Abstract
Yersinia enterocolitica is a pathogen endowed with two adhesins, Inv and YadA, and with the Ysc type III secretion system, which allows extracellular adherent bacteria to inject Yop effectors into the cytosol of animal target cells. We tested the influence of all of these virulence determinants on opsonic and nonopsonic phagocytosis by PU5-1.8 and J774 mouse macrophages, as well as by human polymorphonuclear leukocytes (PMNs). The adhesins contributed to phagocytosis in the absence of opsonins but not in the presence of opsonins. In agreement with previous results, YadA counteracted opsonization. In every instance, the Ysc-Yop system conferred a significant level of resistance to phagocytosis. Nonopsonized single-mutant bacteria lacking either YopE, -H, -T, or -O were phagocytosed significantly more by J774 cells and by PMNs. Opsonized bacteria were phagocytosed more than nonopsonized bacteria, and mutant bacteria lacking either YopH, -T, or -O were phagocytosed significantly more by J774 cells and by PMNs than were wild-type (WT) bacteria. Opsonized mutants lacking only YopE were phagocytosed significantly more than were WT bacteria by PMNs but not by J774 cells. Thus, YopH, -T, and -O were involved in all of the phagocytic processes studied here but YopE did not play a clear role in guarding against opsonic phagocytosis by J774. Mutants lacking YopP and YopM were, in every instance, as resistant as WT bacteria. Overexpression of YopE, -H, -T, or -O alone did not confer resistance to phagocytosis, although it affected the cytoskeleton. These results show that YopH, YopT, YopO, and, in some instances, YopE act synergistically to increase the resistance of Y. enterocolitica to phagocytosis by macrophages and PMNs.
The genus Yersinia includes three species that are pathogenic for rodents and humans, Yersinia pestis, Y. pseudotuberculosis, and Y. enterocolitica, the latter being the most prevalent in humans. These three bacterial species exhibit different degrees of virulence but have in common an ability to multiply in lymphatic tissues and to defeat the primary immune host defenses. This capacity is provided by type III secretion-translocation machinery encoded by a 70-kb plasmid called pYV in Y. enterocolitica. This system allows extracellular bacteria adhering to the surfaces of eukaryotic target cells to inject bacterial proteins into the cytosol of these cells, thereby disarming them and disrupting their communication network. The injected bacterial effectors are called Yops (YopE, -H, -T, -O, -P, and -M). They are secreted by the Ysc secretion apparatus and cross the eukaryotic cell membrane by a pore formed by the other Yops YopB, YopD, and LcrV (for a review, see reference 20).
Adhesion to eukaryotic target cells is crucial for injection of Yops (14), and in Y. enterocolitica, it is mediated by the adhesin YadA (29, 36) or by invasin (Inv) (32). YadA, which is encoded on the pYV plasmid together with the Yops and the Ysc secretion apparatus, is expressed at 37°C (36). It is primarily an adhesin, but it also has the capacity to promote invasion of epithelial cells (11). In addition to its role as an adhesin, it protects Y. enterocolitica against the bactericidal and opsonizing action of complement by binding factor H (17, 18, 51). By this effect, YadA confers some resistance to phagocytosis by human neutrophils (polymorphonuclear leukocytes [PMNs]) and macrophages (17, 59, 66). Inv, which is chromosomally encoded and produced at low temperatures (48), mediates adhesion but also triggers integrin-mediated uptake into nonphagocytic mammalian cells (for a review, see reference 33), promotes self-association (22), and triggers interleukin-8 release (62). In the absence of opsonization, Inv triggers internalization in macrophages via β1 integrins, Cdc42Hs, and WASp (41, 68). The Yop effectors modulate eukaryotic signal transduction pathways. Four of them modify the actin cytoskeleton dynamics. YopH is a tyrosine phosphatase that dephosphorylates focal adhesion kinase (Fak), paxillin, Fyn-binding protein (FYB), p130cas, and SKAP-HOM (4, 7, 9, 15, 28, 49, 50). These eukaryotic proteins regulate the association of actin filaments to the cytoplasmic domain of integrin receptors in focal adhesion complexes (15). YopE causes depolymerization of actin stress fibers by activating the Rho GTPase (8, 57, 67). In addition, it has been proposed to specifically modulate Rac-dependent structures in endothelial cells (5). YopT disrupts the actin stress fibers of the cytoskeleton (31) by an undefined modification of the small GTP-binding protein RhoA and its subsequent redistribution from the membrane to the cytosol (31, 69). YopO, called YpkA in Y. pseudotuberculosis and Y. pestis, is an autophosphorylating serine/threonine kinase that is activated by contact with filamentous actin and phosphorylates it (23, 34). YpkA/YopO also physically interacts with RhoA and Rac, like a RhoA/Rac-binding kinase, resulting in decreased levels of activated RhoA (23). When YpkA/YopO is injected into macrophages, the cells round up but do not detach from the extracellular matrix (27).
Monocytes-macrophages and PMNs represent the first defense mechanisms used by an infected organism against invading bacteria. Two major distinct pathways are involved in the internalization of serum-opsonized particles (1, 2). Both of them are sustained by the Rho GTPase system involved in the reorganization of the actin cytoskeleton (for a review, see reference 16). The first pathway recognizes immunoglobulin G (IgG)-coated particles, on the basis of the immunoglobulin receptor FcγR, and involves actin reorganization mediated by CDC42 and Rac GTPases. The second pathway concerns C3bi-opsonized particles, recognized by complement receptor 3, and involves a remodeling of the cytoskeleton sustained by the Rho GTPases (26). Nonopsonic phagocytosis of microorganisms is also very active in phagocytes. Most of the time, it involves lectin-sugar recognition, which also leads to remodeling of F actin mediated by Rho GTPases (6, 37). Early reports on Y. enterocolitica pathogenesis showed that the pYV plasmid confers the capacity to resist phagocytosis by macrophages or PMNs in vitro (39, 40, 52) YopE and YopH were later shown to be two effectors protecting Y. pseudotuberculosis and Y. enterocolitica from phagocytosis by cultured macrophages (3, 25, 28, 50, 56, 57) or PMNs (4, 65). The two other Yops affecting cytoskeleton dynamics, YopO and YopT, have never been shown to have an antiphagocytic role. In addition, few studies have investigated the role of the adhesins Inv and YadA in phagocytosis by PMNs (17, 54, 66). With some exceptions (25, 59, 65), most of the experiments on phagocytosis were carried out without opsonization. In the present study, we investigated whether YopT and YpkA/YopO are also involved in resistance to phagocytosis. We thus quantified the phagocytosis of an array of Yop effector knockout mutants. We also studied the role of Inv and YadA in phagocytosis. This study was carried out in the absence and in the presence of IgG, complement, or both with freshly isolated human PMNs and with two macrophage cell lines, PU5-1.8 and J774.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Y. enterocolitica MRS40 is a blaA mutant of strain E40 in which the gene encoding β-lactamase A was replaced with the luxAB genes (35, 60). The bacterial strains and plasmids used in this study are listed in Table 1. The strains were routinely grown in tryptic soy broth (Oxoid) and plated on tryptic soy agar (Oxoid) containing the required antibiotics. Selective agents were used at the following concentrations: ampicillin, 200 μg/ml; tetracycline, 10 μg/ml; nalidixic acid, 35 μg/ml; kanamycin, 50 μg/ml; Na+ arsenite, 1 mM (46). For in vitro induction of the Yop virulon and secretion, Y. enterocolitica was grown in brain heart infusion broth (BHI; Remel, Lenexa, Kans.) supplemented with 4 mg of glucose per ml, 20 mM MgCl2, and 20 mM sodium oxalate (BHI-Ox). Cultures were incubated for 2 h at room temperature and then shifted to 37°C for 4 h (19, 43).
TABLE 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Genotype | Reference |
|---|---|---|
| Strains | ||
| E40 | Y. enterocolitica O:9 | |
| MRS40 | blaA luxAB+ mutant of E40 | 60 |
| APB40 | inv::pMS154 mutant of MRS40 | 14 |
| pYV40 plasmids | ||
| pYV40 | WT plasmid of Y. enterocolitica E40 | 60 |
| pAB404 | yopE21yopHΔ1-352 | 13 |
| pAB406 | yopOΔ65-558 | 44 |
| pAB408 | yopM23 | 44 |
| pAB4052 | yopE21 | 44 |
| pAPB4011 | yadAΔ68-326 | 14 |
| pIM409 | yopT135 | 31 |
| pIM424 | yopE21yopT135 | 31 |
| pIM425 | yopHΔ1-352yopT135 | 14 |
| pIM426 | yopE21yopHΔ1-352yopT135 | 14 |
| pIML421 | yopE21yopHΔ1-352yopP23yopM23yopT135yopOΔ65-558 (called ΔHOPEMT) | 31 |
| pMSK41 | yopP23 | 44 |
| pMSL41 | yscNΔ169-177 | 63 |
| pPW401 | yopBΔ89-217 | 13 |
| pSI4008 | yopHΔ1-352 | 44 |
| Expression plasmids | ||
| pIML279 | pBC18R Plac yopT+sycT+ | 31 |
| pMSL34 | pSelect-1 PyopO yopO+ | This work |
| pMSL68 | pBC19R Plac yopE+sycE+ | This work |
| pMSL69 | pBluescriptKS(−) Plac yopH+sycH+ | This work |
| Suicide plasmids | ||
| pINT192 | oriR6K mobRP4 cat; contains 1.4-kb NdeI-BglII internal fragment of inv from Y. enterocolitica 8081 | 48 |
| pMS154 | pKNG160 containing 1.4-kb internal inv fragment subcloned from pINT192 | 64 |
Molecular biology techniques.
