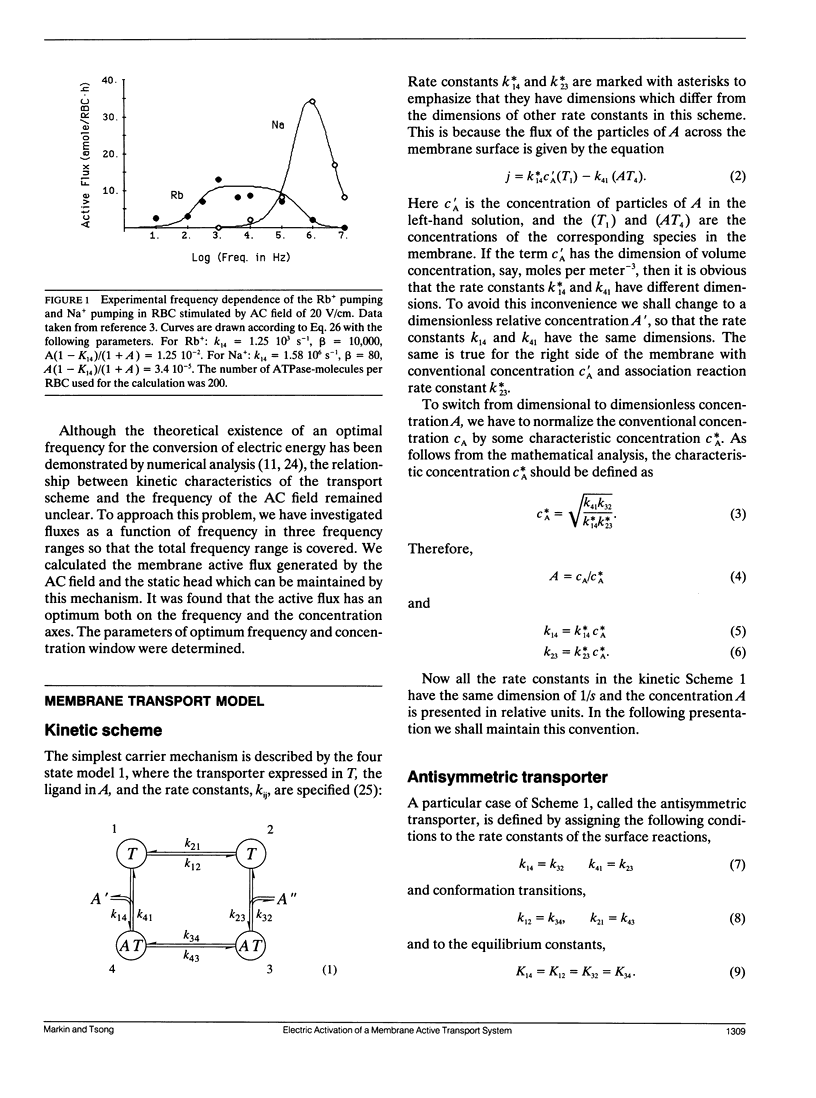
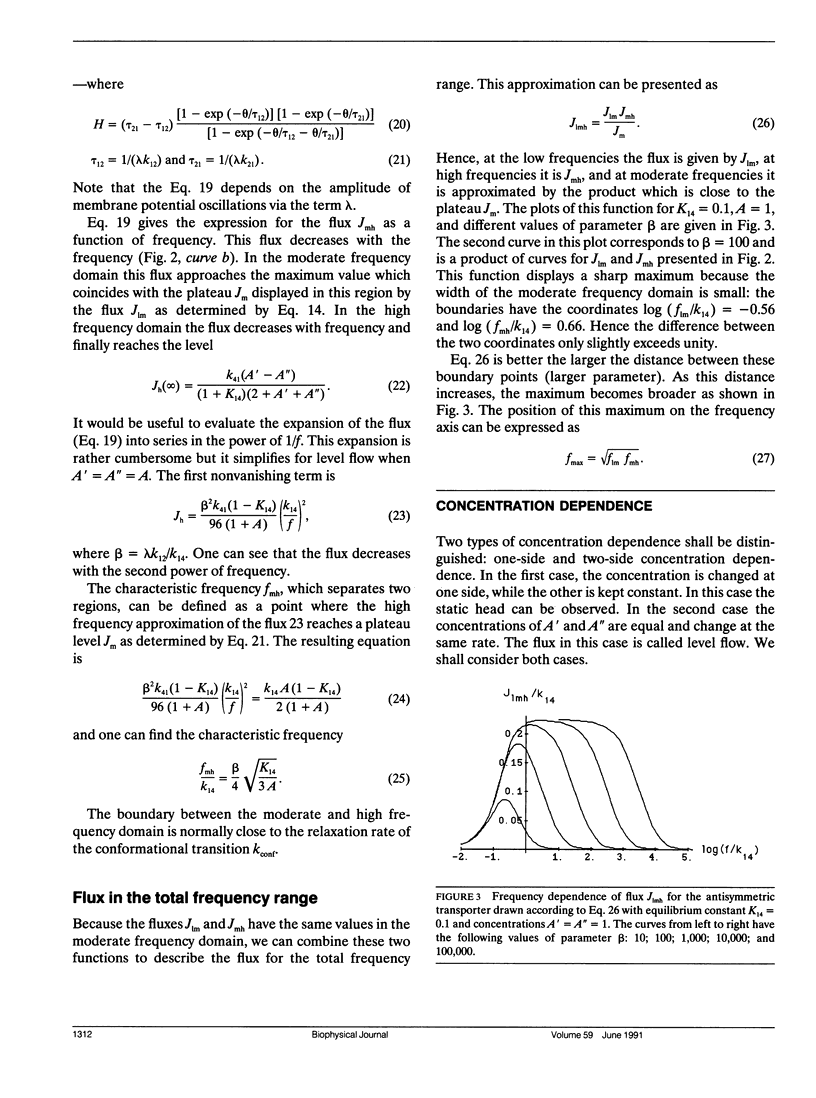
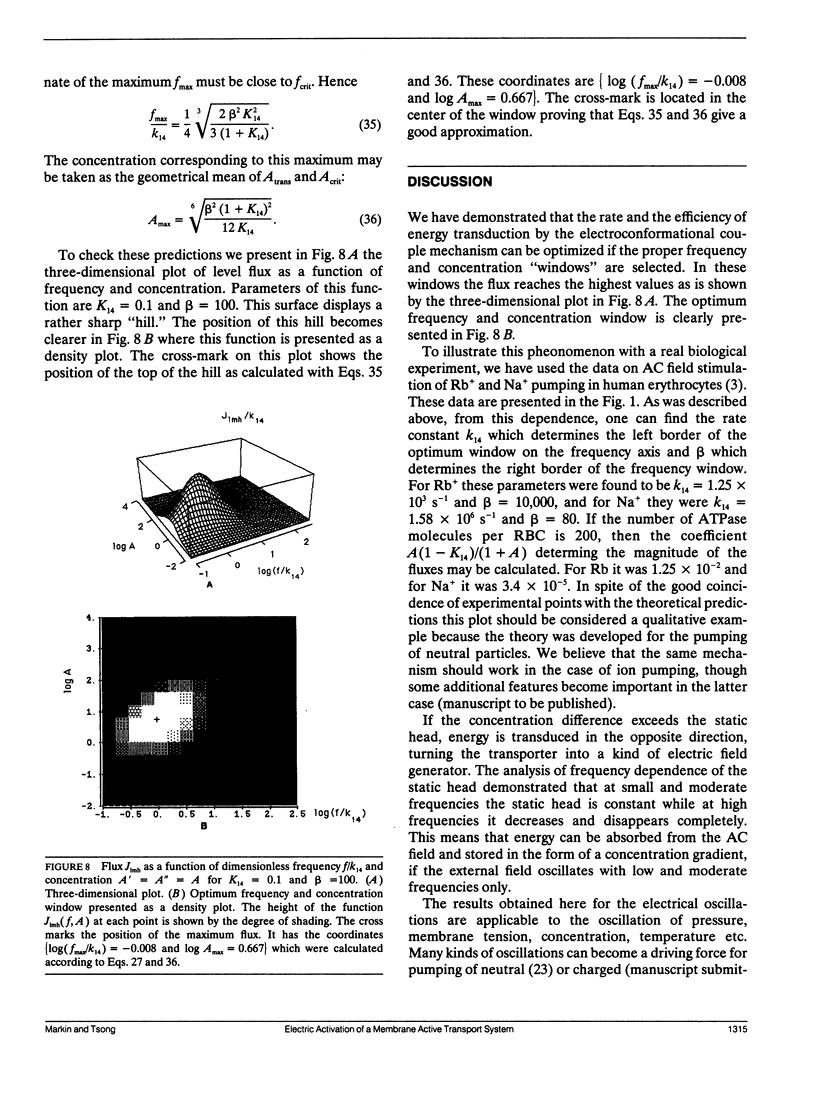
Abstract
Previous work has shown that a simple four-state membrane transport system can interact with an oscillating electric field to become an active transport system if there is charge translocation associated with conformational changes of the transporter and if affinities of the transporter for the ligand on the two sides of membrane are different. The relationship between the transport flux and both the frequency of the applied field and the concentration of ligand have been examined based on the following assumptions: the rate of the electroconformational change of the transporter is much greater than that of the ligand association/dissociation reaction, and the oscillating electric field has a large amplitude. It was found that the transport flux depends strongly on the frequency of the field and on the concentration of the ligand and it displays a window of broad bandwidth both on the frequency and the concentration axes. The maximum concentration gradient, or the static head, which can be supported by this mechanism is shown to be constant for field frequencies smaller than the rate of the electroconformational change. The static head value diminishes completely when the field frequency exceeds the rate of the conformational change. The presence of an optimal field frequency has been shown experimentally in several membrane enzyme systems. The theory was applied to the description of Rb and Na pumping in human erythrocytes stimulated by an AC field. The prediction of a window for a ligand concentration and the static head value may be tested experimentally. In addition, the rate constants and the equilibrium constants of the four state model can be determined by measuring positions of windows, fluxes, and static head values under different experimental conditions. These results are equally applicable to the oscillation of pressure, membrane tension, substrate concentration, or temperature if these external parameters can induce functionally relevant conformational changes of the transporter.