Abstract
The techniques of fluorescence resonance energy transfer (FRET) and cross-linking can provide complementary information concerning the relative separation of a pair of sites. Cross-linking experiments provide an assessment of the distance of closest approach between a pair of sites. FRET measurements, by contrast, yield information about the average distance between the pair of sites. We have taken advantage of hybrid myosins to understand the relationship between distances obtained for a pair of equivalent sites, one on each myosin head, using both FRET (steady-state and time-decay) and cross-linking techniques. The rigid cross-linker, 4-4'-dimaleimidyl-stilbene-2-2'-disulfonic acid (DMSDS), can efficiently cross-link the two myosin regulatory light-chains, each at residue Cys50 of the Mercenaria regulatory light chain (Chantler, P.D., and S. M. Bower. 1988. J. Biol. Chem. 263:938-944), indicating that these sites can come within 18 +/- 2 A of each other. In a complementary set of experiments, steady-state and time-decay measurements using fluorescence donor/acceptor pairs located at these same sites indicate transfer efficiencies of somewhat less than 20%, suggesting an average separation of greater than 50 A between sites (Chantler, P. D., and T. Tao. 1986. J. Mol. Biol. 192:87-99). Here, we present theoretical calculations which show that efficient cross-linking can be achieved readily in dynamic systems such as the heads of myosin, even though the necessary subpopulation of proximate molecules at any instant may be below the detection limits of time-decay-FRET. Therefore, cross-linking experiments