Yops were precipitated from culture supernatants with 10% (wt/vol) trichloroacetic acid, analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and, when appropriate, transferred to nitrocellulose membranes. Immunoblots were developed by using a secondary antibody conjugated to horseradish peroxidase (1:2,000; Dako) and the chemiluminescent substrate Supersignal (Pierce, Rockford, Ill.).
Eukaryotic cell line culture and infection conditions.
PU5-1.8 and J774 A1 mouse monocyte macrophages were grown routinely in RPMI 1640 medium, while Rat-1 fibroblasts were grown in Dulbecco modified Eagle medium supplemented with Na-pyruvate at 1 mM (Life Technologies, Merelbeke, Belgium), all at 37°C in a humidified atmosphere under 5% CO2. Both media were supplemented with 2 mM L-glutamine (Seromed), 10% (vol/vol) fetal bovine serum (FBS), 100 U of penicillin per ml, and 100 μg of streptomycin (Life Technologies) per ml. Twenty hours before infection, PU5-1.8 macrophages, J774 macrophages, and Rat-1 fibroblasts were seeded (107 cells/dish) into 1-cm-diameter tissue culture plates (Greiner, Wemmel, Belgium) unless stated otherwise. Thirty minutes before infection, the cells were washed twice and covered with RPMI 1640 medium or Dulbecco modified Eagle medium lacking FBS and antibiotic. Before infection, overnight cultures of Y. enterocolitica were inoculated in BHI at an optical density at 600 nm of 0.2 and grown for 2 h at 25°C and for 30 min at 37°C with shaking. After washing in prewarmed phosphate-buffered saline (PBS), bacteria were added to the cells at a multiplicity of infection (MOI) of 50:1.
Preparation of human neutrophils and infection conditions.
Neutrophils were isolated from the blood of healthy donors, separated by the dextran-Ficoll method (Amersham Pharmacia, Uppsala, Sweden) as described in reference 45, resuspended in minimal essential medium-10 mM HEPES (pH 7.4), and maintained for 20 min at 37°C prior to infection. Before infection, overnight cultures of Y. enterocolitica were inoculated in BHI at an optical density at 600 nm of 0.2 and grown for 2 h at 25°C and for 30 min at 37°C with shaking. After washing in prewarmed PBS, bacteria were added to the cells at an MOI of 50:1.
Yop translocation experiments.
Twenty hours before infection, J774 macrophages were seeded (107 cells/dish) into 10-cm-diameter tissue culture plates (Greiner). Thirty minutes before infection, the cells were washed and covered with RPMI 1640 medium lacking FBS and antibiotic but containing cytochalasin D (5 μg/ml; stock solution, 2 mg/ml in dimethyl sulfoxide; Sigma Aldrich, Bornem, Belgium). Bacteria preincubated as described before were then added at an MOI of 50:1. After an infection period of 2 h at 37°C under 5% CO2, the culture supernatant was carefully removed and 1 ml of ice-cold extraction buffer (0.1% [vol/vol] Triton X-100 in PBS, 1 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin per ml, 1 mg of pepstatin per ml) prepared freshly before extraction (Sigma Aldrich) was added to lyse the cells. The cell lysate was centrifuged at 158 × g for 15 min to collect the Triton-insoluble fraction. The supernatant was transferred to a new tube, and the centrifugation was repeated. The supernatant proteins were precipitated by the addition of trichloroacetic acid (Merck Eurolab, Leuven, Belgium) at a final concentration of 10% (wt/vol). Supernatant and total cells proteins were resuspended in SDS-PAGE loading buffer and analyzed by Western blotting with polyclonal antibodies directed against YopH, -T, and -O and SycE and monoclonal antibody 13A9 directed against YopE.
Opsonization with immune serum.
Two kinds of sera were used to opsonize bacteria. For phagocytosis by mouse cell lines, bacteria were opsonized with serum from a rabbit immunized by intravenous injections of heat-killed Y. enterocolitica O:9 (gift from G. Wauters, Brussels, Belgium). For phagocytosis by human PMNs, bacteria were opsonized with clinical samples of serum that were found to contain anti-Y. enterocolitica O:9 antibodies as detected by using the Sanofi-Pasteur-Bio-Rad kit (gift from G. Bigaignon, Brussels, Belgium). For specific IgG opsonization, serum was decomplemented after 30 min at 56°C. For specific complement opsonization, bacteria were incubated with guinea pig complement (1/500; Sigma Aldrich). To opsonize bacteria, they were grown as explained before, washed in PBS, and incubated for 30 min at 37°C in serum diluted in PBS. Rabbit antiserum was diluted 1/500, while human serum was diluted 1/800. Opsonized bacteria were washed twice with PBS before infection. Opsonization of Y. enterocolitica was monitored by staining with fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG and anti-human C3c reacting with human complement factors C3c and C3bi (Prosan, Merelbeke, Belgium). Strong staining of the serum-opsonized bacteria with FITC-conjugated anti-IgGs revealed the presence of IgG on the bacterial surface. Similarly, strong staining of the serum-opsonized bacteria with FITC-conjugated anti-C3c revealed the presence of C3bi on the bacterial surface. Survival of bacteria after 30 min of incubation with dilute immune serum was checked by plating dilutions of opsonized and nonopsonized bacteria on tryptic soy broth. Similar numbers of colonies appeared on both plates after 24 h of incubation at 25°C.
Phagocytosis assay for macrophage cell lines.
Bacteria and cells were cultured and placed in contact as described before. After an infection period of 30 min, bacteria associated with the target cells in intra- and extracellular locations were distinguished by the double-immunofluorescence method as described previously (30, 55). To stain extracellularly located bacteria, the coverslips were first washed with PBS and then incubated for 30 min at room temperature with rabbit anti-Y. enterocolitica O:9 LPS serum (diluted 1:500). Thereafter, the excess antiserum was removed by four washes in PBS and the coverslips were covered with tetramethyl rhodamine isothiocyanate-conjugated goat anti-rabbit immunoglobulins (Texas Red; 12 μg/ml; Molecular Probes, Leiden, The Netherlands). After incubation for 20 min at 37°C, cells were washed four times in PBS. Coverslips were air dried, fixed, permeabilized by treatment with ice-cold methanol for 90 s, and dried again. To stain the intracellular and extracellular bacteria, the coverslips were incubated again with rabbit anti-Y. enterocolitica O:9 LPS serum (1:500) for 1 h at 37°C. The coverslips were then washed four times in PBS, overlaid with FITC-conjugated goat anti-rabbit immunoglobulins (24 μg/ml; Prosan), and incubated for 20 min at 37°C. Finally, after another washing, the coverslips were mounted in 50% Mowiol (Polysciences) containing 100 mg of diazabicyclooctane (DABCO) per ml and examined by fluorescence microscopy. Under these conditions, the extracellular bacteria were stained with both FITC and Texas Red and appeared green or red, depending on the filter used, while intracellular bacteria were stained only with FITC and appeared green. At least 100 cells were examined, and the number of extracellularly located bacteria and the total number of cell-associated bacteria were determined. For each condition, the experiments were done three times in duplicate. The phagocytosis percentage represents the ratio of the number of intracellular bacteria to the total number of cell-associated bacteria.
Phagocytosis assay for PMNs.
For monitoring of phagocytosis by PMNs, the protocol was slightly different. Infection was carried out for 30 min at 37°C at an MOI of 50:1 as previously described (37). The neutrophils were then washed to remove free-floating bacteria and fixed for 30 min at room temperature with 3.7% paraformaldehyde in PBS containing 15 mM sucrose (pH 7.4). After neutralization with 50 mM NH4Cl, the coverslips were washed with PBS and incubated for 30 min with rabbit anti-Y. enterocolitica O:9 LPS serum (diluted 1:500) and then for 30 min with goat anti-rabbit Texas Red antibodies (12 μg/ml) to stain the extracellular bacteria. The cells were then permeabilized by 2 min of treatment at room temperature with 0.5% Triton X-100 in PBS and subsequently washed in PBS. Intracellular and extracellular bacteria were then stained by sequential addition of anti-Y. enterocolitica O:9 LPS serum (1:100) and goat anti-rabbit antibodies coupled to FITC (24 μg/ml). The coverslips were air dried, mounted on Mowiol-DABCO, and examined by fluorescence microscopy.
