Abstract
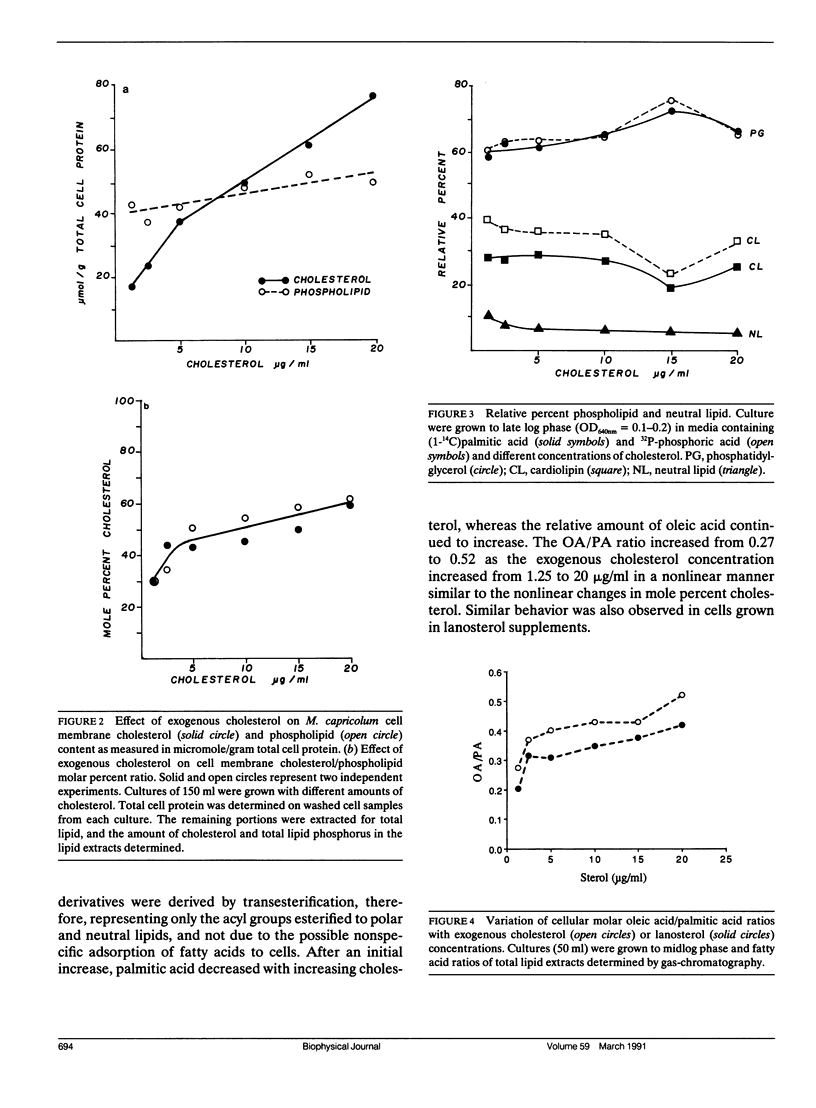
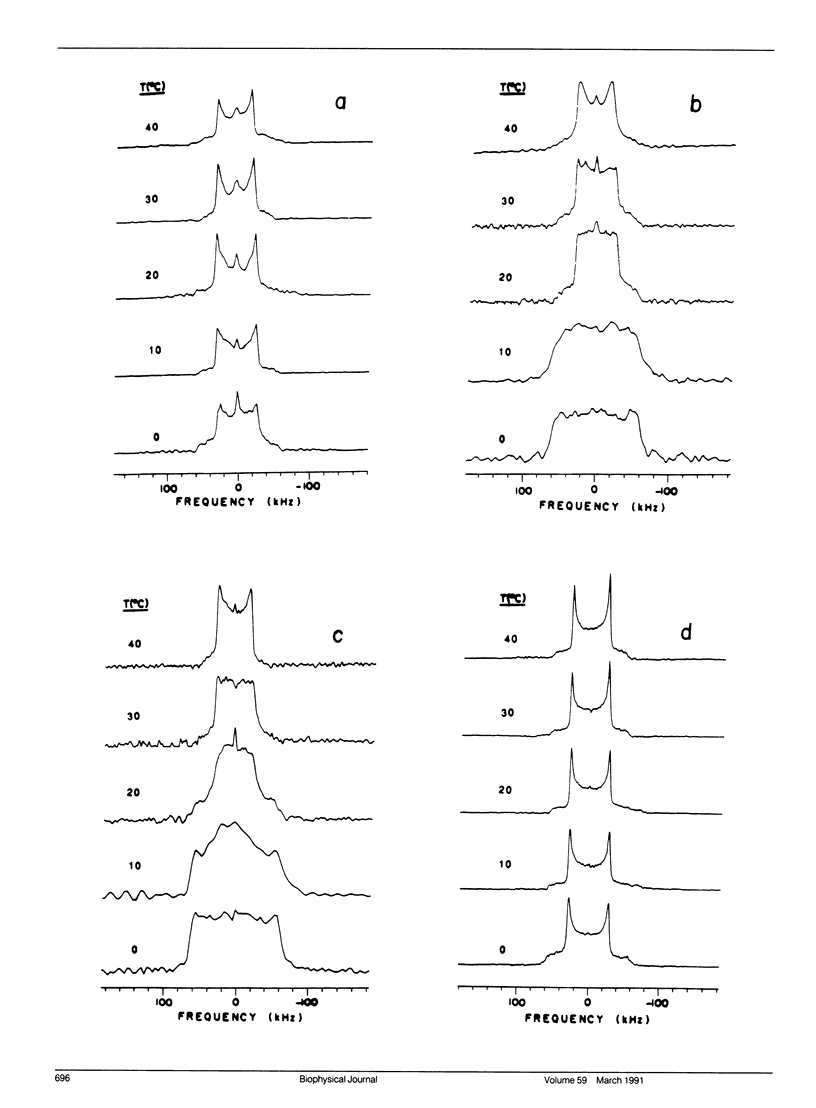
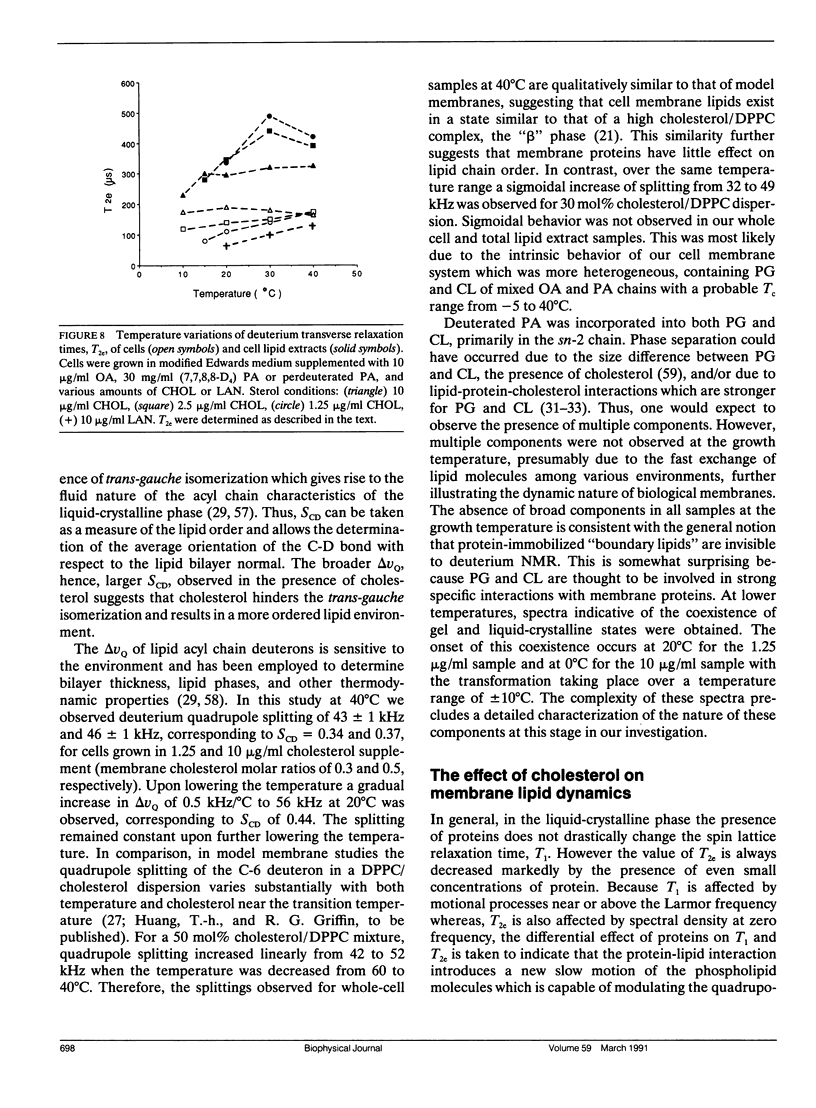
Deuterium nuclear magnetic resonance (NMR) techniques were employed to study the effect of sterols on the composition and dynamics of the membrane lipids of Mycoplasma capricolum, a natural fatty acid auxotroph that requires sterols for growth. The membrane lipids of cells grown in modified Edwards medium supplemented with cholesterol, oleic acid (OA), and palmitic acid (PA) were composed primarily of phosphatidylglycerol (PG) (60%) and cardiolipin (CL) (35%). The incorporation of cholesterol and the cellular OA/PA ratio increased nonlinearly with increases in exogenous cholesterol level, whereas the levels of phospholipid increased only slightly. At the growth temperature, 37 degrees C, the residual deuterium quadrupole splittings were found to be 43-46 kHz for cells grown with (7,7,8,8-2H4) PA and 1.25 micrograms/ml (30 mol%) to 10 micrograms/ml (50 mol%) cholesterol, respectively, similar to that found in the cholesterol/lecithin binary dispersions of similar cholesterol contents. Deuterium T2e of these samples were found to be 170 +/- 10 microseconds and were independent of cellular cholesterol content. In comparison, T2e of the corresponding lipid extracts were longer (320-420 microseconds) and dependent on cholesterol content. Thus, lipid-protein interactions in the cell membrane is the dominant mechanism responsible for the reduced T2e. At lower temperatures, spectra indicative of the coexistence of gel and liquid-crystalline states were observed for cells having low cholesterol levels. For both cell membrane and membrane lipid extract containing 50 mol% cholesterol, T2e was found to be constant at the temperature range from 15 to 40 degrees C. On the other hand, T2e of cell membrane containing 30 mol% cholesterol decreased linearly at 3.2 microseconds/degrees C. T2e of the corresponding lipid extract showed much stronger temperature variation. Cells containing 39 mol% lanosterol were found to have a quadrupole splitting of 39 kHz, broader than that of the cholesterol-free lecithin dispersion (less than 30 kHz) but