Abstract
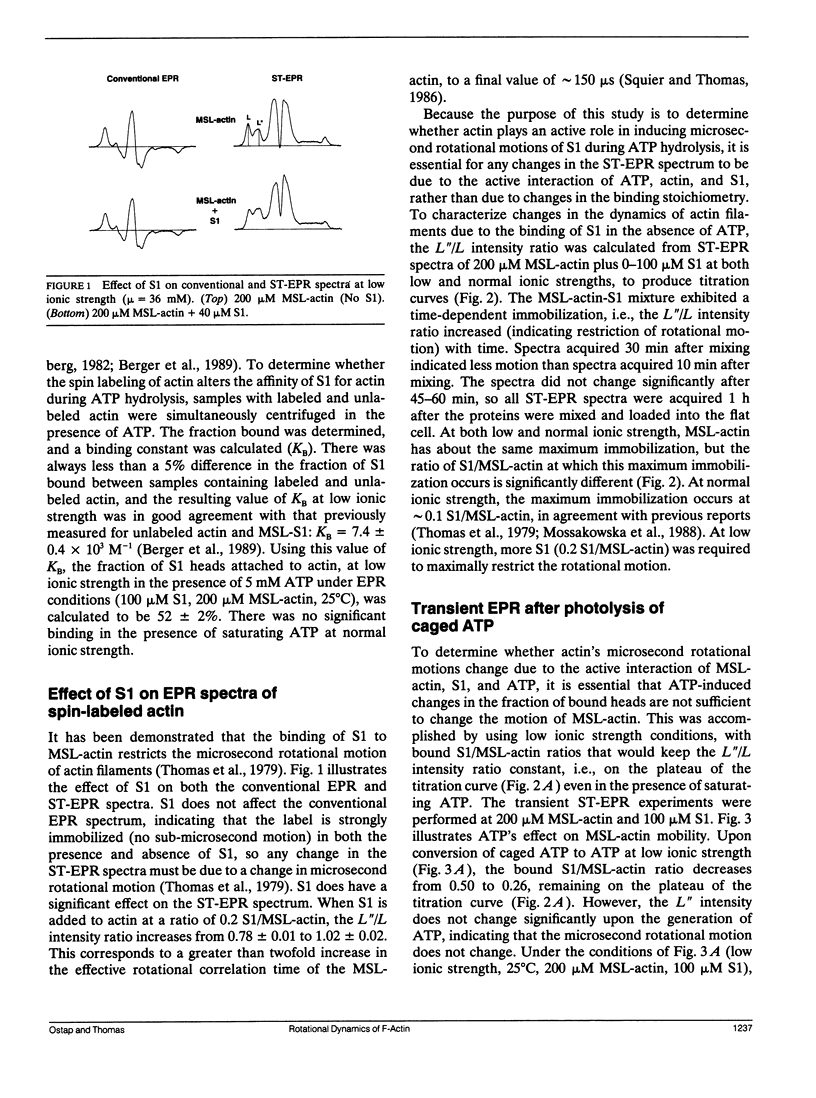
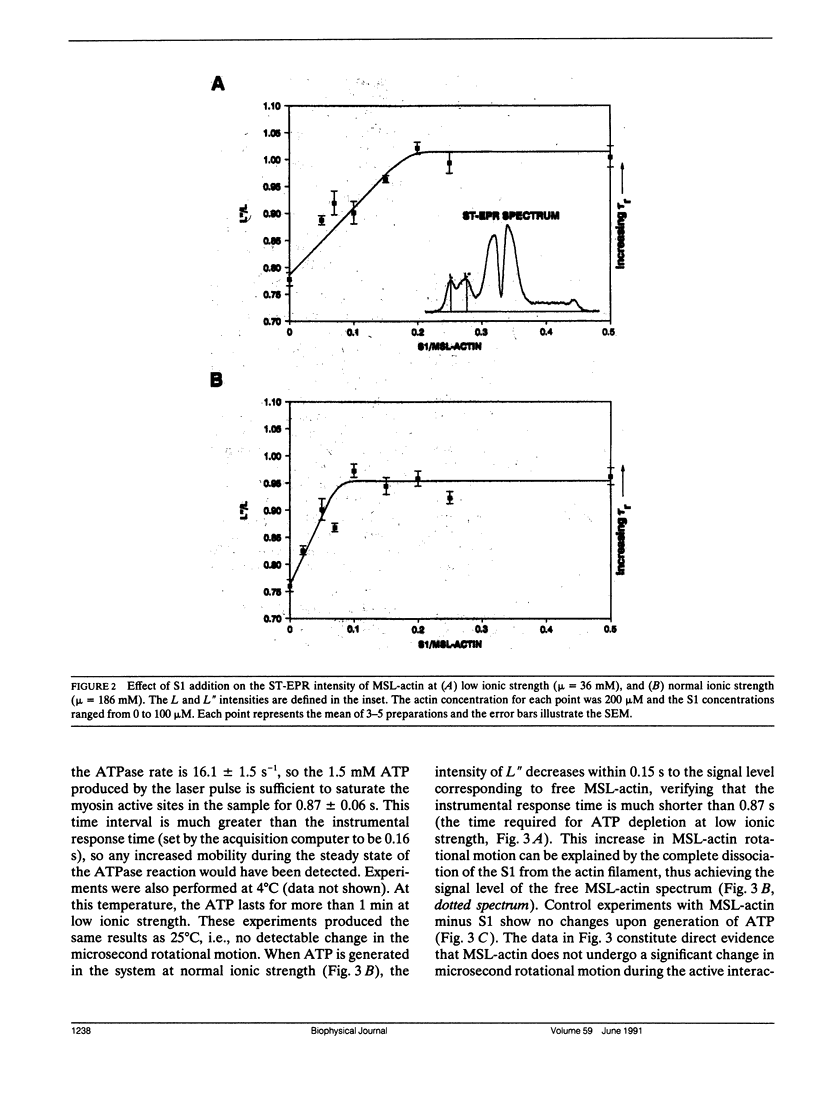
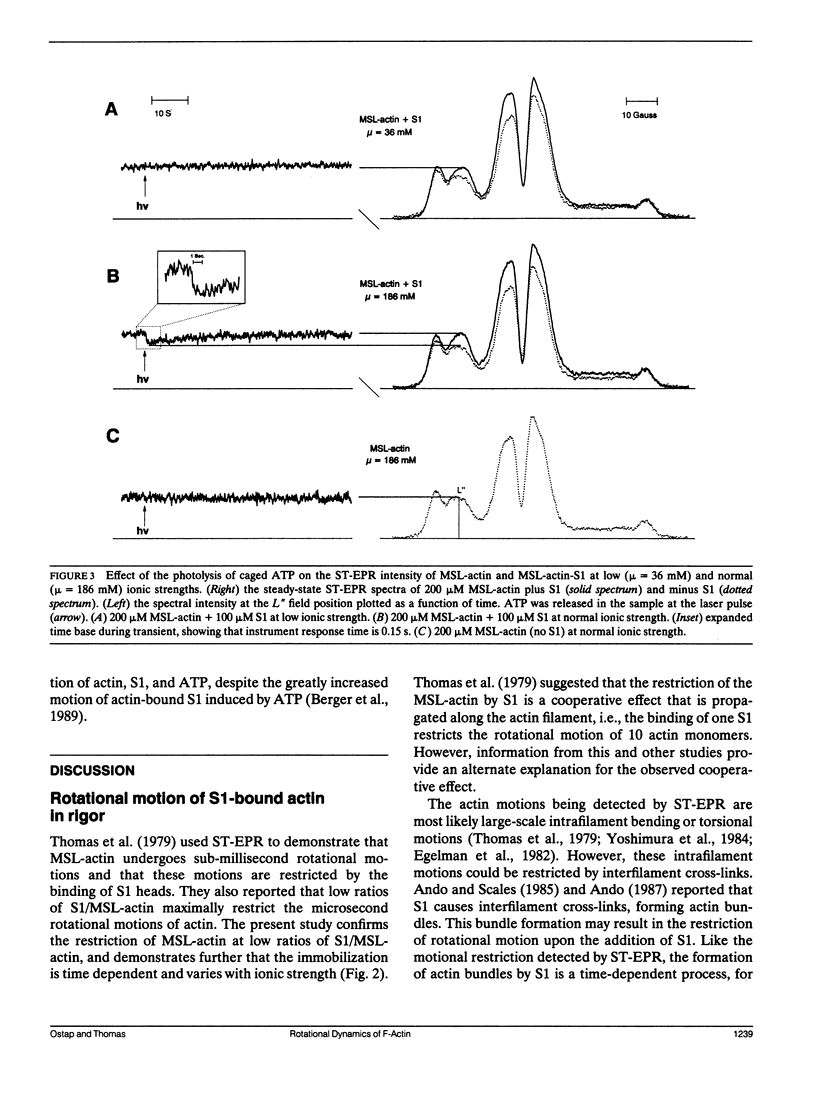
The most probable source of force generation in muscle fibers in the rotation of the myosin head when bound to actin. This laboratory has demonstrated that ATP induces microsecond rotational motions of spin-labeled myosin heads bound to actin (Berger, C. L. E. C. Svensson, and D. D. Thomas. 1989. Proc. Natl. Acad. Sci. USA. 86:8753-8757). Our goal is to determine whether the observed ATP-induced rotational motions of actin-bound heads are accompanied by changes in actin rotational motions. We have used saturation transfer electron paramagnetic resonance (ST-EPR) and laser-induced photolysis of caged ATP to monitor changes in the microsecond rotational dynamics of spin-labeled F-actin in the presence of myosin subfragment-1 (S1). A maleimide spin label was attached selectively to cys-374 on actin. In the absence of ATP (with or without caged ATP), the ST-EPR spectrum (corresponding to an effective rotational time of approximately 150 microseconds) was essentially the same as observed for the same spin label bound to cys-707 (SH1) on S1, indicating that S1 is rigidly bound to actin in rigor. At normal ionic strength (micro = 186 mM), a decrease in ST-EPR intensity (increase in microsecond F-actin mobility) was clearly indicated upon photolysis of 1 mM caged ATP with a 50-ms, 351-nm laser pulse. This increase in mobility is due to the complete dissociation of Si from the actin filament. At low ionic strength (micro, = 36 mM), when about half the Si heads remain bound during ATP hydrolysis, no change in the actin mobility was detected, despite much faster motions of labeled S1 bound to actin. Therefore, we conclude that the active interaction of Si, actin,and ATP induces rotation of myosin heads relative to actin, but does not affect the microsecond rotational motion of actin itself, as detected at cys-374 of actin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando T. Bundling of myosin subfragment-1-decorated actin filaments. J Mol Biol. 1987 May 20;195(2):351–358. doi: 10.1016/0022-2836(87)90656-5. [DOI] [PubMed] [Google Scholar]