can provide important ancillary information about the extent of motions within a marcomolecular system when used in conjunction with FRET.As a corollary, demonstration of extensive cross-linking does not necessarily indicate a static proximity; the mean separation distance should be ascertained by other methods such as FRET.
Full text
PDF

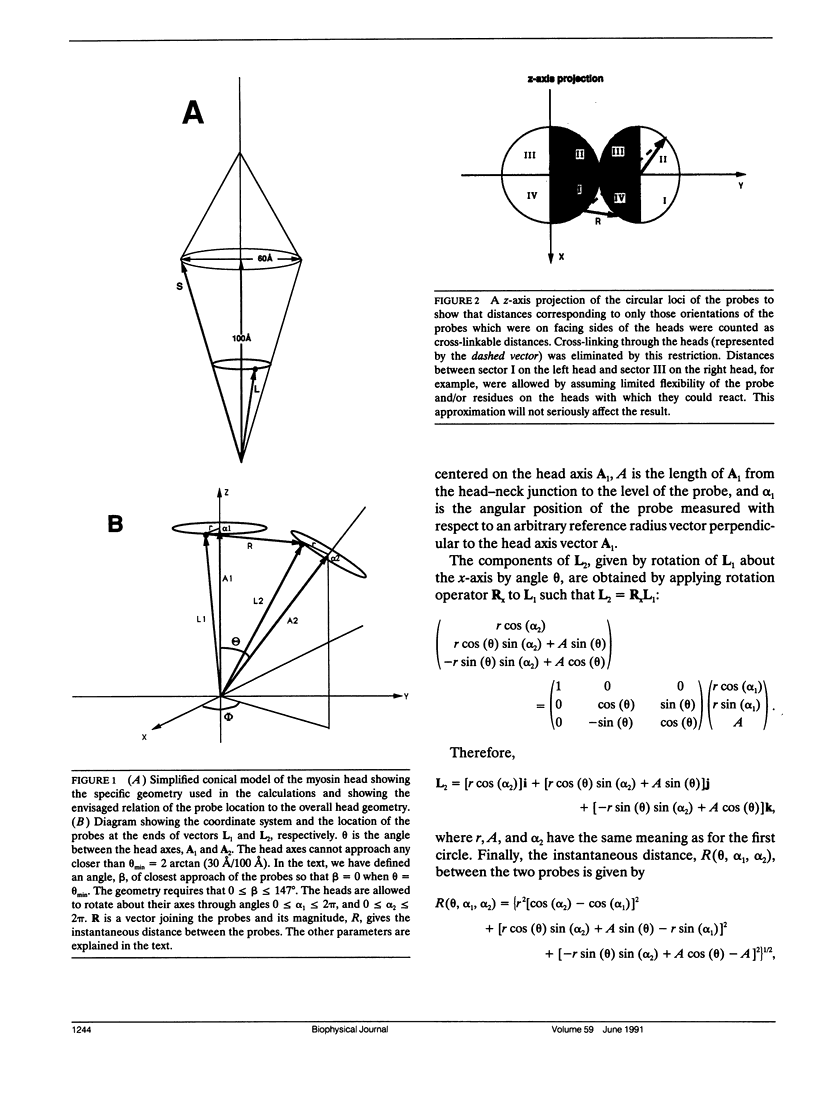
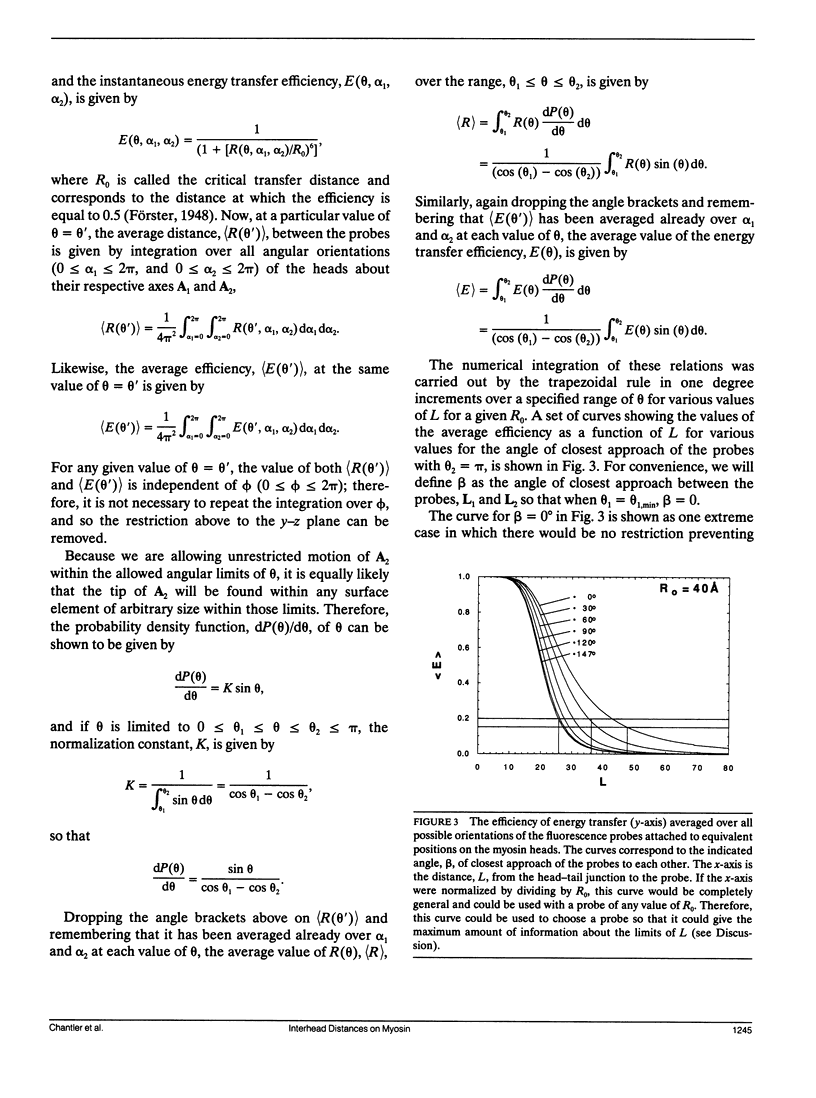
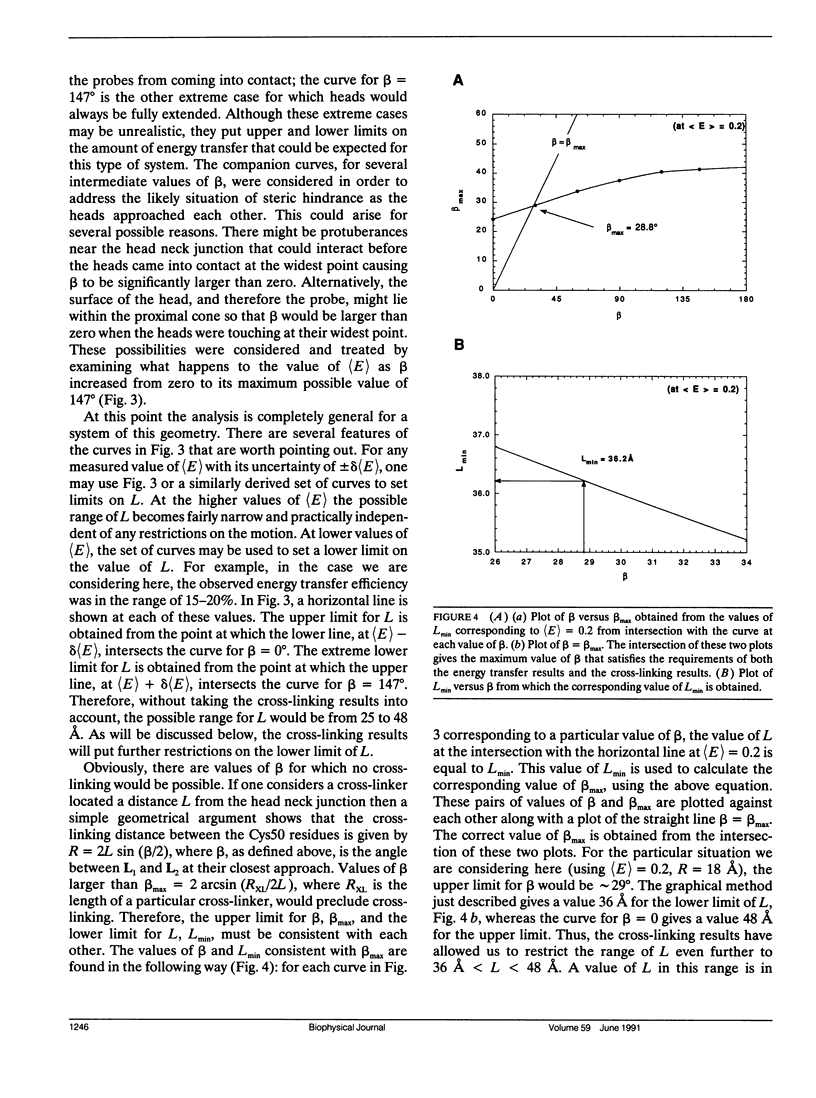


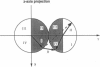
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke M., Reisler E. Effect of nucleotide binding on the proximity of the essential sulfhydryl groups of myosin. Chemical probing of movement of residues during conformational transitions. Biochemistry. 1977 Dec 13;16(25):5559–5563. doi: 10.1021/bi00644a026. [DOI] [PubMed] [Google Scholar]
- Chantler P. D., Bower S. M. Cross-linking between translationally equivalent sites on the two heads of myosin. Relationship to energy transfer results between the same pair of sites. J Biol Chem. 1988 Jan 15;263(2):938–944. [PubMed] [Google Scholar]
- Chantler P. D., Kensler R. W. Position of Mercenaria regulatory light-chain Cys50 site on the surface of myosin visualized by electron microscopy. J Mol Biol. 1989 Jun 5;207(3):631–636. doi: 10.1016/0022-2836(89)90472-5. [DOI] [PubMed] [Google Scholar]
- Chantler P. D., Tao T. Interhead fluorescence energy transfer between probes attached to translationally equivalent sites on the regulatory light chains of scallop myosin. J Mol Biol. 1986 Nov 5;192(1):87–99. doi: 10.1016/0022-2836(86)90466-3. [DOI] [PubMed] [Google Scholar]