Immunofluorescence staining and confocal microscopy analysis.
Rat-1 fibroblasts were grown as monolayers on coverslips and infected with Y. enterocolitica at an MOI of 50:1. After 2 h 30 min of infection, the first sign of cytotoxicity appeared and the cells were fixed in 2% paraformaldehyde for 20 min. After washing with PBS, membranes were permeabilized with PBS-0.5% Triton X-100 for 10 min. Actin microfilaments were labeled by incubation for 40 min at 37°C with FITC-conjugated phalloidin. Samples were mounted in (Mowiol-DABCO), and cells were examined on a confocal laser scanning microscope equipped with dual detectors and an argon-krypton laser (MRC 1024; Bio-Rad Laboratories).
Statistical analysis.
The number of samples in each experimental condition is indicated in the figure legends. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Bonferroni's multiple-comparison test.
RESULTS
Resistance of Y. enterocolitica MRS40 to phagocytosis by J774 cells and PMNs.
We first monitored the behavior of Y. enterocolitica MRS40 in contact with J774 mouse macrophage-like cells at an MOI of 50:1. After 30 min of infection at 37°C, we counted the cell-associated bacteria and calculated the percentage of bacteria that were phagocytosed by using the double-immunofluorescence method (30). In parallel, we tested wild-type (WT) Y. enterocolitica and a mutant deprived of YscN, the ATPase of the type III Ysc machinery. Adherence of both nonopsonized bacteria to J774 was in the same range (Fig. 1A). The WT bacteria adhered somewhat better, presumably because they produce more YadA (42). However, only 30% of the WT Y. enterocolitica MRS40 bacteria associated with J774 cells were ingested (Fig. 1C) while 80% of the cell-associated yscN bacteria were taken up. This demonstrated that the Ysc-Yop system allows Y. enterocolitica MRS40 to resist phagocytosis by J774 cells. Since Y. enterocolitica encounters not only macrophages but also PMNs during infection, we also tested the resistance of Y. enterocolitica bacteria to ingestion by PMNs freshly isolated from a healthy human donor. As shown in Fig. 1D, only 40% of the WT bacteria were phagocytosed while 82% of the YscN-deficient bacteria were phagocytosed. PMNs were thus more efficient than J774 cells at ingesting Y. enterocolitica but, again, the Ysc-Yop-deficient bacteria were more phagocytosed than WT bacteria. This result is in agreement with a previous study (65) and illustrates the reactivity of freshly isolated neutrophils in comparison with immortalized macrophage cell lines.
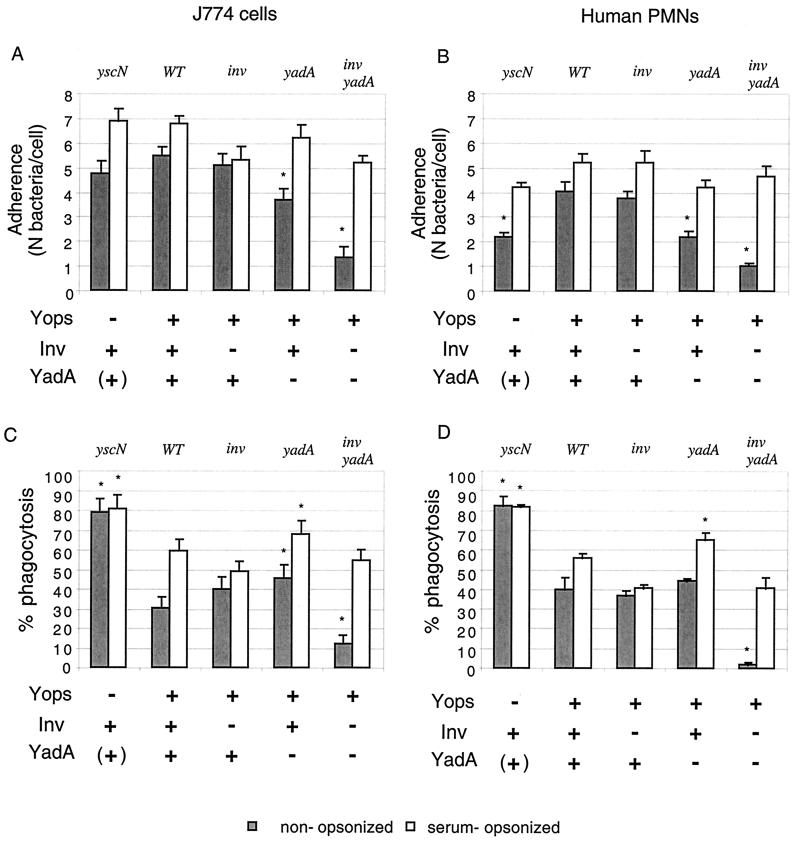
FIG. 1.
(A and B) Influence of Inv and YadA on adherence and phagocytosis of Y. enterocolitica MRS40 by J774 macrophages (A and C) and human PMNs (B and D). Nonopsonized or serum-opsonized WT Y. enterocolitica [MRS40(pYV40)], the yscN mutant [MRS40(pMSL41)], the inv mutant [APB40(pYV40)], the yadA mutant [MRS40(pAPB4011)], and the inv yadA mutant [APB40(pAPB4011)] were exposed to J774 cells (A and C) or PMNs (B and D) at a calculated MOI of 50:1 for 30 min at 37°C. The number of adherent bacteria per cell and the percentage of phagocytosis were determined by the double-immunofluorescence technique. Results of adherence are given as the mean plus the standard error of the mean. Phagocytosis results are given as percentages of phagocytosed bacteria relative to the total number of cell-associated bacteria (∗, P < 0.05 compared to the WT; n = 6). The values are the mean plus the standard error of the mean. For each experiment, at least 100 cells were examined.
Role of bacterial adhesins in the internalization process.
Y. enterocolitica adheres to many different cell types by means of its two adhesins, Inv and YadA. With most cell types, but not with macrophages, the presence of Inv or YadA is an absolute prerequisite for activation of the Ysc-Yop system (14, 68). In the case of phagocytes, the adhesins could have two antagonistic effects. By promoting contact with the phagocyte, they may trigger injection of antiphagocytic Yops but, on the contrary, they could also favor phagocytosis. We thus tested the role of Inv and/or YadA in both adherence and phagocytosis by J774 macrophages and PMNs. This was done by using inv or yadA single mutants, as well as an inv yadA double mutant.
Under our experimental conditions and in the absence of opsonization (Fig. 1A, grey bars), adherence of the inv mutant to J774 was almost equivalent to the adherence of WT bacteria (5.1 ± 0.4 and 5.5 ± 0.3, respectively) and adherence of yadA mutant bacteria was slightly decreased (3.7 ± 0.4; Fig. 1A, grey bars). In contrast, the adherence of the inv yadA double-mutant bacteria was decreased fourfold compared to the adherence of WT bacteria (1.4 ± 0.4). These values confirmed that both adhesins, especially YadA, play a role in the adherence of nonopsonized Y. enterocolitica MRS40 to phagocytic cells. Notice that adherence is significantly lower on PMNs than on J774 cells, but the ratio of mutant to WT bacteria is conserved (Fig. 1B, grey bars). Although the double-mutant bacteria adhered less than the other bacteria, they still interacted with phagocytes. This suggests that another element, different from Inv and YadA, also leads to adherence. It could be either a phagocyte receptor acting in the absence of opsonization or another, unidentified, bacterial adhesin.
The percentage of phagocytosis of nonopsonized inv or yadA single-mutant bacteria by J774 macrophages (Fig. 1C) or by PMNs (Fig. 1D) was not significantly different from the percentage of phagocytosis of WT bacteria. In contrast, the few inv yadA double-mutant bacteria that interacted with phagocytic cells were very poorly internalized. This indicates that, in the absence of opsonization, Inv or YadA favors entry into phagocytes. It also indicates that the unidentified adherence mechanism does not trigger efficient uptake of bacteria.
In vivo, recognition of the invading bacteria by phagocytes involves opsonins such as C3bi and IgG. We thus tested the influence of Inv and YadA on adherence and phagocytosis after incubation of bacteria with nonheated immune serum containing opsonins like IgG and C3bi (Fig. 1, white bars). For experiments with J774 mouse cells, bacteria were opsonized with anti-Y. enterocolitica O:9 LPS rabbit serum, while for experiments with human PMNs, they were opsonized with human serum containing antibodies directed against Y. enterocolitica O:9. Opsonization increased the number of cell-associated bacteria for every strain tested, including the inv yadA double mutant (Fig. 1A and B, white bars). These double-mutant bacteria even associated with phagocytes as efficiently as WT bacteria, which confirmed the efficacy of opsonization by the immune serum. Opsonized yadA bacteria were taken-up more efficiently than opsonized inv bacteria and opsonized WT bacteria. This presumably results from the capacity of YadA to prevent C3bi deposition on bacteria, as shown by China et al. (18). This protective effect of YadA can also explain the strong increase in the phagocytosis percentage caused by opsonization of inv yadA double-mutant bacteria. Indeed, the increase in phagocytosis due to opsonization is much more pronounced for the inv yadA double mutant than for the inv single mutant.