- Ando T. Propagation of Acto-S-1 ATPase reaction-coupled conformational change in actin along the filament. J Biochem. 1989 May;105(5):818–822. doi: 10.1093/oxfordjournals.jbchem.a122751. [DOI] [PubMed] [Google Scholar]
- Ando T., Scales D. Skeletal muscle myosin subfragment-1 induces bundle formation by actin filaments. J Biol Chem. 1985 Feb 25;260(4):2321–2327. [PubMed] [Google Scholar]
- Barnett V. A., Fajer P., Polnaszek C. F., Thomas D. D. High-Resolution Detection of muscle Crossbridge Orientation by Electron Paramagnetic Resonance. Biophys J. 1986 Jan;49(1):144–147. doi: 10.1016/S0006-3495(86)83628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett V. A., Thomas D. D. Microsecond rotational motion of spin-labeled myosin heads during isometric muscle contraction. Saturation transfer electron paramagnetic resonance. Biophys J. 1989 Sep;56(3):517–523. doi: 10.1016/S0006-3495(89)82698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C. L., Svensson E. C., Thomas D. D. Photolysis of a photolabile precursor of ATP (caged ATP) induces microsecond rotational motions of myosin heads bound to actin. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8753–8757. doi: 10.1073/pnas.86.22.8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Eisenberg E. Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin. J Biol Chem. 1982 Mar 10;257(5):2432–2437. [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Crowder M. S., Thomas D. D. Orientation of spin labels attached to cross-bridges in contracting muscle fibres. Nature. 1982 Dec 23;300(5894):776–778. doi: 10.1038/300776a0. [DOI] [PubMed] [Google Scholar]
