Abstract
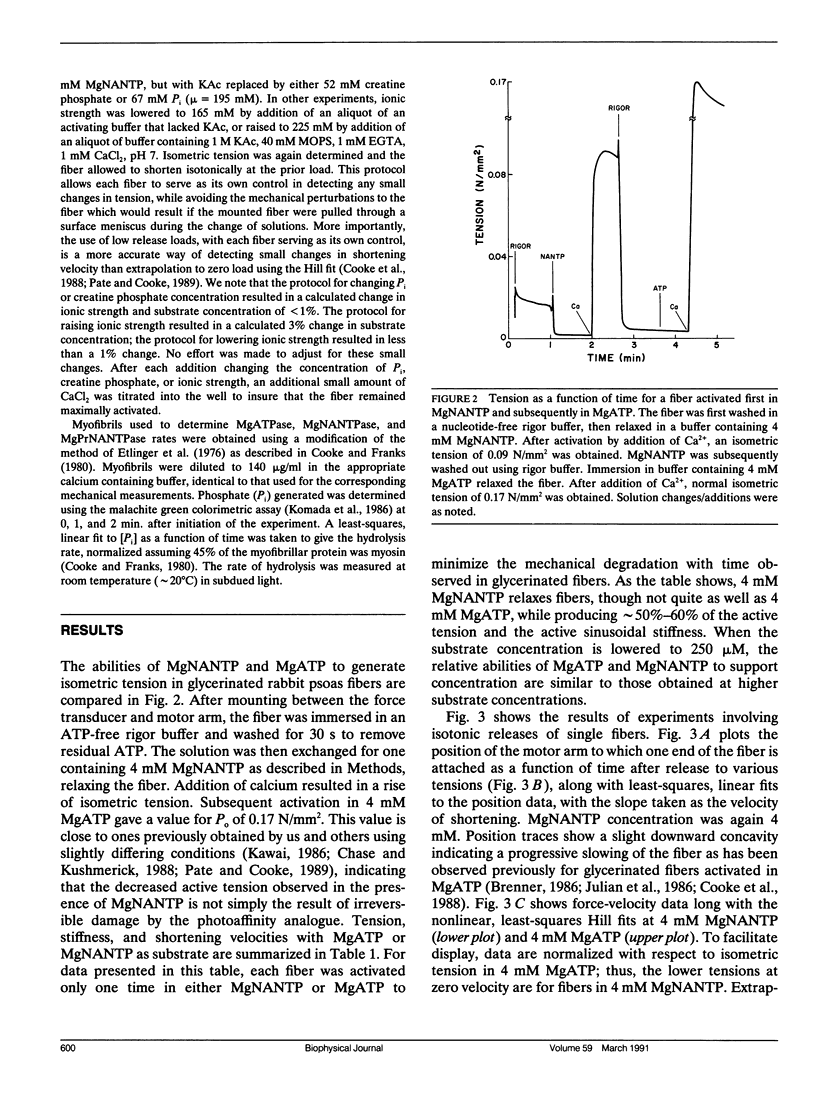
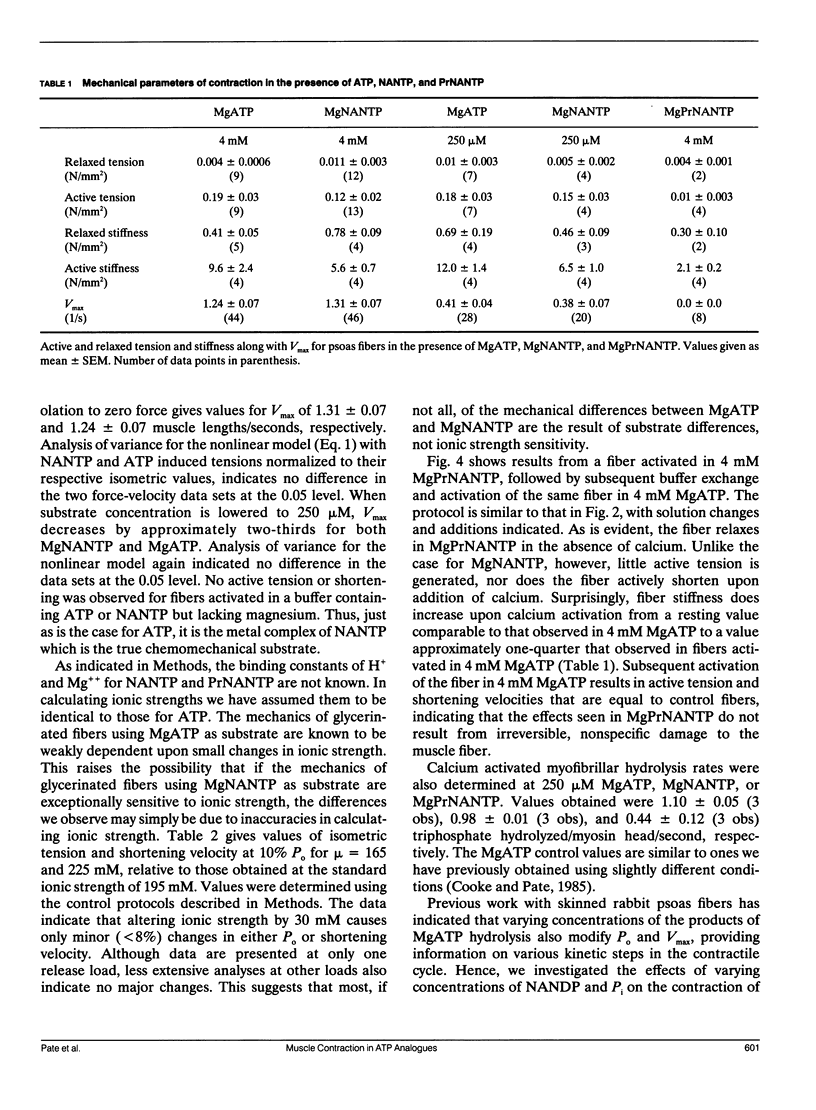
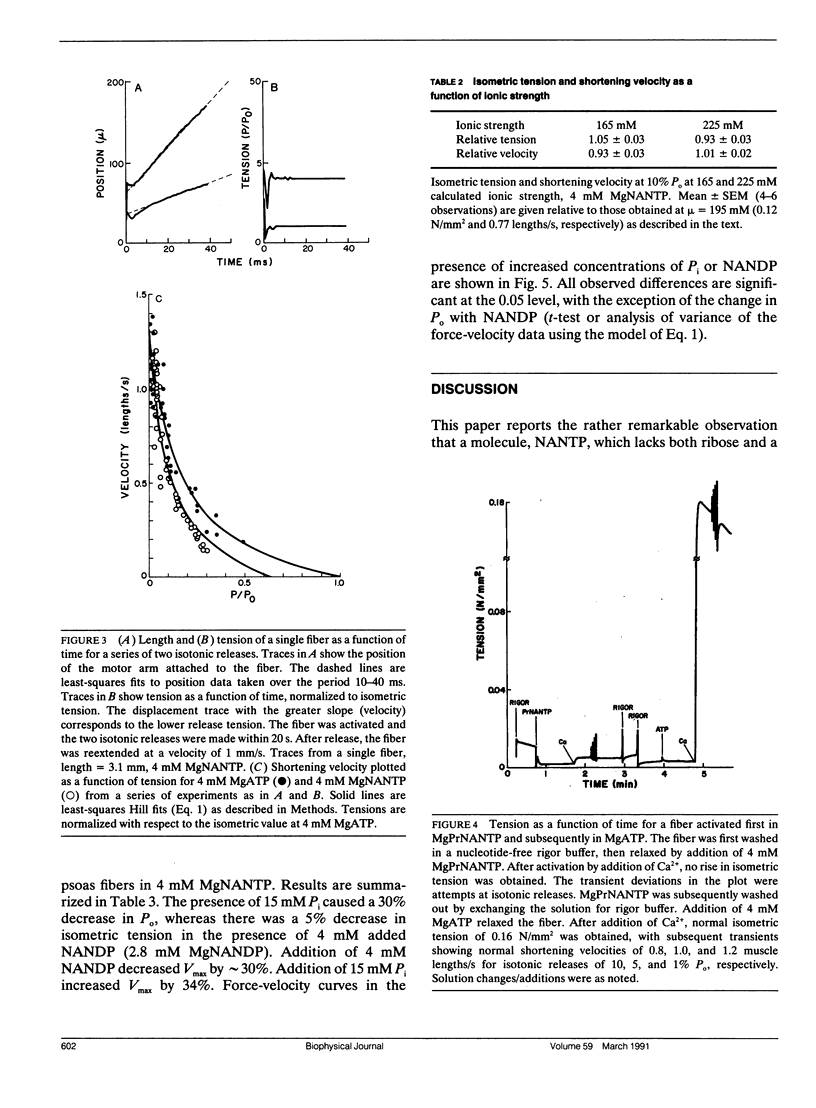
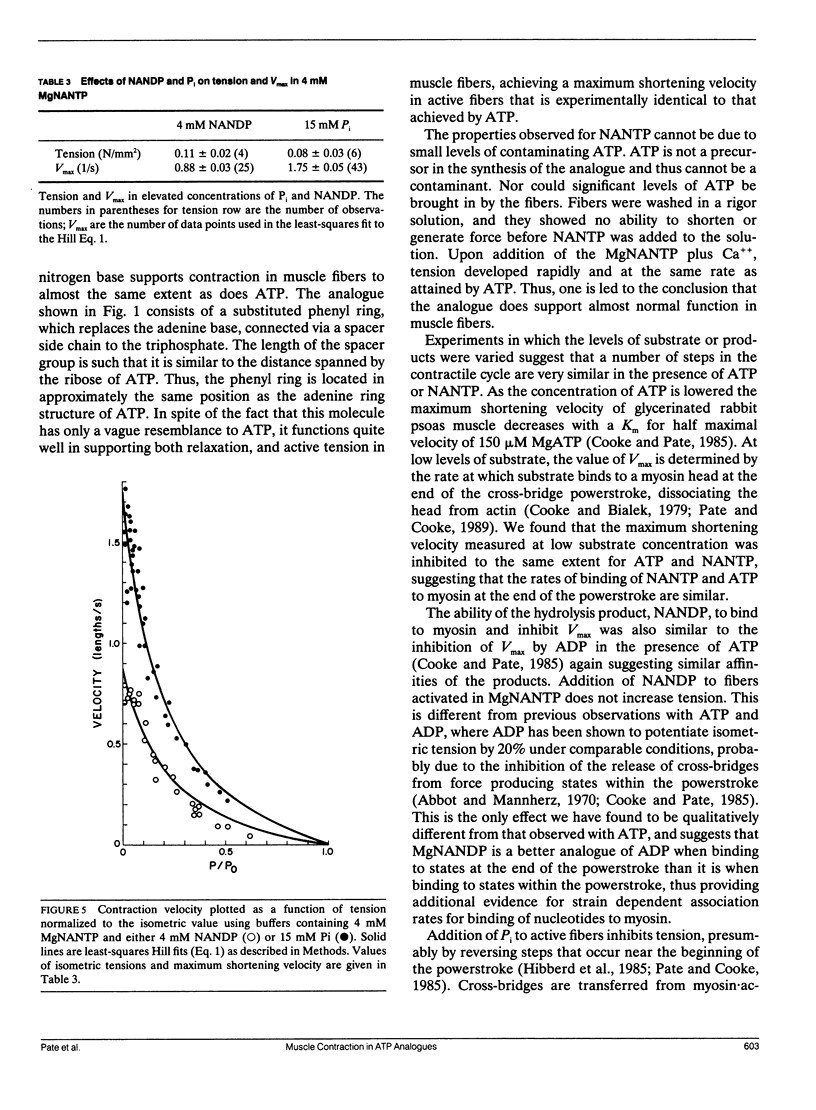
We have investigated the ability of the photoaffinity, nonnucleotide ATP analogues, 2-[(4-azido-2-nitrophenyl) amino] ethyl triphosphate (NANTP) and 2-[(4-azido-2-nitrophenyl) amino] propyl triphosphate (PrNANTP), to support active contraction in glycerinated rabbit psoas fibers. At millimolar concentrations, in the absence of calcium, both analogues relaxed fibers. In the presence of calcium, MgNANTP produced isometric tension and stiffness that were one-half to two-thirds the values obtained in MgATP. Maximum shortening velocity and the calcium-activated, myofibrillar catalyzed rate of hydrolysis were approximately the same for MgNANTP as for MgATP. With MgNANTP as the substrate, increasing concentrations of the diphosphate analogue, MgNANDP, inhibited shortening velocity but did not change isometric tension. The addition of increased concentrations of orthophosphate (P) decreased tension while shortening velocity increased. Thus, the effects of the hydrolysis products of NANTP were quite similar to those observed previously for ADP and P in the presence of MgATP. Taken together, these observations show that MgNANTP binds to, and functions in the active site of myosin in a manner quite analogous to MgATP. Thus, the aryl azido group should serve as a valid photoaffinity label for the purine portion of the active site. In contrast, MgPrNANTP, which