less than that of cell membrane containing 30 mol% cholesterol (43 kHz). T2e of the lanosterol sample was found to be 130 +/- 10 microseconds which decreased linearly at a slope similar to that observed for the low cholesterol sample. Therefore, although lanosterol appeared to be capable of modulating cell membrane physical properties it is less effective than cholesterol. When growth rates were correlated with NMR parameters, we found that the membranes of faster growing cells were also more ordered. In contrast, the T2e of the cells of M. capricolum seemed to be maintained at a relatively constant value around 170 microseconds.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bloch K. E. Sterol structure and membrane function. CRC Crit Rev Biochem. 1983;14(1):47–92. doi: 10.3109/10409238309102790. [DOI] [PubMed] [Google Scholar]
- Brown M. F., Seelig J. Influence of cholesterol on the polar region of phosphatidylcholine and phosphatidylethanolamine bilayers. Biochemistry. 1978 Jan 24;17(2):381–384. doi: 10.1021/bi00595a029. [DOI] [PubMed] [Google Scholar]
- Burnell E. E., Cullis P. R., de Kruijff B. Effects of tumbling and lateral diffusion on phosphatidylcholine model membrane 31P-NMR lineshapes. Biochim Biophys Acta. 1980 Dec 2;603(1):63–69. doi: 10.1016/0005-2736(80)90391-0. [DOI] [PubMed] [Google Scholar]
- Calhoun W. I., Shipley G. G. Sphingomyelin--lecithin bilayers and their interaction with cholesterol. Biochemistry. 1979 May 1;18(9):1717–1722. doi: 10.1021/bi00576a013. [DOI] [PubMed] [Google Scholar]
- Chen H. W., Kandutsch A. A., Heiniger H. J. The role of cholesterol in malignancy. Prog Exp Tumor Res. 1978;22:275–316. doi: 10.1159/000401203. [DOI] [PubMed] [Google Scholar]
- Copeland B. R., McConnel H. M. The rippled structure in bilayer membranes of phosphatidylcholine and binary mixtures of phosphatidylcholine and cholesterol. Biochim Biophys Acta. 1980 Jun 20;599(1):95–109. doi: 10.1016/0005-2736(80)90059-0. [DOI] [PubMed] [Google Scholar]
- Dahl J. S., Dahl C. E., Bloch K. Effect of cholesterol on macromolecular synthesis and fatty acid uptake by Mycoplasma capricolum. J Biol Chem. 1981 Jan 10;256(1):87–91. [PubMed] [Google Scholar]
