Abstract
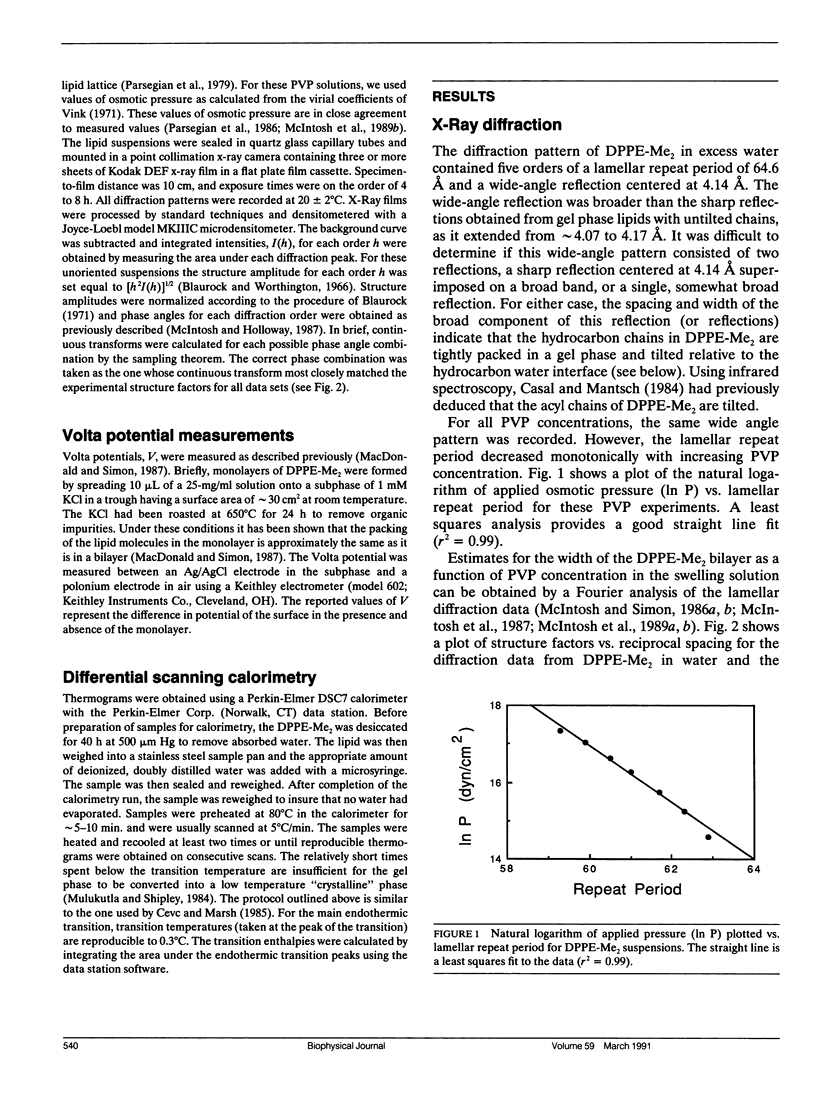
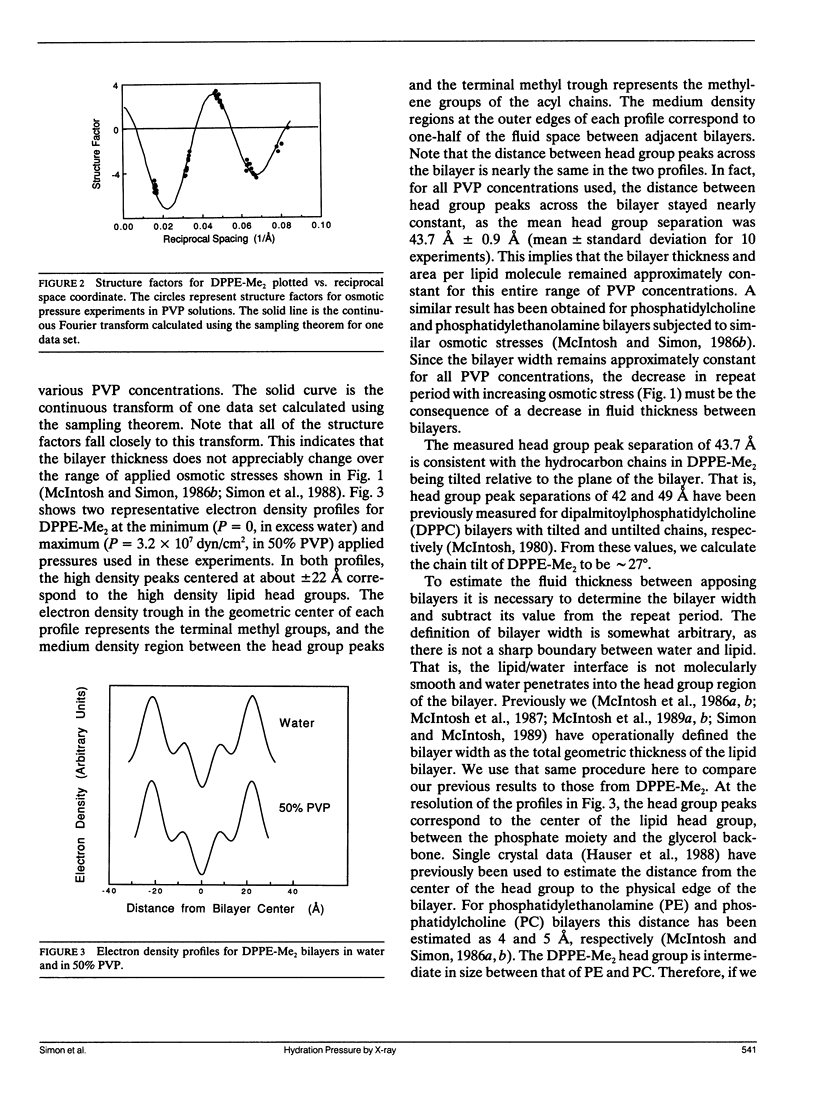
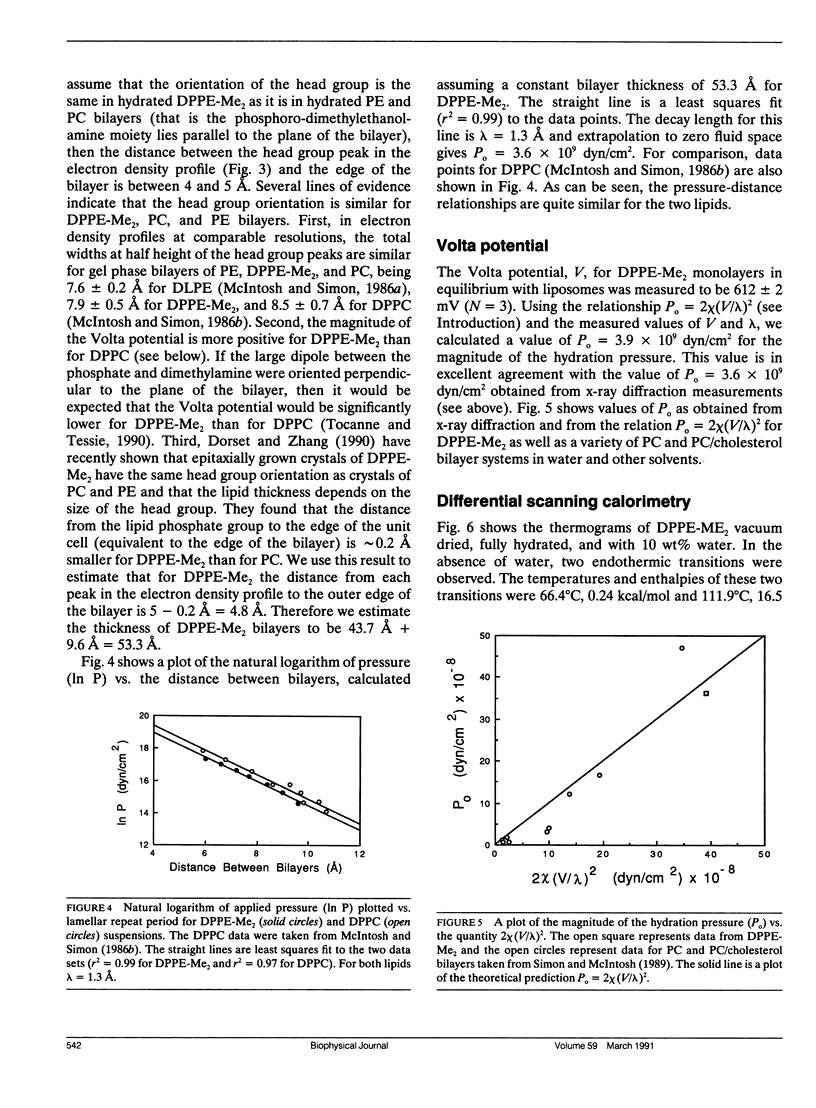
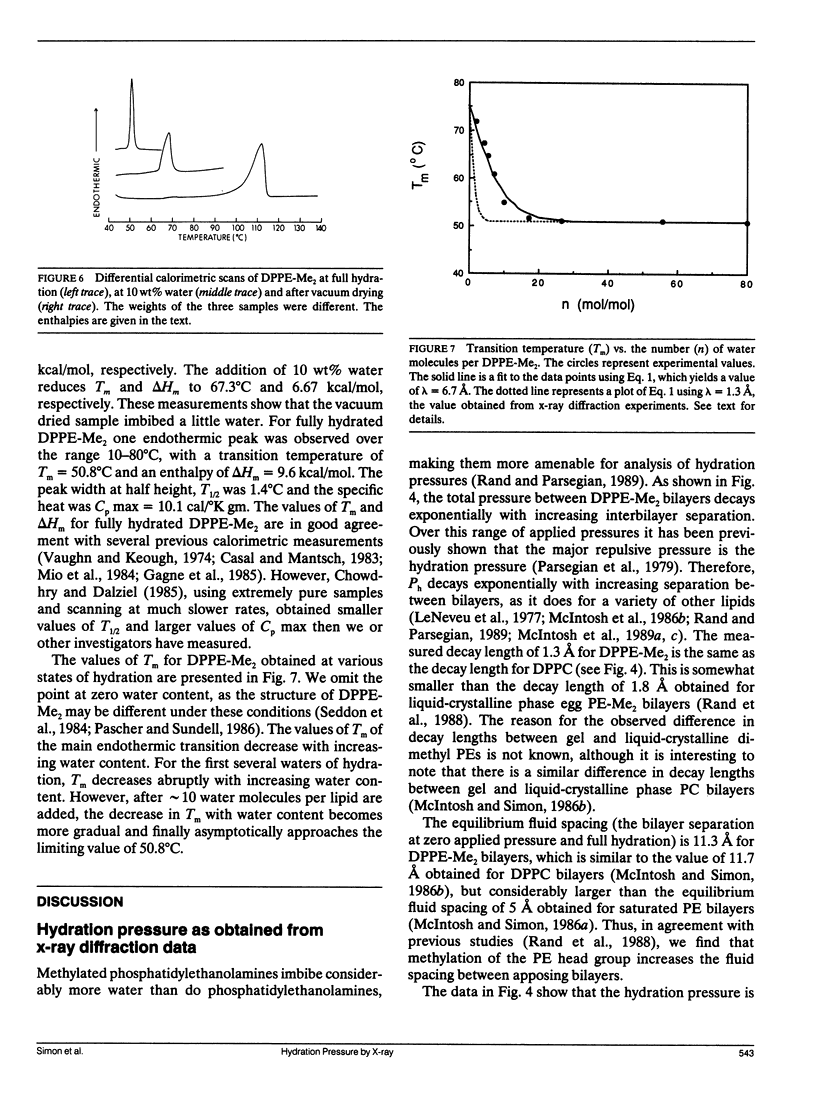
The hydration pressure between dipalmitoyl phosphatidyl-N,N-dimethylethanolamine (DPPE-Me2) bilayers has been analyzed by both x-ray diffraction measurements of osmotically stressed liposomes and by differential scanning calorimetry. By the x-ray method, we obtain a magnitude (Po) and decay length (lambda) for the hydration pressure which are both quite similar to those found for bilayers of other zwitterionic lipids, such as phosphatidylcholines. That is, x-ray analysis of DPPE-Me2 in the gel phase gives lambda = 1.3 A, the same as that previously measured for the analogous gel phase lipid dipalmitoylphosphatidylcholine (DPPC), and Po = 3.9 x 10(9) dyn/cm2, which is in excellent agreement with the value of 3.6 x 10(9) dyn/cm2 calculated from the measured Volta potential of DPPE-Me2 monolayers in equilibrium with liposomes. These results indicate that the removal of one methyl group to convert DPPC to DPPE-Me2 does not markedly alter the range or magnitude of the hydration pressure. Calorimetry shows that the main gel to liquid-crystalline phase transition temperature of DPPE-Me2 is approximately constant for water contents ranging from 80 to 10 water molecules per lipid molecule, but increases monotonically with decreasing water content below 10 waters per lipid. A theoretical fit to these temperature vs. water content data predicts lambda = 6.7 A. The difference in observed values of lambda for x-ray and calorimetry measurements can be explained by effects on the thermograms of additional intra- and intermolecular interactions which occur at low water contents where apposing bilayers are in contact. We conclude that, although calorimetry provides important data on the energetics of bilayer hydration, it is difficult to obtain quantitative information on the hydration pressure from this technique.