We concluded from all of this that, in the absence of opsonization, entry of Y. enterocolitica into phagocytes is essentially a phenomenon driven by YadA or Inv. In contrast, opsonization leads to phagocytosis independently of YadA and Inv. In the latter situation, YadA tends to reduce phagocytosis. In any instance, yscN mutant bacteria were internalized more than WT bacteria, indicating that the Ysc-Yop system counteracts the engulfment mechanism.
Influence of opsonization on Yop injection.
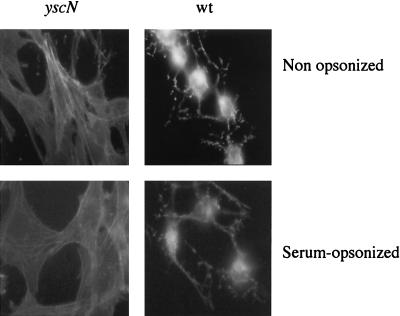
It is not known whether opsonization reduces the efficiency of the type III secretion-translocation system. To address this question, we used Rat-1 fibroblasts because they have a developed actin cytoskeleton and because the destruction of the cytoskeleton is the most sensitive test with which to monitor the delivery of Yops. We thus infected Rat-1 cells at an MOI of 50:1, and after 2.5 h of infection, we fixed the cells and stained actin with FITC-phalloidin. As shown in Fig. 2, incubation with rabbit anti-Y. enterocolitica O:9 immune serum, used at the same dilution as for opsonization, did not prevent or even delay the destruction of the actin cytoskeleton. Thus, we concluded that serum opsonization does not inhibit the activity of the type III Ysc-Yop system.
FIG. 2.
Cytotoxic effect of serum-opsonized WT Y. enterocolitica MRS40 bacteria. Rat-1 fibroblasts were infected with Y. enterocolitica [MRS40(pYV40)] bacteria or yscN mutant [MRS40(pMSL41)] bacteria, and cytotoxicity was monitored by staining with FITC-phalloidin after 2 h 30 min of infection. Opsonized and nonopsonized WT Y. enterocolitica MRS40 bacteria were cytotoxic for Rat-1 fibroblasts, in contrast to opsonized or nonopsonized yscN mutant bacteria.
Role of Yop effectors in resistance to nonopsonic phagocytosis.
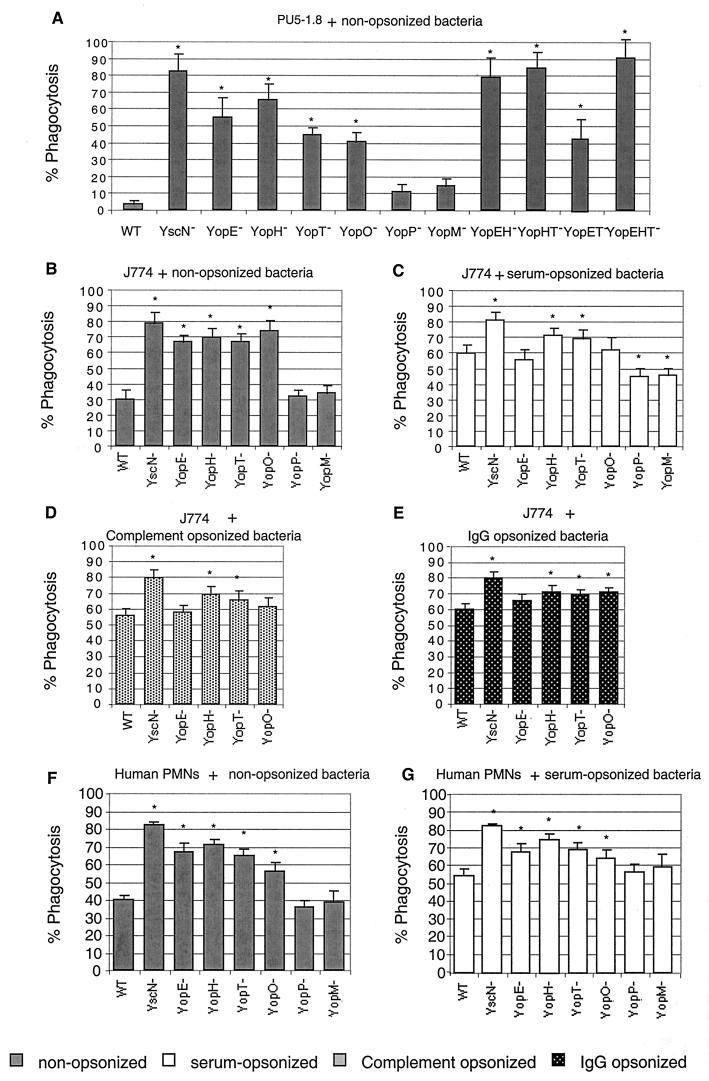
To determine the involvement of each Yop effector in resistance to phagocytosis, a collection of six single mutants (Table 1) was used. For each experiment, we measured the total number of bacteria associated with PU5-1.8 macrophages and observed that it was similar for the different strains tested (data not shown). In the absence of opsonization, the yopE, yopH, yopT, and yopO single-mutant bacteria were phagocytosed by PU5-1.8 macrophages at least 10-fold more than WT bacteria (Fig. 3A ). In contrast, yopP and yopM single-mutant bacteria resisted phagocytosis as well as did WT bacteria, showing that these effectors do not play any role in resistance to phagocytosis. Double or triple mutants affected in YopE, YopH, and YopT (Fig. 3A) were more phagocytosed than single mutants, as already described for Y. pseudotuberculosis (25). The yopET double-mutant bacteria seemed to be less phagocytosed than the yopE single-mutant bacteria. However, this difference was not significant according to the statistical analysis (P < 0.05). We concluded from this that YopE, -H, -T, and -O are all necessary for Y. enterocolitica MRS40 to fully resist engulfment by PU5-1.8 macrophages.
FIG. 3.
Phagocytosis of nonopsonized (grey bars, A, B, and F), serum-opsonized (white bars, C and G), complement-opsonized (D), and IgG-opsonized (E) Y. enterocolitica MRS40 and effector mutants by PU5-1.8 macrophages (A), J774 macrophages (B, C, D, and E), and PMNs (F and G). Y. enterocolitica MRS40(pYV40), the yscN mutant [MRS40(pMSL41)], the yopE mutant [MRS40(pAB4052)], the yopH mutant [MRS40(pSI4008)], the yopT mutant [MRS40(pIM409)], the yopO mutant [MRS40(pAB406)], the yopP mutant [MRS40(pMSK41)], the yopM mutant [MRS40(pAB408)], the yopEH mutant [MRS40(pAB404)], the yopHT mutant [MRS40(pIM425)], the yopET mutant [MRS40 (pIM424)], and the yopEHT mutant [MRS40(pIM426)] were exposed to phagocytic cells at a calculated MOI of 50:1 for 30 min at 37°C. Phagocytosis percentages were determined by double-immunofluorescence assay. Phagocytosis results are given as percentages of phagocytosed bacteria relative to the total number of cell-associated bacteria (∗, P < 0.05 compared to the WT; n = 6). The values are the mean plus the standard error of the mean. For each experiment, at least 100 cells were counted.
yopE, yopH, yopT, and yopO single mutants were also phagocytosed more than WT bacteria by J774 macrophages (Fig. 3B, grey bars). The percentage of phagocytosis increased from 30% (WT) to 66% (yopE or yopT), 70% (yopH), and even 74% (yopO), confirming that each of these four effectors contributes significantly to resistance to phagocytosis. In J774 infection, as well as in PU5-1.8 infection, removal of YopP or YopM did not increase the percentage of phagocytosis of cell-associated bacteria compared to WT Y. enterocolitica MRS40. Single mutations affecting YopE, YopH, YopT, and YopO also significantly increased phagocytosis by PMNs. Again, mutations affecting YopP and YopM had no effect (Fig. 3F, grey bars). Thus, single mutations in any of the Yop effectors known to act on the cytoskeleton significantly increased the phagocytosis of nonopsonized bacteria, indicating that each of them plays an important role. Single-mutant bacteria were phagocytosed almost as efficiently as bacteria deprived of the whole Yop virulon.
Role of Yop effectors in resistance to opsonic phagocytosis.