- Cheung H. C., Gonsoulin F., Garland F. An investigation of the SH1-SH2 and SH1-ATPase distances in myosin subfragment-1 by resonance energy transfer using nanosecond fluorimetry. Biochim Biophys Acta. 1985 Nov 8;832(1):52–62. doi: 10.1016/0167-4838(85)90173-6. [DOI] [PubMed] [Google Scholar]
- Cheung H. C., Gonsoulin F., Garland F. Fluorescence energy transfer studies on the proximity of the two essential thiols of myosin subfragment-1. J Biol Chem. 1983 May 10;258(9):5775–5786. [PubMed] [Google Scholar]
- Dalbey R. E., Weiel J., Yount R. G. Förster energy transfer measurements of thiol 1 to thiol 2 distances in myosin subfragment 1. Biochemistry. 1983 Sep 27;22(20):4696–4706. doi: 10.1021/bi00289a014. [DOI] [PubMed] [Google Scholar]
- Dobrovol'sky A. B., Gusev N. B., Friedrich P. Crosslinking of troponin complex with 1,3-difluoro-4,6-dinitrobenzene. Identification of the crosslink formed between troponin C and troponin I in the absence of Ca2+. Biochim Biophys Acta. 1984 Sep 11;789(2):144–151. doi: 10.1016/0167-4838(84)90198-5. [DOI] [PubMed] [Google Scholar]
- Hillel Z., Wu C. W. Statistical interpretation of fluorescence energy transfer measurements in macromolecular systems. Biochemistry. 1976 May 18;15(10):2105–2113. doi: 10.1021/bi00655a012. [DOI] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Mendelson R. A., Morales M. F., Botts J. Segmental flexibility of the S-1 moiety of myosin. Biochemistry. 1973 Jun 5;12(12):2250–2255. doi: 10.1021/bi00736a011. [DOI] [PubMed] [Google Scholar]