differs from MgNANTP only in an extra CH2 spacer between the nitrophenyl ring and the triphosphate moiety did not support isometric tension or active shortening in the presence of calcium. Fiber stiffness increased in the presence of calcium and MgPrNANTP, with a calcium-activated, myofibrillar MgPrNANTPase which was about half that obtained with MgATP. Thus, in the presence of MgPrNANTP, cross-bridges appeared to be cycling through states that were attached to actin, but not producing force.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott R. H., Mannherz G. H. Activation by ADP and the correlation between tension and ATPase activity in insect fibrillar muscle. Pflugers Arch. 1970;321(3):223–232. doi: 10.1007/BF00588443. [DOI] [PubMed] [Google Scholar]
- Brenner B. The necessity of using two parameters to describe isotonic shortening velocity of muscle tissues: the effect of various interventions upon initial shortening velocity (vi) and curvature (b). Basic Res Cardiol. 1986 Jan-Feb;81(1):54–69. doi: 10.1007/BF01907427. [DOI] [PubMed] [Google Scholar]
- Brozovich F. V., Yates L. D., Gordon A. M. Muscle force and stiffness during activation and relaxation. Implications for the actomyosin ATPase. J Gen Physiol. 1988 Mar;91(3):399–420. doi: 10.1085/jgp.91.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase P. B., Kushmerick M. J. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys J. 1988 Jun;53(6):935–946. doi: 10.1016/S0006-3495(88)83174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Bialek W. Contraction of glycerinated muscle fibers as a function of the ATP concentration. Biophys J. 1979 Nov;28(2):241–258. doi: 10.1016/S0006-3495(79)85174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Franks K. All myosin heads form bonds with actin in rigor rabbit skeletal muscle. Biochemistry. 1980 May 13;19(10):2265–2269. doi: 10.1021/bi00551a042. [DOI] [PubMed] [Google Scholar]
- Cooke R., Franks K., Luciani G. B., Pate E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J Physiol. 1988 Jan;395:77–97. doi: 10.1113/jphysiol.1988.sp016909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985 Nov;48(5):789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. The mechanism of muscle contraction. CRC Crit Rev Biochem. 1986;21(1):53–118. doi: 10.3109/10409238609113609. [DOI] [PubMed] [Google Scholar]
- Etlinger J. D., Zak R., Fischman D. A. Compositional studies of myofibrils from rabbit striated muscle. J Cell Biol. 1976 Jan;68(1):123–141. doi: 10.1083/jcb.68.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig J. W., Peterson J. W., Rüegg J. C., Solaro R. J. Vanadate and phosphate ions reduce tension and increase cross-bridge kinetics in chemically skinned heart muscle. Biochim Biophys Acta. 1981 Jan 21;672(2):191–196. doi: 10.1016/0304-4165(81)90392-5. [DOI] [PubMed] [Google Scholar]
- Hibberd M. G., Dantzig J. A., Trentham D. R., Goldman Y. E. Phosphate release and force generation in skeletal muscle fibers. Science. 1985 Jun 14;228(4705):1317–1319. doi: 10.1126/science.3159090. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Rome L. C., Stephenson D. G., Striz S. The maximum speed of shortening in living and skinned frog muscle fibres. J Physiol. 1986 Jan;370:181–199. doi: 10.1113/jphysiol.1986.sp015929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M. The role of orthophosphate in crossbridge kinetics in chemically skinned rabbit psoas fibres as detected with sinusoidal and step length alterations. J Muscle Res Cell Motil. 1986 Oct;7(5):421–434. doi: 10.1007/BF01753585. [DOI] [PubMed] [Google Scholar]
- Kentish J. C. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J Physiol. 1986 Jan;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T., Fukui K., Kometani K. The initial phosphate burst in ATP hydrolysis by myosin and subfragment-1 as studied by a modified malachite green method for determination of inorganic phosphate. J Biochem. 1986 May;99(5):1465–1472. doi: 10.1093/oxfordjournals.jbchem.a135616. [DOI] [PubMed] [Google Scholar]
- Nakamaye K. L., Wells J. A., Bridenbaugh R. L., Okamoto Y., Yount R. G. 2-[(4-Azido-2-nitrophenyl)amino]ethyl triphosphate, a novel chromophoric and photoaffinity analogue of ATP. Synthesis, characterization, and interaction with myosin subfragment 1. Biochemistry. 1985 Sep 10;24(19):5226–5235. doi: 10.1021/bi00340a041. [DOI] [PubMed] [Google Scholar]
- Nosek T. M., Fender K. Y., Godt R. E. It is diprotonated inorganic phosphate that depresses force in skinned skeletal muscle fibers. Science. 