Phagocytosis of Y. enterocolitica by J774 and by PMNs was also monitored after opsonization by immune serum providing both complement and specific IgG. The results obtained with both types of cells were very similar (Fig. 3C and G, white bars). The opsonization of WT Y. enterocolitica increased by about 30% the number of bacteria engulfed by J774 and by about 15% the number of bacteria engulfed by PMNs, indicating that WT Y. enterocolitica bacteria resist phagocytosis less when they are opsonized. The yscN mutant bacteria were still phagocytosed significantly more than WT bacteria, indicating that the Ysc-Yop virulon also counteracts opsonic phagocytosis. However, the effect of single mutations was not so clear in the presence of opsonins as in the absence of opsonins. Compared to opsonized WT bacteria, opsonized yopE and yopO mutant bacteria were not phagocytosed significantly more. In contrast, opsonized yopH and yopT bacteria were phagocytosed slightly but significantly (P < 0.05) more than WT bacteria. Since the four Yops involved in phagocytosis resistance control different host cell GTPases that are differentially implicated in IgG or complement opsonic phagocytosis (1, 2), we wondered whether the different Yops could be differently involved in the two types of opsonic phagocytosis. To address this question, we performed phagocytosis experiments with either complement- or IgG-opsonized bacteria. The results, presented in Fig. 3D and E, respectively, were not different from those obtained with double opsonization (Fig. 3C). In both cases, phagocytosis of the yopH, YopT, and yopO mutants was significantly increased compared to that of WT bacteria. Again, no significant difference was observed for the yopE mutant.
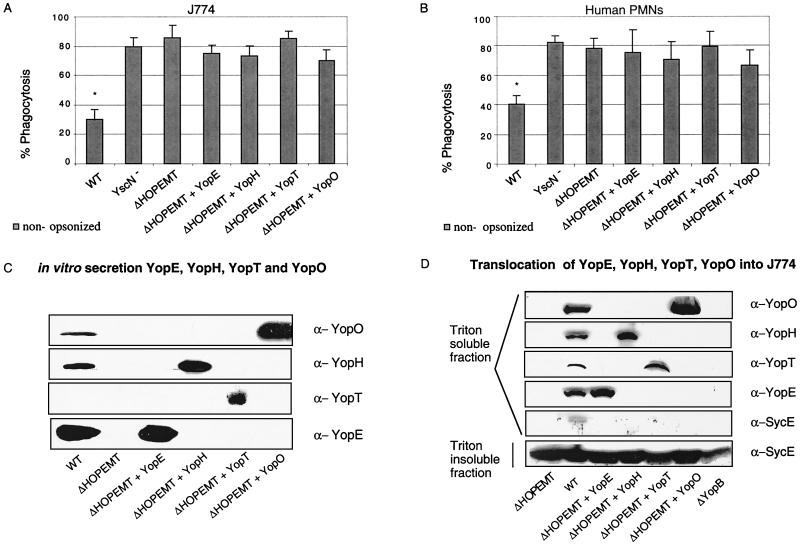
YopE, -H, -T, or -O alone is not sufficient for resistance to phagocytosis.
We have shown that each of the four effectors YopE, -H, -T, or -O contributes to resistance to phagocytosis. To investigate whether each of them could confer some level of protection when overproduced alone, we used a strain mutated in all six effector genes and called ΔHOPEMT (31). We introduced separately into this multimutant strain high-copy plasmids containing yopE and its sycE chaperone gene, yopH and sycH, yopT and sycT, and yopO alone. No chaperone could be identified for this Yop (Table 1). We first controlled expression of these different constructions for their capacity to secrete the relevant Yop under calcium chelation conditions. YopE, -H, -T, and -O were clearly visible in the supernatants (Fig. 4C), but, as already observed by Iriarte and Cornelis (31), YopT was detected in the supernatant of the MRS40 ΔHOPEMT-overproducing YopT strains but not in the supernatant of the WT strain. Translocation of these effectors into J774 macrophages was also checked by Triton fractionation of infected cells (Fig. 4D). As seen in Fig. 4D, all four Yops, including YopT, were detected in the Triton-soluble fraction, indicating that all four Yops were indeed injected. After infection with yopB mutant bacteria, we could not see any of the four effectors in the Triton-soluble fraction, confirming that we only detected injected proteins. We then compared the phagocytosis of these bacteria producing only one effector to the phagocytosis of ΔHOPEMT. As shown in Fig. 4A and B, after 30 min of contact, there was no significant reduction of phagocytosis by J774 or PMNs due to the injection of only one effector. Thus, we concluded that none of the antiphagocytic Yops alone can confer a significant degree of resistance.
FIG. 4.
(A and B) Phagocytosis of the Y. enterocolitica ΔHOPEMT mutant overexpressing the effectors by J774 macrophages (A) or PMNs (B). J774 macrophages or PMNs were infected at an MOI of 50:1 for 30 min at 37°C with WT [MRS40(pYV40)] Y. enterocolitica, the yscN mutant [MRS4(pMSL41)], the ΔHOPEMT mutant [MRS40(pIML421)], or the ΔHOPEMT strain overexpressing YopE (pMSL68), YopH (pMSL69), YopT (pIML279), or YopO (pMSL34). Phagocytosis percentages were determined by double-immunofluorescence assay. Phagocytosis results are given as percentages of phagocytosed bacteria relative to the total number of cell-associated bacteria (∗, P < 0.05 compared to the ΔHOPEMT strain; n = 6). The values are the mean plus the standard error of the mean. For each experiment, at least 100 cells were counted. (C) Yop secretion by the Y. enterocolitica ΔHOPEMT mutant overexpressing the effectors. Western blot analysis (chemiluminescence detection) of proteins from culture supernatant corresponding to 2 ml of a culture of WT [MRS40(pYV40)] Y. enterocolitica, the yscN mutant [MRS40(pMSL41)], the ΔHOPEMT mutant [MRS40(pIML421)], or the ΔHOPEMT mutant overexpressing YopE (pMSL68), YopH (pMSL69), YopT (pIML279), or YopO (pMSL34) incubated for 4 h at 37°C in BHI-Ox. Immunoblot assay with rabbit anti-YopH, anti-YopT, and anti-YopO polyclonal antibodies and rat anti-YopE monoclonal antibodies (13A9). (D) Injection of the effectors by the various Y. enterocolitica ΔHOPEMT recombinant strains. J774 macrophages were infected at an MOI of 50:1 with WT Y. enterocolitica [MRS40(pYV40)], the ΔHOPEMT mutant [MRS40(pIML421)], the ΔHOPEMT mutant expressing YopE (pMSL68), the ΔHOPEMT mutant expressing YopH (pMSL69), the ΔHOPEMT mutant expressing YopT (pIML279), the ΔHOPEMT mutant expressing YopO (pMSL34), or the yopB mutant [MRS40(pPW401)] producing the same individual Yops. After 2 h, cytosolic fractions of J774 were prepared by Triton (0.1%) lysis and analyzed by SDS-PAGE and Western blotting with rabbit anti-YopH, anti-YopT, anti-YopO, and anti-SycE polyclonal antibodies and rat anti-YopE monoclonal antibodies (13A9). The absence of detection of SycE in the Triton-soluble fraction demonstrates that this fraction does not contain cytoplasmic proteins from Y. enterocolitica.
Actin cytoskeleton disruption by effectors YopE, YopH, YopT, and YopO.
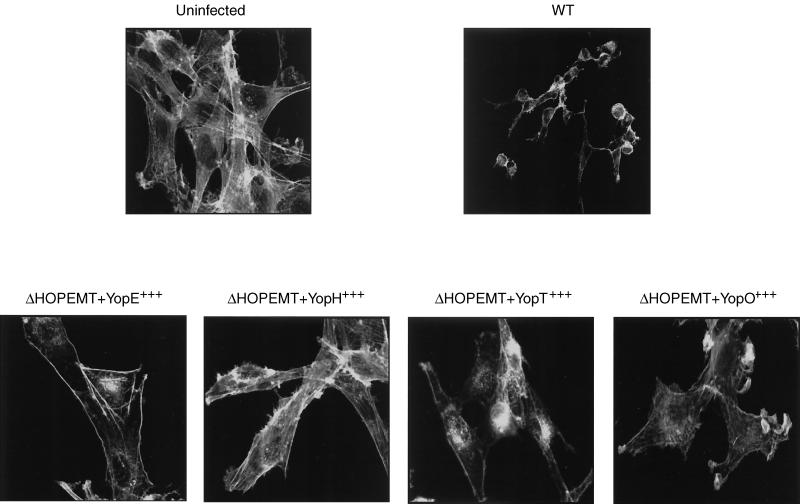
Since we could not see any resistance of our recombinant ΔHOPEMT clones to phagocytosis, we wondered whether they exerted their effect on the cytoskeleton. To address this question, we again used Rat-1 fibroblasts instead of phagocytes because their actin cytoskeleton is more developed than that of phagocytes. We infected Rat-1 fibroblasts for 2.5 h and stained the cytoskeleton with FITC-phalloidin (Fig. 5). The cytoskeleton of cells infected with Y. enterocolitica MRS40 expressing only one effector was affected less than that of cells infected with WT bacteria. Cells did not round up, remained spread, and attached to the coverslips even though the cytoskeleton was modified. Injection of YopE led to disruption of actin stress fibers and induced accumulation of peripheral actin. YopH induced retraction of Rat-1 fibroblast and the appearance of cellular protrusions like microspikes. After injection of YopT, the actin cytoskeleton was disrupted and some aggregates of actin appeared within the Rat-1 fibroblast cells. The fibroblasts were still adherent and stayed attached after YopO injection but presented ruffle structures. Finally, after 4 h of infection, Rat-1 cells infected with these different strains rounded up and detached, as normally seen after 2.5 h of infection with WT bacteria (data not shown).