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astumian RD, Chock PB, Tsong TY, Westerhoff HV. Effects of oscillations and energy-driven fluctuations on the dynamics of enzyme catalysis and free-energy transduction. Phys Rev A Gen Phys. 1989 Jun 15;39(12):6416–6435. doi: 10.1103/physreva.39.6416. [DOI] [PubMed] [Google Scholar]
- Devreotes P. Dictyostelium discoideum: a model system for cell-cell interactions in development. Science. 1989 Sep 8;245(4922):1054–1058. doi: 10.1126/science.2672337. [DOI] [PubMed] [Google Scholar]
- Drachev A. L., Markin V. S., Skulachev V. P. delta-mu H-buffering by Na+ and K+ gradients in bacteria. Model and experimental systems. Biochim Biophys Acta. 1985 Jun 3;811(2):197–215. doi: 10.1016/0304-4173(85)90018-7. [DOI] [PubMed] [Google Scholar]
- Knox B. E., Devreotes P. N., Goldbeter A., Segel L. A. A molecular mechanism for sensory adaptation based on ligand-induced receptor modification. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2345–2349. doi: 10.1073/pnas.83.8.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Goldbeter A. Frequency specificity in intercellular communication. Influence of patterns of periodic signaling on target cell responsiveness. Biophys J. 1989 Jan;55(1):125–145. doi: 10.1016/S0006-3495(89)82785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D. S., Astumian R. D., Tsong T. Y. Activation of Na+ and K+ pumping modes of (Na,K)-ATPase by an oscillating electric field. J Biol Chem. 1990 May 5;265(13):7260–7267. [PubMed] [Google Scholar]
- Mandelkow E., Mandelkow E. M., Hotani H., Hess B., Müller S. C. Spatial patterns from oscillating microtubules. Science. 1989 Dec 8;246(4935):1291–1293. doi: 10.1126/science.2588005. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Keilin's respiratory chain concept and its chemiosmotic consequences. Science. 1979 Dec 7;206(4423):1148–1159. doi: 10.1126/science.388618. [DOI] [PubMed] [Google Scholar]
- Okamura N., Ishiwata S. Spontaneous oscillatory contraction of sarcomeres in skeletal myofibrils. J Muscle Res Cell Motil. 1988 Apr;9(2):111–119. doi: 10.1007/BF01773733. [DOI] [PubMed] [Google Scholar]