- Criddle A. H., Geeves M. A., Jeffries T. The use of actin labelled with N-(1-pyrenyl)iodoacetamide to study the interaction of actin with myosin subfragments and troponin/tropomyosin. Biochem J. 1985 Dec 1;232(2):343–349. doi: 10.1042/bj2320343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eads T. M., Thomas D. D., Austin R. H. Microsecond rotational motions of eosin-labeled myosin measured by time-resolved anisotropy of absorption and phosphorescence. J Mol Biol. 1984 Oct 15;179(1):55–81. doi: 10.1016/0022-2836(84)90306-1. [DOI] [PubMed] [Google Scholar]
- Egelman E. H., Francis N., DeRosier D. J. F-actin is a helix with a random variable twist. Nature. 1982 Jul 8;298(5870):131–135. doi: 10.1038/298131a0. [DOI] [PubMed] [Google Scholar]
- Fajer P. G., Fajer E. A., Thomas D. D. Myosin heads have a broad orientational distribution during isometric muscle contraction: time-resolved EPR studies using caged ATP. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5538–5542. doi: 10.1073/pnas.87.14.5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselgrove J. C., Huxley H. E. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. 1973 Jul 15;77(4):549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- Huxley A. F. Muscular contraction. J Physiol. 1974 Nov;243(1):1–43. [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Kress M. Crossbridge behaviour during muscle contraction. J Muscle Res Cell Motil. 1985 Apr;6(2):153–161. doi: 10.1007/BF00713057. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- McCray J. A., Herbette L., Kihara T., Trentham D. R. A new approach to time-resolved studies of ATP-requiring biological systems; laser flash photolysis of caged ATP. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7237–7241. doi: 10.1073/pnas.77.12.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan R. A., Whittaker M., Safer D. Molecular structure of F-actin and location of surface binding sites. Nature. 1990 Nov 15;348(6298):217–221. doi: 10.1038/348217a0. [DOI] [PubMed] [Google Scholar]
