Abstract
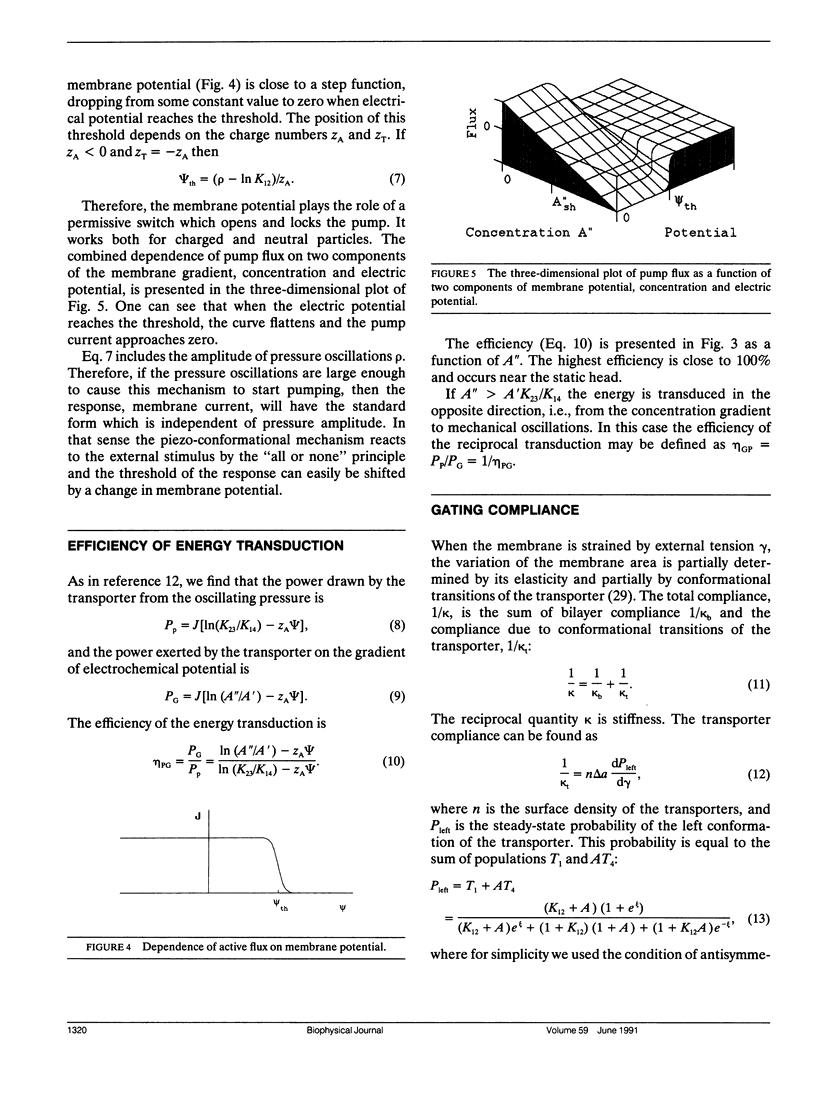
To explain the ability of some mechanosensitive cells to reverse the process of mechanotransduction and to generate mechanical oscillations and emit sound, a piezo-conformational coupling model (PCC model) is proposed. The model includes a transport protein which changes either its volume (PV-coupling) or its area in the membrane (gamma A-coupling) when undergoing conformational transitions. Such a protein can interact with an oscillating pressure to pump ions and create a transmembrane gradient if the affinities of the protein for ions are different at the two sides of membrane. The frequency and concentration windows for mechanical energy transduction were determined. Under optimal conditions, the efficiency of energy transduction can approach the theoretical maximum of 100%. If the concentration gradient exceeds the static head value (quasi-equilibrium which can be built up and maintained by this transport system), the energy transduction reverses and the transporter becomes a generator of mechanical oscillations at the expense of a concentration gradient. Estimation of thermodynamic parameters of the pump shows that the PV-coupling model would require large pressure oscillations to work while the gamma A-coupling model could work in physiological conditions. The gamma A-coupling mechanism may be used by cells for two purposes. In the reverse mode, it can be a force generator for various applications. In the direct mode, it may serve bioenergetic purposes by harvesting the energy of mechanical oscillations and storing it in the form of a concentration gradient. This pump has an unusual thermodynamic feature: it can distinguish the two components of the electrochemical potential gradient,i.e., the concentration gradient and the electrical potential, the latter serving as a permissive switch to open, or close, the pump when the potential reaches the threshold value.Predictions of the PCC model and its probable involvement in biological mechanotransduction are dicussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astumian RD, Chock PB, Tsong TY, Westerhoff HV. Effects of oscillations and energy-driven fluctuations on the dynamics of enzyme catalysis and free-energy transduction. Phys Rev A Gen Phys. 1989 Jun 15;39(12):6416–6435. doi: 10.1103/physreva.39.6416. [DOI] [PubMed] [Google Scholar]
- Brehm P., Kullberg R., Moody-Corbett F. Properties of non-junctional acetylcholine receptor channels on innervated muscle of Xenopus laevis. J Physiol. 1984 May;350:631–648. doi: 10.1113/jphysiol.1984.sp015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell W. E., Bader C. R., Bertrand D., de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985 Jan 11;227(4683):194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Conti F., Fioravanti R., Segal J. R., Stühmer W. Pressure dependence of the sodium currents of squid giant axon. J Membr Biol. 1982;69(1):23–34. doi: 10.1007/BF01871238. [DOI] [PubMed] [Google Scholar]
- Denk W., Webb W. W., Hudspeth A. J. Mechanical properties of sensory hair bundles are reflected in their Brownian motion measured with a laser differential interferometer. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5371–5375. doi: 10.1073/pnas.86.14.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachev A. L., Markin V. S., Skulachev V. P. delta-mu H-buffering by Na+ and K+ gradients in bacteria. Model and experimental systems. Biochim Biophys Acta. 1985 Jun 3;811(2):197–215. doi: 10.1016/0304-4173(85)90018-7. [DOI] [PubMed] [Google Scholar]
- Guharay F., Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984 Jul;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J., Hudspeth A. J. Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog's saccular hair cell. Neuron. 1988 May;1(3):189–199. doi: 10.1016/0896-6273(88)90139-0. [DOI] [PubMed] [Google Scholar]
