H]epiryanodine binding capacities, whereas in toadfish muscle the isoforms differ in their levels of ligand binding. Additionally, chicken thigh and breast muscles differ in the relative amounts of the two isoforms they contain, the amounts being similar in breast muscle and markedly different in thigh muscle. In conclusion, in contrast to mammalian skeletal muscle, two foot protein isoforms are present in amphibian, avian, and piscine skeletal muscles. This may represent a general difference in the architecture and/or a functional specialization of the triad junction in mammalian and nonmammalian vertebrate muscles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S. N., Ellisman M. H., Deerinck T. J., Maulet Y., Gentry M. K., Doctor B. P., Taylor P. Differences in structure and distribution of the molecular forms of acetylcholinesterase. J Cell Biol. 1989 Jun;108(6):2301–2311. doi: 10.1083/jcb.108.6.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airey J. A., Beck C. F., Murakami K., Tanksley S. J., Deerinck T. J., Ellisman M. H., Sutko J. L. Identification and localization of two triad junctional foot protein isoforms in mature avian fast twitch skeletal muscle. J Biol Chem. 1990 Aug 25;265(24):14187–14194. [PubMed] [Google Scholar]
- Block B. A., Imagawa T., Campbell K. P., Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988 Dec;107(6 Pt 2):2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. F., Lawson-Wendling K., Pugsley T. A. A rapid filtration assay for soluble receptors using polyethylenimine-treated filters. Anal Biochem. 1983 Jul 1;132(1):74–81. doi: 10.1016/0003-2697(83)90427-x. [DOI] [PubMed] [Google Scholar]
- Campbell K. P., Knudson C. M., Imagawa T., Leung A. T., Sutko J. L., Kahl S. D., Raab C. R., Madson L. Identification and characterization of the high affinity [3H]ryanodine receptor of the junctional sarcoplasmic reticulum Ca2+ release channel. J Biol Chem. 1987 May 15;262(14):6460–6463. [PubMed] [Google Scholar]
- Caswell A. H., Brunschwig J. P. Identification and extraction of proteins that compose the triad junction of skeletal muscle. J Cell Biol. 1984 Sep;99(3):929–939. doi: 10.1083/jcb.99.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisman M. H., Deerinck T. J., Ouyang Y., Beck C. F., Tanksley S. J., Walton P. D., Airey J. A., Sutko J. L. Identification and localization of ryanodine binding proteins in the avian central nervous system. Neuron. 1990 Aug;5(2):135–146. doi: 10.1016/0896-6273(90)90304-x. [DOI] [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem. 1987 Feb 5;262(4):1740–1747. [PubMed] [Google Scholar]
- Kaplan R. S., Pedersen P. L. Determination of microgram quantities of protein in the presence of milligram levels of lipid with amido black 10B. Anal Biochem. 1985 Oct;150(1):97–104. doi: 10.1016/0003-2697(85)90445-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Otsu K., Willard H. F., Khanna V. K., Zorzato F., Green N. M., MacLennan D. H. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J Biol Chem. 1990 Aug 15;265(23):13472–13483. [PubMed] [Google Scholar]
- Saito A., Seiler S., Chu A., Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol. 1984 Sep;99(3):875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima H., Nishimura S., Matsumoto T., Ishida H., Kangawa K., Minamino N., Matsuo H., Ueda M., Hanaoka M., Hirose T. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989 Jun 8;339(6224):439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- Zorzato F., Fujii J., Otsu K., Phillips M., Green N. M., Lai F. A., Meissner G., MacLennan D. H. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1990 Feb 5;265(4):2244–2256. [PubMed] [Google Scholar]
- Zorzato F., Volpe P. Calcium binding proteins of junctional sarcoplasmic reticulum: detection by 45Ca ligand overlay. Arch Biochem Biophys. 1988 Mar;261(2):324–329. doi: 10.1016/0003-9861(88)90347-5. [DOI] [PubMed] [Google Scholar]