FIG. 5.
Actin disruption by YopE, YopH, YopT, and YopO. Rat-1 cells were infected with Y. enterocolitica MRS40(pYV40) for 2 h 30 min in the presence of 10% FBS. After fixing and permeabilization of the cells, actin was stained with phalloidin-FITC. The cells were then analyzed by confocal scanning laser microscopy. Uninfected cells display elongated actin filaments (stress fibers) throughout the cells. Infection with WT Y. enterocolitica [MRS40(pYV40)], the ΔHOPEMT mutant [MRS40(pIML421)], and the ΔHOPEMT mutant expressing YopE (pMSL68), YopH (pMSL69), YopT (pIML279), or YopO (pMSL34) results in different modifications of the actin cytoskeleton.
Therefore, each effector injected alone manifested its effect on the cytoskeleton. In agreement with the known diversity of targets of YopE, -H, -T, and -O, the effects were different from each other. Thus, the lack of resistance to phagocytosis observed in Fig. 4A and B was not due to lack of Yop action. We thus concluded that YopE, -H, -T, or -O alone is not sufficient to inhibit phagocytosis.
DISCUSSION
Previous studies have unambiguously demonstrated that YopH and YopE play a major role in the defense of Y. pseudotuberculosis against phagocytosis (10, 25, 56, 57). In this study, we focused on Y. enterocolitica and addressed the question of the role of the other four known Yop effectors against opsonic or nonopsonic phagocytosis by cultured monocytes-macrophages and by PMNs. We also wanted to study whether there was redundancy in this system. Finally, we also reinvestigated the role of the two major adhesins. We first tested a set of single-knockout mutants and confirmed that, indeed, YopH and YopE are involved in resistance to phagocytosis (10, 25, 56, 57). The antiphagocytic role of YopH from Y. pseudotuberculosis has been documented for a long time (3, 25), as well as its role in the dephosphorylation of several focal adhesion proteins, including Fak, paxillin, FYB, p130cas, and SKAP-HOM (4, 7, 9, 15, 24, 28, 49, 50). More recent reports made the link between phosphorylation of paxillin and Fak and cytoskeletal assembly (15) and between focal adhesion and phagocytosis (49, 50). It has been known for a long time that microinjection of YopE into epithelial cells leads to disruption of the actin microfilaments (57, 67). More recently, it appeared that YopE acts as a GTPase-activating protein active toward GTPases of the Rho family (g67), more precisely, toward Rac (5). Since selective inhibition of Rac abolishes ruffle formation (5), one of the engulfment steps, here again, the two kinds of observations can be combined. In this work, we confirmed that YopH contributes to the inhibition of phagocytosis of both opsonized and nonopsonized Y. enterocolitica bacteria by J774 mouse macrophages and by human PMNs. We also confirmed that YopE is involved in protection against nonopsonic phagocytosis. However, we did not observe any clear role of YopE against opsonic phagocytosis by J774 cells. In addition, we demonstrated that YopO and YopT are involved in the inhibition of both types of phagocytosis, even though they are probably injected in smaller amounts. Although this observation fits perfectly with the known cellular action of YopO and YopT, this role had never been reported before. It is somehow surprising that YopT plays such a clear role in defending against phagocytosis in vitro while it does not seem to contribute to the capacity of Y. enterocolitica to multiply in Peyer's patches (30). We have no explanation for this discrepancy, which is, however, not unprecedented: the same occurs with YopP, which has very strong in vitro activity but a minor role in vivo (61). These discrepancies probably only point to the fact that the animal models have their limitations, possibly because they only allow monitoring of acute infections. YopT inactivates RhoA by inducing an unknown biochemical modification (69). Since RhoA is necessary for the actin rearrangement underlying the phagocytic event (16), this could explain the antiphagocytic role of YopT. YpkA, the homologue of YopO in Y. pseudotuberculosis, has been shown to localize to the inner surface of the plasma membrane, where, upon activation by actin, it works like a RhoA- and Rac-binding kinase (23, 34). As a result of this, it is expected to prevent the actin cytoskeleton rearrangement that must occur upon contact with the parasite and lead to its engulfment.
According to our results, the other two effectors YopM and YopP do not contribute to resistance to phagocytosis. This again fits quite well with the present knowledge of these effectors. YopM is a leucine-rich repeat protein to which neither a target nor a function has been assigned. It is known to migrate to the nucleus (38, 53). As for YopP, called YopJ in Y. pestis and Y. pseudotuberculosis, it inhibits transcription of proinflammatory genes by blocking the mitogen-activated protein kinase and NF-κB pathways, presumably acting as a protease (21, 47, 58, 61). Our data thus show that of six known effectors, four play a role in defense against phagocytosis. Interestingly, they also show that single mutants are severely affected in their resistance capacity. Thus, there is no redundancy among these anti-cytoskeleton effectors and each of them has a role to play in phagocyte neutralization. This conclusion is reinforced by our complementary experiments showing that none of the four effectors alone, even when produced in larger amounts, can confer a significant level of resistance. Thus, the four effectors act in a synergistic way by blocking different key steps in the phagocytic pathway.
There was no difference in the profiles of resistance of the various single mutants to phagocytosis after opsonization either with complement or with IgG. This result is somehow surprising, as these two different opsonizations lead to phagocytosis driven by different mechanisms, in particular, by different GTPases (1, 2). The result obtained with YopT, which was only shown to affect RhoA, suggests that either RhoA is always involved or that YopT also affects other GTPases. Even more surprising is our result showing that YopE does not seem to protect at all against opsonic phagocytosis, whatever the opsonin used. This might be explained by the fact that nonopsonic phagocytosis involves a different signaling cascade than opsonic phagocytosis. YopE has been shown to inactivate Rho, Rac, and Cdc42 in vitro (8, 67) but only activity toward RhoA and Rac has been indirectly demonstrated in vivo (8). Our results thus suggest that Cdc42 contributes more to opsonic phagocytosis than to nonopsonic phagocytosis and that Rac and RhoA are involved in the nonopsonic pathway of phagocytosis. Adherence is a prerequisite for activation of the Yop virulon in contact with epithelial cells, endothelial cells, and fibroblasts (14, 68). In the absence of opsonization, the inv yadA double mutant has fourfold reduced adherence to phagocytes, indicating that these two bacterial adhesins can play a major role in establishing contact with phagocytes. This is in perfect agreement with previous reports by Roggenkamp et al. (54) and Ruckdeschel et al. (59) showing that YadA plays a specific role during interaction of nonopsonized Y. enterocolitica and PMNs. Inv has been shown to trigger phagocytosis of nonopsonized Y. enterocolitica after contact with β1 integrins, which activates actin polymerization via Cdc42Hs, its effector WASp, and the Arp2/3 complex (68). In our experiments, loss of YadA resulted in a more pronounced reduction of adherence than did loss of Inv. However, one cannot draw a conclusion from this about the relative importance of these two adhesins in vivo because they differ in relative abundance, depending on the experimental conditions. Indeed, Inv is produced at low temperatures while YadA is produced at 37°C and here the experiments were conducted with bacteria grown at room temperature and subsequently shifted to 37°C for 30 min. Under these conditions, both adhesins are expected to be present at the bacterial surface. In addition to being temperature regulated, yadA belongs to the yop regulon and hence is subject to feedback inhibition when the Ysc secretion channel is not functional (19). This may explain why the yscN mutant interacted less with phagocytes than did WT bacteria. Looking at the internalization data in the absence of opsonization, it thus appears that Inv or YadA promotes contact and internalization, which could be considered detrimental for the bacterium.
It has previously been shown that nonopsonized Y. enterocolitica and macrophages can interact independently of YadA and Inv (14). In agreement with these previous findings, the inv yadA double-mutant bacteria still interacted with phagocytes, indicating that another element, different from Inv and YadA, leads to some adherence. It could be either an unidentified bacterial adhesin or a phagocyte receptor recognizing another bacterial surface component, such lipoglycans, mannose, or other sugar moieties. However, the Inv- and YadA-independent adherence leads to very poor internalization but our data do not reveal whether this results from poor stimulation of phagocytic activity or from excellent Yop virulon activity.