- Dahl J. S., Dahl C. E., Bloch K. Sterols in membranes: growth characteristics and membrane properties of Mycoplasma capricolum cultured on cholesterol and lanosterol. Biochemistry. 1980 Apr 1;19(7):1467–1472. doi: 10.1021/bi00548a032. [DOI] [PubMed] [Google Scholar]
- Dahl J. S., Dahl C. E. Coordinate regulation of unsaturated phospholipid, RNA, and protein synthesis in Mycoplasma capricolum by cholesterol. Proc Natl Acad Sci U S A. 1983 Feb;80(3):692–696. doi: 10.1073/pnas.80.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Siervo A. J. High levels of glycolipid and low levels of phospholipid in a marine caulobacter. J Bacteriol. 1985 Nov;164(2):684–688. doi: 10.1128/jb.164.2.684-688.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Siervo A. J., Homola A. D. Analysis of caulobacter crescentus lipids. J Bacteriol. 1980 Sep;143(3):1215–1222. doi: 10.1128/jb.143.3.1215-1222.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux P. F., Seigneuret M. Specificity of lipid-protein interactions as determined by spectroscopic techniques. Biochim Biophys Acta. 1985 Jun 12;822(1):63–125. doi: 10.1016/0304-4157(85)90004-8. [DOI] [PubMed] [Google Scholar]
- Estep T. N., Freire E., Anthony F., Barenholz Y., Biltonen R. L., Thompson T. E. Thermal behavior of stearoylsphingomyelin-cholesterol dispersions. Biochemistry. 1981 Dec 8;20(25):7115–7118. doi: 10.1021/bi00528a010. [DOI] [PubMed] [Google Scholar]
- Gallay J., Vincent M. Cardiolipin-cholesterol interactions in the liquid-crystalline phase: a steady-state and time-resolved fluorescence anisotropy study with cis- and trans-parinaric acids as probes. Biochemistry. 1986 May 6;25(9):2650–2656. doi: 10.1021/bi00357a054. [DOI] [PubMed] [Google Scholar]
- Gally H. U., Seelig A., Seelig J. Cholesterol-induced rod-like motion of fatty acyl chains in lipid bilayers a deuterium magnetic resonance study. Hoppe Seylers Z Physiol Chem. 1976 Dec;357(10):1447–1450. [PubMed] [Google Scholar]
- Griffin R. G. Solid state nuclear magnetic resonance of lipid bilayers. Methods Enzymol. 1981;72:108–174. doi: 10.1016/s0076-6879(81)72010-x. [DOI] [PubMed] [Google Scholar]