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaurock A. E. Structure of the nerve myelin membrane: proof of the low-resolution profile. J Mol Biol. 1971 Feb 28;56(1):35–52. doi: 10.1016/0022-2836(71)90082-9. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E., Worthington C. R. Treatment of low angle x-ray data from planar and concentric multilayered structures. Biophys J. 1966 May;6(3):305–312. doi: 10.1016/S0006-3495(66)86658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs J. M. Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function. Biochim Biophys Acta. 1987 Oct 5;906(3):353–404. doi: 10.1016/0304-4157(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Casal H. L., Mantsch H. H. Polymorphic phase behaviour of phospholipid membranes studied by infrared spectroscopy. Biochim Biophys Acta. 1984 Dec 4;779(4):381–401. doi: 10.1016/0304-4157(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Cevc G., Marsh D. Hydration of noncharged lipid bilayer membranes. Theory and experiments with phosphatidylethanolamines. Biophys J. 1985 Jan;47(1):21–31. doi: 10.1016/S0006-3495(85)83872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhry B. Z., Dalziel A. W. Phase transition properties of 1,2- and 1,3-diacylphosphatidylethanolamines with modified head groups. Biochemistry. 1985 Jul 16;24(15):4109–4117. doi: 10.1021/bi00336a045. [DOI] [PubMed] [Google Scholar]
- Gagné J., Stamatatos L., Diacovo T., Hui S. W., Yeagle P. L., Silvius J. R. Physical properties and surface interactions of bilayer membranes containing N-methylated phosphatidylethanolamines. Biochemistry. 1985 Jul 30;24(16):4400–4408. doi: 10.1021/bi00337a022. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Sundell S. Preferred conformation and dynamics of the glycerol backbone in phospholipids. An NMR and X-ray single-crystal analysis. Biochemistry. 1988 Dec 27;27(26):9166–9174. doi: 10.1021/bi00426a014. [DOI] [PubMed] [Google Scholar]