In the presence of opsonins, bacteria lacking both adhesins were efficiently associated with phagocytes and phagocytosed. This indicates that, in the presence of opsonins, bacterial adhesins do not facilitate ingestion. It is even the opposite, since YadA-deficient bacteria appear to be phagocytosed more than WT bacteria, as expected from the observations of China et al. that YadA binds factor H (17). The fact that YadA has a protective role against opsonic phagocytosis is also apparent from the effect of opsonization on phagocytosis of the inv yadA double-mutant bacteria: opsonization increases the percentage of phagocytosed inv yadA double-mutant bacteria by 45% but that of inv yadA+ mutant bacteria by only 10%. We infer that this difference reflects some protective activity of YadA against opsonization.
In conclusion, in the absence of opsonins, YopE, -H, -T, and -O act synergistically to counteract phagocytosis of Y. enterocolitica by macrophages and PMNs. There is no redundancy in the system since the loss of any of these Yops leads to a significant increase in phagocytosis. In the presence of opsonins, YopH, YopT, and YopO play a clear role but their synergistic action is insufficient to block phagocytosis completely. Presumably, this reduction allows sufficient escape to sustain infection. YopE does not seem to contribute to resistance to opsonic phagocytosis by macrophages. As for the adhesins, they favor phagocytosis in the absence of opsonization. However, in the presence of opsonins, the situation is different: the adhesins do not contribute to phagocytosis, and YadA even has a protective effect.
Acknowledgments
We thank Christine Bordier for excellent technical assistance and Pierre Masson, Nathalie Sauvonnet, and Geertrui Denecker for discussions and critical reading of the manuscript.
N.G. is research assistant funded by the Belgian Fonds National de la Recherche Scientifique. This work was supported by the Belgian Fonds National de la Recherche Scientifique Médicale (Convention 3.4595.97) and the Direction générale de la Recherche Scientifique-Communauté Française de Belgium (Action de Recherche Concertée 94/99-172).
Editor: B. B. Finlay
REFERENCES
- 1.Aderem, A., and D. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593-623. [DOI] [PubMed] [Google Scholar]
- 2.Allen, L., and A. Aderem. 1996. Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. J. Exp. Med. 184:627-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson, K., N. Carballeira, K. Magnusson, C. Persson, O. Stendahl, H. Wolf-Watz, and M. Fallman. 1996. YopH of Yersinia pseudotuberculosis interrupts early phosphotyrosine signalling associated with phagocytosis. Mol. Microbiol. 20:1057-1069. [DOI] [PubMed] [Google Scholar]
- 4.Andersson, K., K. E. Magnusson, M. Majeed, O. Stendahl, and M. Fallman. 1999. Yersinia pseudotuberculosis-induced calcium signaling in neutrophils is blocked by the virulence effector YopH. Infect. Immun. 67:2567-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andor, A., K. Trulzsch, M. Essler, A. Roggenkamp, A. Wiedemann, J. Heesemann, and M. Aepfelbacher. 2001. YopE of yersinia, a GAP for Rho GTPases, selectively modulates Rac-dependent actin structures in endothelial cells. Cell Microbiol. 3:301-310. [DOI] [PubMed] [Google Scholar]
- 6.Astarie-Dequeker, C., E. N'Diaye, V. Le Cabec, M. Rittig, J. Prandi, and I. Maridonneau-Parini. 1999. The mannose receptor mediates uptake of pathogenic and nonpathogenic mycobacteria and bypasses bactericidal responses in human macrophages. Infect. Immun. 67:469-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black, D., L. Montagna, S. Zitsmann, and J. Bliska. 1998. Identification of an amino-terminal substrate-binding domain in the yersinia tyrosine phosphatase that is required for efficient recognition of focal adhesion targets. Mol. Microbiol. 29:1263-1274. [DOI] [PubMed] [Google Scholar]
- 8.Black, D. S., and J. B. Bliska. 2000. The RhoGAP activity of the yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37:515-527. [DOI] [PubMed] [Google Scholar]
- 9.Black, D. S., A. Marie-Cardine, B. Schraven, and J. B. Bliska. 2000. The yersinia tyrosine phosphatase YopH targets a novel adhesion-regulated signalling complex in macrophages. Cell Microbiol. 2:401-414. [DOI] [PubMed] [Google Scholar]
- 10.Bliska, J., and D. Black. 1995. Inhibition of the Fc receptor-mediated oxidative burst in macrophages by the Yersinia pseudotuberculosis tyrosine phosphatase. Infect. Immun. 63:681-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bliska, J. B., M. C. Copass, and S. Falkow. 1993. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infect. Immun. 61:3914-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boland, A., and G. R. Cornelis. 1998. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during yersinia infection. Infect. Immun. 66:1878-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boland, A., M. P. Sory, M. Iriarte, C. Kerbourch, P. Wattiau, and G. R. Cornelis. 1996. Status of YopM and YopN in the yersinia Yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5-1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 15:5191-5201. [PMC free article] [PubMed] [Google Scholar]
- 14.Boyd, A., N. Grosdent, S. Totemeyer, C. Geuijen, S. Bleves, M. Iriarte, I. Lambermont, J. Octave, and G. Cornelis. 2000. Yersinia enterocolitica can deliver Yop proteins into a wide range of cell types: development of a delivery system for heterologous proteins. Eur. J. Cell Biol. 79:659-671. [DOI] [PubMed] [Google Scholar]
- 15.Burridge, K., C. Turner, and L. Romer. 1992. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J. Cell Biol. 119:893-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caron, E., and A. Hall. 1998. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282:1717-1721. [DOI] [PubMed] [Google Scholar]
- 17.China, B., B. T. N'Guyen, M. de Bruyere, and G. R. Cornelis. 1994. Role of YadA in resistance of Yersinia enterocolitica to phagocytosis by human polymorphonuclear leukocytes. Infect. Immun. 62:1275-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.China, B., M. P. Sory, B. T. N'Guyen, M. De Bruyere, and G. R. Cornelis. 1993. Role of the YadA protein in prevention of opsonization of Yersinia enterocolitica by C3b molecules. Infect. Immun. 61:3129-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornelis, G., J. Vanootegem, and C. Sluiters. 1987. Transcription of the yop regulon from Y. enterocolitica requires trans acting pYV and chromosomal genes. Microb. Pathog. 2:367-379. [DOI] [PubMed] [Google Scholar]
- 20.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M. P. Sory, and I. Stainier. 1998. The virulence plasmid of yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denecker, G., W. Declercq, C. Geuijen, A. Boland, R. Benabdillah, M. van Gurp, M. Sory, P. Vandenabeele, and G. Cornelis. 2001. Yersinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of bid. J. Biol. Chem. 276:19706-19714. [DOI] [PubMed] [Google Scholar]
- 22.Dersch, P., and R. R. Isberg. 1999. A region of the Yersinia pseudotuberculosis invasin protein enhances integrin-mediated uptake into mammalian cells and promotes self-association. EMBO J. 18:1199-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dukuzumuremyi, J., R. Rosqvist, B. Hallberg, B. Akerstrom, H. Wolf-Watz, and K. Schesser. 2000. The yersinia protein kinase A is a host factor inducible RhoA/Rac-binding virulence factor. J. Biol. Chem. 275:35281-35290. [DOI] [PubMed] [Google Scholar]
- 24.Evdokimov, A., J. Tropea, K. Routzahn, and T. Copeland. 2001. Structure of the N-terminal domain of Yersinia pestis YopH at 2.0 Å resolution. Acta Crystallogr. D Biol. Crystallogr. 57:793-799. [DOI] [PubMed] [Google Scholar]
- 25.Fallman, M., K. Andersson, S. Hakansson, K. Magnusson, O. Stendahl, and H. Wolf-Watz. 1995. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect. Immun. 63:3117-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hackam, D., O. Rotstein, A. Schreiber, W. Zhang, and S. Grinstein. 1997. Rho is required for the initiation of calcium signaling and phagocytosis by Fcγ receptors in macrophages. J. Exp. Med. 186:955-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hakansson, S., E. Galyov, R. Rosqvist, and H. Wolf-Watz. 1996. The yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol. Microbiol. 20:593-603. [DOI] [PubMed] [Google Scholar]
- 28.Hamid, N., A. Gustavsson, K. Andersson, K. McGee, C. Persson, C. Rudd, and M. Fallman. 1999. YopH dephosphorylates Cas and Fyn-binding protein in macrophages. Microb. Pathog. 27:231-242. [DOI] [PubMed] [Google Scholar]
- 29.Heesemann, J., and L. Gruter. 1987. Genetic evidence that the outer membrane protein YOP1 of Yersinia enterocolitica mediates adherence and phagocytosis resistance to human epithelial cells. FEMS Microbiol. Lett. 40:37-41. [Google Scholar]