- Gross Z., Rottem S., Bittman R. Phospholipid interconversions in Mycoplasma capricolum. Eur J Biochem. 1982 Feb;122(1):169–174. doi: 10.1111/j.1432-1033.1982.tb05863.x. [DOI] [PubMed] [Google Scholar]
- Haberkorn R. A., Griffin R. G., Meadows M. D., Oldfield E. Deuterium nuclear magnetic resonance investigation of the dipalmitoyl lecithin-cholesterol-water system. J Am Chem Soc. 1977 Oct 26;99(22):7353–7355. doi: 10.1021/ja00464a043. [DOI] [PubMed] [Google Scholar]
- Hui S. W., He N. B. Molecular organization in cholesterol-lecithin bilayers by X-ray and electron diffraction measurements. Biochemistry. 1983 Mar 1;22(5):1159–1164. doi: 10.1021/bi00274a026. [DOI] [PubMed] [Google Scholar]
- Jacobs R., Oldfield E. Deuterium nuclear magnetic resonance investigation of dimyristoyllecithin--dipalmitoyllecithin and dimyristoyllecithin--cholesterol mixtures. Biochemistry. 1979 Jul 24;18(15):3280–3285. doi: 10.1021/bi00582a013. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ladbrooke B. D., Williams R. M., Chapman D. Studies on lecithin-cholesterol-water interactions by differential scanning calorimetry and X-ray diffraction. Biochim Biophys Acta. 1968 Apr 29;150(3):333–340. doi: 10.1016/0005-2736(68)90132-6. [DOI] [PubMed] [Google Scholar]
- Lajeunesse D., Le Grimellec C. Phosphate distribution and transport in mycoplasma. Can J Biochem Cell Biol. 1984 Nov;62(11):1041–1045. doi: 10.1139/o84-133. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Barrow D. A., Hoechli M. Cholesterol-phosphatidylcholine interactions in multilamellar vesicles. Biochemistry. 1980 Apr 29;19(9):1943–1954. doi: 10.1021/bi00550a034. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Mateo P. L., Sturtevant J. M. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1978 Jun 13;17(12):2464–2468. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J. The effect of cholesterol on the structure of phosphatidylcholine bilayers. Biochim Biophys Acta. 1978 Oct 19;513(1):43–58. doi: 10.1016/0005-2736(78)90110-4. [DOI] [PubMed] [Google Scholar]