1987 Apr 10;236(4798):191–193. doi: 10.1126/science.3563496. [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Sekine T., Grammer J., Yount R. G. The essential light chains constitute part of the active site of smooth muscle myosin. Nature. 1986 Nov 6;324(6092):78–80. doi: 10.1038/324078a0. [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Yount R. G. Identification of an active site peptide of skeletal myosin after photoaffinity labeling with N-(4-azido-2-nitrophenyl)-2-aminoethyl diphosphate. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1575–1579. doi: 10.1073/pnas.82.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate E., Cooke R. Addition of phosphate to active muscle fibers probes actomyosin states within the powerstroke. Pflugers Arch. 1989 May;414(1):73–81. doi: 10.1007/BF00585629. [DOI] [PubMed] [Google Scholar]
- Podolsky R. J., Teichholz L. E. The relation between calcium and contraction kinetics in skinned muscle fibres. J Physiol. 1970 Nov;211(1):19–35. doi: 10.1113/jphysiol.1970.sp009263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb M. R., Hibberd M. G., Goldman Y. E., Trentham D. R. Oxygen exchange between Pi in the medium and water during ATP hydrolysis mediated by skinned fibers from rabbit skeletal muscle. Evidence for Pi binding to a force-generating state. J Biol Chem. 1986 Nov 25;261(33):15557–15564. [PubMed] [Google Scholar]
- Yount R. G., Ojala D., Babcock D. Interaction of P--N--P and P--C--P analogs of adenosine triphosphate with heavy meromyosin, myosin, and actomyosin. Biochemistry. 1971 Jun 22;10(13):2490–2496. doi: 10.1021/bi00789a010. [DOI] [PubMed] [Google Scholar]