- 30.Heesemann, J., and R. Laufs. 1985. Double immunofluorescence microscopic technique for accurate differentiation of extracellularly and intracellularly located bacteria in cell culture. J. Clin. Microbiol. 22:168-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iriarte, M., and G. R. Cornelis. 1998. YopT, a new yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol. Microbiol. 29:915-929. [DOI] [PubMed] [Google Scholar]
- 32.Isberg, R., and J. M. Leong. 1990. Multiple β1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell 60:861-871. [DOI] [PubMed] [Google Scholar]
- 33.Isberg, R., and G. Tran Van Nhieu. 1994. Binding and internalization of microorganisms by integrin receptors. Trends Microbiol. 2:10-14. [DOI] [PubMed] [Google Scholar]
- 34.Juris, S., A. Rudolph, D. Huddler, K. Orth, and J. Dixon. 2000. A distinctive role for the yersinia protein kinase: actin binding, kinase activation, and cytoskeleton disruption. Proc. Natl. Acad. Sci. USA 97:9431-9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaniga, K., I. Delor, and G. R. Cornelis. 1991. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109:137-141. [DOI] [PubMed] [Google Scholar]
- 36.Kapperud, G., E. Namork, M. Skurnik, and T. Nesbakken. 1987. Plasmid-mediated surface fibrillae of Yersinia pseudotuberculosis and Yersinia enterocolitica: relationship to the outer membrane protein YOP1 and possible importance for pathogenesis. Infect. Immun. 55:2247-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Cabec, V., C. Cols, and I. Maridonneau-Parini. 2000. Nonopsonic phagocytosis of zymosan and Mycobacterium kansasii by CR3 (CD11b/CD18) involves distinct molecular determinants and is or is not coupled with NADPH oxidase activation. Infect. Immun. 68:4736-4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leung, K. Y., and S. C. Straley. 1989. The yopM gene of Yersinia pestis encodes a released protein having homology with the human platelet surface protein GPIbα. J. Bacteriol. 171:4623-4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lian, C., W. Hwang, and C. Pai. 1987. Plasmid-mediated resistance to phagocytosis in Yersinia enterocolitica. Infect. Immun. 55:1176-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lian, C., and C. Pai. 1985. Inhibition of human neutrophil chemiluminescence by plasmid-mediated outer membrane proteins of Yersinia enterocolitica. Infect. Immun. 49:145-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGee, K., M. Zettl, M. Way, and M. Fallman. 2001. A role for N-WASP in invasin-promoted internalisation. FEBS Lett. 509:59-65. [DOI] [PubMed] [Google Scholar]
- 42.Michiels, T., J. C. Vanooteghem, C. Lambert de Rouvroit, B. China, A. Gustin, P. Boudry, and G. R. Cornelis. 1991. Analysis of virC, an operon involved in the secretion of Yop proteins by Yersinia enterocolitica. J. Bacteriol. 173:4994-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michiels, T., P. Wattiau, R. Brasseur, J. Ruysschaert, and G. Cornelis. 1990. Secretion of Yop proteins by yersiniae. Infect. Immun. 58:2840-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mills, S. D., A. Boland, M. P. Sory, P. van der Smissen, C. Kerbourch, B. B. Finlay, and G. R. Cornelis. 1997. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc. Natl. Acad. Sci. USA 94:12638-12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.N'Diaye, E., X. Darzacq, C. Astarie-Dequeker, M. Daffe, J. Calafat, and I. Maridonneau-Parini. 1998. Fusion of azurophil granules with phagosomes and activation of the tyrosine kinase Hck are specifically inhibited during phagocytosis of mycobacteria by human neutrophils. J. Immunol. 161:4983-4991. [PubMed] [Google Scholar]
- 46.Neyt, C., M. Iriarte, V. H. Thi, and G. R. Cornelis. 1997. Virulence and arsenic resistance in yersiniae. J. Bacteriol. 179:612-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orth, K., L. Palmer, Z. Bao, S. Stewart, A. Rudolph, J. Bliska, and J. Dixon. 1999. Inhibition of the mitogen-activated protein kinase kinase superfamily by a yersinia effector. Science 285:1920-1923. [DOI] [PubMed] [Google Scholar]
- 48.Pepe, J., and V. Miller. 1993. Yersinia enterocolitica invasin: a primary role in the initiation of infection. Proc. Natl. Acad. Sci. USA 90:6473-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Persson, C., N. Carballeira, H. Wolf-Watz, and M. Fallman. 1997. The PTPase YopH inhibits uptake of yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 16:2307-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Persson, C., R. Nordfelth, K. Andersson, A. Forsberg, H. Wolf-Watz, and M. Fallman. 1999. Localization of the yersinia PTPase to focal complexes is an important virulence mechanism. Mol. Microbiol. 33:828-838. [DOI] [PubMed] [Google Scholar]
- 51.Pilz, D., T. Vocke, J. Heesemann, and V. Brade. 1992. Mechanism of YadA-mediated serum resistance of Yersinia enterocolitica serotype O3. Infect. Immun. 60:189-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Portnoy, D., S. Moseley, and S. Falkow. 1981. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect. Immun. 31:775-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reisner, B., and S. Straley. 1992. Yersinia pestis YopM: thrombin binding and overexpression. Infect. Immun. 60:5242-5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roggenkamp, A., K. Ruckdeschel, L. Leitritz, R. Schmitt, and J. Heesemann. 1996. Deletion of amino acids 29 to 81 in adhesion protein YadA of Yersinia enterocolitica serotype O:8 results in selective abrogation of adherence to neutrophils. Infect. Immun. 64:2506-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosqvist, R., I. Bolin, and H. Wolf-Watz. 1988. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect. Immun. 56:2139-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosqvist, R., A. Forsberg, M. Rimpilainen, T. Bergman, and H. Wolf-Watz. 1990. The cytotoxic protein YopE of yersinia obstructs the primary host defence. Mol. Microbiol. 4:657-667. [DOI] [PubMed] [Google Scholar]
- 57.Rosqvist, R., Å. Forsberg, and H. Wolf-Watz. 1991. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect. Immun. 59:4562-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruckdeschel, K., S. Harb, A. Roggenkamp, M. Hornef, R. Zumbihl, S. Kohler, J. Heesemann, and B. Rouot. 1998. Yersinia enterocolitica impairs activation of transcription factor NF-κB: involvement in the induction of programmed cell death and in the suppression of the macrophage tumor necrosis factor alpha production. J. Exp. Med. 187:1069-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruckdeschel, K., A. Roggenkamp, S. Schubert, and J. Heesemann. 1996. Differential contribution of Yersinia enterocolitica virulence factors to evasion of microbicidal action of neutrophils. Infect. Immun. 64:724-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarker, M. R., C. Neyt, I. Stainier, and G. R. Cornelis. 1998. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J. Bacteriol. 180:1207-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schesser, K., A. Spiik, J. Dukuzumuremyi, M. Neurath, S. Pettersson, and H. Wolf-Watz. 1998. The yopJ locus is required for yersinia-mediated inhibition of NF-κB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol. Microbiol. 28:1067-1079. [DOI] [PubMed] [Google Scholar]
- 62.Schulte, R., G. Grassl, S. Preger, S. Fessele, C. Jacobi, M. Schaller, P. Nelson, and I. Autenrieth. 2000. Yersinia enterocolitica invasin protein triggers IL-8 production in epithelial cells via activation of Rel p65-p65 homodimers. FASEB J. 14:1471-1484. [DOI] [PubMed] [Google Scholar]
- 63.Sory, M. P., A. Boland, I. Lambermont, and G. R. Cornelis. 1995. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach Proc. Natl. Acad. Sci. USA 92:11998-12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sory, M. P., and G. R. Cornelis. 1994. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol. Microbiol. 14:583-594. [DOI] [PubMed] [Google Scholar]
- 65.Visser, L., A. Annema, and R. van Furth. 1995. Role of Yops in inhibition of phagocytosis and killing of opsonized Yersinia enterocolitica by human granulocytes. Infect. Immun. 63:2570-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Visser, L., P. Hiemstra, M. van den Barselaar, P. Ballieux, and R. van Furth. 1996. Role of YadA in resistance to killing of Yersinia enterocolitica by antimicrobial polypeptides of human granulocytes. Infect. Immun. 64:1653-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Von Pawel-Rammingen, U., M. Telepnev, G. Schmidt, K. Aktories, H. Wolf-Watz, and R. Rosqvist. 2000. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol. Microbiol. 36:737-748. [DOI] [PubMed] [Google Scholar]
- 68.Wiedemann, A., S. Linder, G. Grassl, M. Albert, I. Autenrieth, and A. M. 2001. Yersinia enterocolitica invasin triggers phagocytosis via beta1 integrins, CDC42Hs and WASp in macrophages. Cell Microbiol. 3:693-702. [DOI] [PubMed] [Google Scholar]
- 69.Zumbihl, R., M. Aepfelbacher, A. Andor, C. Jacobi, K. Ruckdeschel, B. Rouot, and J. Heesemann. 1999. The cytotoxin YopT of Yersinia enterocolitica induces modification and cellular redistribution of the small GTP-binding protein RhoA. J. Biol. Chem. 274:29289-29293. [DOI] [PubMed] [Google Scholar]