- Mossakowska M., Belágyi J., Strzelecka-Gołaszewska H. An EPR study of the rotational dynamics of actins from striated and smooth muscle and their complexes with heavy meromyosin. Eur J Biochem. 1988 Aug 15;175(3):557–564. doi: 10.1111/j.1432-1033.1988.tb14228.x. [DOI] [PubMed] [Google Scholar]
- Prochniewicz E., Yanagida T. Inhibition of sliding movement of F-actin by crosslinking emphasizes the role of actin structure in the mechanism of motility. J Mol Biol. 1990 Dec 5;216(3):761–772. doi: 10.1016/0022-2836(90)90397-5. [DOI] [PubMed] [Google Scholar]
- Próchniewicz-Nakayama E., Yanagida T. The effect of crosslinking of thin filament with glutaraldehyde on the contractility of muscle fiber. J Biochem. 1982 Oct;92(4):1269–1277. doi: 10.1093/oxfordjournals.jbchem.a134045. [DOI] [PubMed] [Google Scholar]
- Squier T. C., Thomas D. D. Methodology for increased precision in saturation transfer electron paramagnetic resonance studies of rotational dynamics. Biophys J. 1986 Apr;49(4):921–935. doi: 10.1016/S0006-3495(86)83720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. A., Ludescher R. D., Dahlberg P. S., Fajer P. G., Bennett R. L., Thomas D. D. Time-resolved rotational dynamics of phosphorescent-labeled myosin heads in contracting muscle fibers. Biochemistry. 1990 Oct 30;29(43):10023–10031. doi: 10.1021/bi00495a003. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Seidel J. C., Gergely J. Rotational dynamics of spin-labeled F-actin in the sub-millisecond time range. J Mol Biol. 1979 Aug 15;132(3):257–273. doi: 10.1016/0022-2836(79)90259-6. [DOI] [PubMed] [Google Scholar]
- Yoshimura H., Nishio T., Mihashi K., Kinosita K., Jr, Ikegami A. Torsional motion of eosin-labeled F-actin as detected in the time-resolved anisotropy decay of the probe in the sub-millisecond time range. J Mol Biol. 1984 Nov 5;179(3):453–467. doi: 10.1016/0022-2836(84)90075-5. [DOI] [PubMed] [Google Scholar]