- Olson E. S., Smullin L. D. Frequency tuning in the electroreceptive periphery. Biophys J. 1989 Jun;55(6):1191–1204. doi: 10.1016/S0006-3495(89)82915-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B., Astumian R. D. Kinetics of a multistate enzyme in a large oscillating field. Biophys J. 1990 Apr;57(4):689–696. doi: 10.1016/S0006-3495(90)82590-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B., Astumian R. D. Michaelis-Menten equation for an enzyme in an oscillating electric field. Biophys J. 1990 Oct;58(4):969–974. doi: 10.1016/S0006-3495(90)82441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpersu E. H., Tsong T. Y. Activation of electrogenic Rb+ transport of (Na,K)-ATPase by an electric field. J Biol Chem. 1984 Jun 10;259(11):7155–7162. [PubMed] [Google Scholar]
- Serpersu E. H., Tsong T. Y. Stimulation of a ouabain-sensitive Rb+ uptake in human erthrocytes with an external electric field. J Membr Biol. 1983;74(3):191–201. doi: 10.1007/BF02332123. [DOI] [PubMed] [Google Scholar]
- Teissié J. Adenosine 5'-triphosphate synthesis in Escherichia coli submitted to a microsecond electric pulse. Biochemistry. 1986 Jan 28;25(2):368–373. doi: 10.1021/bi00350a014. [DOI] [PubMed] [Google Scholar]
- Toko K., Souda M., Matsuno T., Yamafuji K. Oscillations of electrical potential along a root of a higher plant. Biophys J. 1990 Feb;57(2):269–279. doi: 10.1016/S0006-3495(90)82529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong T. Y. Electrical modulation of membrane proteins: enforced conformational oscillations and biological energy and signal transductions. Annu Rev Biophys Biophys Chem. 1990;19:83–106. doi: 10.1146/annurev.bb.19.060190.000503. [DOI] [PubMed] [Google Scholar]
- Weaver J. C., Astumian R. D. The response of living cells to very weak electric fields: the thermal noise limit. Science. 1990 Jan 26;247(4941):459–462. doi: 10.1126/science.2300806. [DOI] [PubMed] [Google Scholar]
- Witt H. T., Schlodder E., Gräber P. Membrane-bound ATP synthesis generated by an external electrical field. FEBS Lett. 1976 Oct 15;69(1):272–276. doi: 10.1016/0014-5793(76)80702-8. [DOI] [PubMed] [Google Scholar]