- Meier P., Sachse J. H., Brophy P. J., Marsh D., Kothe G. Integral membrane proteins significantly decrease the molecular motion in lipid bilayers: a deuteron NMR relaxation study of membranes containing myelin proteolipid apoprotein. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3704–3708. doi: 10.1073/pnas.84.11.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior D. L., Rottem S. The organization of cholesterol esters in membranes of Mycoplasma capricolum. Eur J Biochem. 1981 Jun;117(1):147–153. doi: 10.1111/j.1432-1033.1981.tb06313.x. [DOI] [PubMed] [Google Scholar]
- Mortensen K., Pfeiffer W., Sackmann E., Knoll W. Structural properties of a phosphatidylcholine-cholesterol system as studied by small-angle neutron scattering: ripple structure and phase diagram. Biochim Biophys Acta. 1988 Nov 22;945(2):221–245. doi: 10.1016/0005-2736(88)90485-3. [DOI] [PubMed] [Google Scholar]
- Mouritsen O. G., Bloom M. Mattress model of lipid-protein interactions in membranes. Biophys J. 1984 Aug;46(2):141–153. doi: 10.1016/S0006-3495(84)84007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddy M. R., Dahlquist F. W., Davis J. H., Bloom M. Dynamical and temperature-dependent effects of lipid-protein interactions. Application of deuterium nuclear magnetic resonance and electron paramagnetic resonance spectroscopy to the same reconstitutions of cytochrome c oxidase. Biochemistry. 1981 May 26;20(11):3152–3162. doi: 10.1021/bi00514a026. [DOI] [PubMed] [Google Scholar]
- Rance M., Jeffrey K. R., Tulloch A. P., Butler K. W., Smith I. C. Effects of cholesterol on the orientational order of unsaturated lipids in the membranes of acholeplasma laidlawii. A 2H-NMR study. Biochim Biophys Acta. 1982 May 21;688(1):191–200. doi: 10.1016/0005-2736(82)90594-6. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Parsegian V. A., Henry J. A., Lis L. J., McAlister M. The effect of cholesterol on measured interaction and compressibility of dipalmitoylphosphatidylcholine bilayers. Can J Biochem. 1980 Oct;58(10):959–968. doi: 10.1139/o80-131. [DOI] [PubMed] [Google Scholar]
- Recktenwald D. J., McConnell H. M. Phase equilibria in binary mixtures of phosphatidylcholine and cholesterol. Biochemistry. 1981 Jul 21;20(15):4505–4510. doi: 10.1021/bi00518a042. [DOI] [PubMed] [Google Scholar]