- Milligan R. A., Flicker P. F. Structural relationships of actin, myosin, and tropomyosin revealed by cryo-electron microscopy. J Cell Biol. 1987 Jul;105(1):29–39. doi: 10.1083/jcb.105.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins W. J., Weiel J., Grammer J., Yount R. G. Introduction of a donor-acceptor pair by a single protein modification. Förster energy transfer distance measurements from trapped 1,N6-ethenoadenosine diphosphate to chromophoric cross-linking reagents on the critical thiols of myosin subfragment. J Biol Chem. 1984 Jul 25;259(14):8786–8793. [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Tao T., Gowell E., Strasburg G. M., Gergely J., Leavis P. C. Ca2+ dependence of the distance between Cys-98 of troponin C and Cys-133 of troponin I in the ternary troponin complex. Resonance energy transfer measurements. Biochemistry. 1989 Jul 11;28(14):5902–5908. doi: 10.1021/bi00440a029. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Seidel J. C., Hyde J. S., Gergely J. Motion of subfragment-1 in myosin and its supramolecular complexes: saturation transfer electron paramagnetic resonance. Proc Natl Acad Sci U S A. 1975 May;72(5):1729–1733. doi: 10.1073/pnas.72.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M., Knight P., Trinick J. Negative staining of myosin molecules. J Mol Biol. 1985 Aug 5;184(3):535–542. doi: 10.1016/0022-2836(85)90300-6. [DOI] [PubMed] [Google Scholar]
- Wells J. A., Yount R. G. Reaction of 5,5'-dithiobis(2-nitrobenzoic acid) with myosin subfragment one: evidence for formation of a single protein disulfide with trapping of metal nucleotide at the active site. Biochemistry. 1980 Apr 15;19(8):1711–1717. doi: 10.1021/bi00549a030. [DOI] [PubMed] [Google Scholar]