- LeNeveu D. M., Rand R. P. Measurement and modification of forces between lecithin bilayers. Biophys J. 1977 May;18(2):209–230. doi: 10.1016/S0006-3495(77)85608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. C., Simon S. A. Lipid monolayer states and their relationships to bilayers. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4089–4093. doi: 10.1073/pnas.84.12.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra J., Israelachvili J. Direct measurements of forces between phosphatidylcholine and phosphatidylethanolamine bilayers in aqueous electrolyte solutions. Biochemistry. 1985 Aug 13;24(17):4608–4618. doi: 10.1021/bi00338a020. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J. Differences in hydrocarbon chain tilt between hydrated phosphatidylethanolamine and phosphatidylcholine bilayers. A molecular packing model. Biophys J. 1980 Feb;29(2):237–245. doi: 10.1016/S0006-3495(80)85128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh T. J., Holloway P. W. Determination of the depth of bromine atoms in bilayers formed from bromolipid probes. Biochemistry. 1987 Mar 24;26(6):1783–1788. doi: 10.1021/bi00380a042. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Magid A. D., Simon S. A. Cholesterol modifies the short-range repulsive interactions between phosphatidylcholine membranes. Biochemistry. 1989 Jan 10;28(1):17–25. doi: 10.1021/bi00427a004. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Magid A. D., Simon S. A. Range of the solvation pressure between lipid membranes: dependence on the packing density of solvent molecules. Biochemistry. 1989 Sep 19;28(19):7904–7912. doi: 10.1021/bi00445a053. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Magid A. D., Simon S. A. Repulsive interactions between uncharged bilayers. Hydration and fluctuation pressures for monoglycerides. Biophys J. 1989 May;55(5):897–904. doi: 10.1016/S0006-3495(89)82888-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh T. J., Magid A. D., Simon S. A. Steric repulsion between phosphatidylcholine bilayers. Biochemistry. 1987 Nov 17;26(23):7325–7332. doi: 10.1021/bi00397a020. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Area per molecule and distribution of water in fully hydrated dilauroylphosphatidylethanolamine bilayers. Biochemistry. 1986 Aug 26;25(17):4948–4952. doi: 10.1021/bi00365a034. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Hydration force and bilayer deformation: a reevaluation. Biochemistry. 1986 Jul 15;25(14):4058–4066. doi: 10.1021/bi00362a011. [DOI] [PubMed] [Google Scholar]
- Mio M., Okamoto M., Akagi M., Tasaka K. Effect of N-methylation of phosphatidylethanolamine on the fluidity of phospholipid bilayers. Biochem Biophys Res Commun. 1984 May 16;120(3):989–995. doi: 10.1016/s0006-291x(84)80204-1. [DOI] [PubMed] [Google Scholar]
- Mulukutla S., Shipley G. G. Structure and thermotropic properties of phosphatidylethanolamine and its N-methyl derivatives. Biochemistry. 1984 May 22;23(11):2514–2519. doi: 10.1021/bi00306a030. [DOI] [PubMed] [Google Scholar]
- Nagle J. F., Wilkinson D. A. Lecithin bilayers. Density measurement and molecular interactions. Biophys J. 1978 Aug;23(2):159–175. doi: 10.1016/S0006-3495(78)85441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian V. A., Fuller N., Rand R. P. Measured work of deformation and repulsion of lecithin bilayers. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2750–2754. doi: 10.1073/pnas.76.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian V. A., Rand R. P., Fuller N. L., Rau D. C. Osmotic stress for the direct measurement of intermolecular forces. Methods Enzymol. 1986;127:400–416. doi: 10.1016/0076-6879(86)27032-9. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Fuller N., Parsegian V. A., Rau D. C. Variation in hydration forces between neutral phospholipid bilayers: evidence for hydration attraction. Biochemistry. 1988 Oct 4;27(20):7711–7722. doi: 10.1021/bi00420a021. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Cevc G., Kaye R. D., Marsh D. X-ray diffraction study of the polymorphism of hydrated diacyl- and dialkylphosphatidylethanolamines. Biochemistry. 1984 Jun 5;23(12):2634–2644. doi: 10.1021/bi00307a015. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Harlos K., Marsh D. Metastability and polymorphism in the gel and fluid bilayer phases of dilauroylphosphatidylethanolamine. Two crystalline forms in excess water. J Biol Chem. 1983 Mar 25;258(6):3850–3854. [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J. Magnitude of the solvation pressure depends on dipole potential. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9263–9267. doi: 10.1073/pnas.86.23.9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocanne J. F., Teissié J. Ionization of phospholipids and phospholipid-supported interfacial lateral diffusion of protons in membrane model systems. Biochim Biophys Acta. 1990 Feb 28;1031(1):111–142. doi: 10.1016/0304-4157(90)90005-w. [DOI] [PubMed] [Google Scholar]
- Vaughan D. J., Keough K. M. Changes in phase transitions of phosphatidylethanolamine- and phosphatidylcholine-water dispersions induced by small modifications in the headgroup and backbone regions. FEBS Lett. 1974 Oct 1;47(1):158–161. doi: 10.1016/0014-5793(74)80449-7. [DOI] [PubMed] [Google Scholar]
- Xu H., Stephenson F. A., Lin H. N., Huang C. H. Phase metastability and supercooled metastable state of diundecanoylphosphatidylethanolamine bilayers. Biochim Biophys Acta. 1988 Aug 4;943(1):63–75. doi: 10.1016/0005-2736(88)90347-1. [DOI] [PubMed] [Google Scholar]