- Rigaud J. L., Leblanc G. Effect of membrane cholesterol on action of phospholipase A2 in Mycoplasma mycoides var. Capri. Evidence for lysophospholipase activity. Eur J Biochem. 1980 Sep;110(1):77–84. doi: 10.1111/j.1432-1033.1980.tb04842.x. [DOI] [PubMed] [Google Scholar]
- Rottem S. Membrane lipids of mycoplasmas. Biochim Biophys Acta. 1980 May 27;604(1):65–90. doi: 10.1016/0005-2736(80)90585-4. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Smith B. A., McConnell H. M. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):15–18. doi: 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel L. L., Morris M. D. Determination of cholesterol using o-phthalaldehyde. J Lipid Res. 1973 May;14(3):364–366. [PubMed] [Google Scholar]
- Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Q Rev Biophys. 1977 Aug;10(3):353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- Shah D. O., Schulman J. H. Influence of calcium, cholesterol, and unsaturation on lecithin monolayers. J Lipid Res. 1967 May;8(3):215–226. [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separations in binary mixtures of cholesterol and phospholipids. Biochem Biophys Res Commun. 1973 Jul 17;53(2):446–451. doi: 10.1016/0006-291x(73)90682-7. [DOI] [PubMed] [Google Scholar]
- Unwin N., Henderson R. The structure of proteins in biological membranes. Sci Am. 1984 Feb;250(2):78–94. doi: 10.1038/scientificamerican0284-78. [DOI] [PubMed] [Google Scholar]
- Vist M. R., Davis J. H. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 1990 Jan 16;29(2):451–464. doi: 10.1021/bi00454a021. [DOI] [PubMed] [Google Scholar]
- Wittebort R. J., Blume A., Huang T. H., Das Gupta S. K., Griffin R. G. Carbon-13 nuclear magnetic resonance investigations of phase transitions and phase equilibria in pure and mixed phospholipid bilayers. Biochemistry. 1982 Jul 6;21(14):3487–3502. doi: 10.1021/bi00257a036. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L. Cholesterol and the cell membrane. Biochim Biophys Acta. 1985 Dec 9;822(3-4):267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L. Lanosterol and cholesterol have different effects on phospholipid acyl chain ordering. Biochim Biophys Acta. 1985 Apr 26;815(1):33–36. doi: 10.1016/0005-2736(85)90470-5. [DOI] [PubMed] [Google Scholar]