- Howard J., Roberts W. M., Hudspeth A. J. Mechanoelectrical transduction by hair cells. Annu Rev Biophys Biophys Chem. 1988;17:99–124. doi: 10.1146/annurev.bb.17.060188.000531. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. J. How the ear's works work. Nature. 1989 Oct 5;341(6241):397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- Kachar B., Brownell W. E., Altschuler R., Fex J. Electrokinetic shape changes of cochlear outer hair cells. Nature. 1986 Jul 24;322(6077):365–368. doi: 10.1038/322365a0. [DOI] [PubMed] [Google Scholar]
- Kemp D. T. Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am. 1978 Nov;64(5):1386–1391. doi: 10.1121/1.382104. [DOI] [PubMed] [Google Scholar]
- Koslov M. M., Markin V. S. A theory of osmotic lysis of lipid vesicles. J Theor Biol. 1984 Jul 7;109(1):17–39. doi: 10.1016/s0022-5193(84)80108-3. [DOI] [PubMed] [Google Scholar]
- Kwok R., Evans E. Thermoelasticity of large lecithin bilayer vesicles. Biophys J. 1981 Sep;35(3):637–652. doi: 10.1016/S0006-3495(81)84817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kössl M., Vater M. Evoked acoustic emissions and cochlear microphonics in the mustache bat, Pteronotus parnellii. Hear Res. 1985;19(2):157–170. doi: 10.1016/0378-5955(85)90120-0. [DOI] [PubMed] [Google Scholar]
- Liu D. S., Astumian R. D., Tsong T. Y. Activation of Na+ and K+ pumping modes of (Na,K)-ATPase by an oscillating electric field. J Biol Chem. 1990 May 5;265(13):7260–7267. [PubMed] [Google Scholar]
- Markin V. S., Tsong T. Y. Frequency and concentration windows for the electric activation of a membrane active transport system. Biophys J. 1991 Jun;59(6):1308–1316. doi: 10.1016/S0006-3495(91)82345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac B., Buechner M., Delcour A. H., Adler J., Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. E. Mechanosensitive ion channels. J Membr Biol. 1990 Feb;113(2):93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- Robertson B., Astumian R. D. Kinetics of a multistate enzyme in a large oscillating field. Biophys J. 1990 Apr;57(4):689–696. doi: 10.1016/S0006-3495(90)82590-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F. Mechanical transduction in biological systems. Crit Rev Biomed Eng. 1988;16(2):141–169. [PubMed] [Google Scholar]
- Serpersu E. H., Tsong T. Y. Activation of electrogenic Rb+ transport of (Na,K)-ATPase by an electric field. J Biol Chem. 1984 Jun 10;259(11):7155–7162. [PubMed] [Google Scholar]
- Tsong T. Y., Astumian R. D. Electroconformational coupling: how membrane-bound ATPase transduces energy from dynamic electric fields. Annu Rev Physiol. 1988;50:273–290. doi: 10.1146/annurev.ph.50.030188.001421. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Deciphering the language of cells. Trends Biochem Sci. 1989 Mar;14(3):89–92. doi: 10.1016/0968-0004(89)90127-8. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Electrical modulation of membrane proteins: enforced conformational oscillations and biological energy and signal transductions. Annu Rev Biophys Biophys Chem. 1990;19:83–106. doi: 10.1146/annurev.bb.19.060190.000503. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y., Liu D. S., Chauvin F., Astumian R. D. Resonance electroconformational coupling: a proposed mechanism for energy and signal transductions by membrane proteins. Biosci Rep. 1989 Feb;9(1):13–26. doi: 10.1007/BF01117508. [DOI] [PubMed] [Google Scholar]
- Westerhoff H. V., Tsong T. Y., Chock P. B., Chen Y. D., Astumian R. D. How enzymes can capture and transmit free energy from an oscillating electric field. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4734–4738. doi: 10.1073/pnas.83.13.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J., Parsegian V. A. Polymer inaccessible volume changes during opening and closing of a voltage-dependent ionic channel. Nature. 1986 Sep 4;323(6083):36–39. doi: 10.1038/323036a0. [DOI] [PubMed] [Google Scholar]
- Zurek P. M. Spontaneous narrowband acoustic signals emitted by human ears. J Acoust Soc Am. 1981 Feb;69(2):514–523. doi: 10.1121/1.385481. [DOI] [PubMed] [Google Scholar]


