Abstract
Practically and ethically attractive as model systems, invertebrate organisms are increasingly recognized as relevant for the study of bacterial pathogenesis. We show here that the nematode Caenorhabditis elegans is susceptible to a surprisingly broad range of bacteria and may constitute a useful model for the study of both pathogens and symbionts.
There is a continuing need for the development of simple animal models for the study of host-pathogen interactions (8). As well as facilitating the identification and study of virulence mechanisms (13), simple model systems may also permit direct genetic approaches for the study of host defenses (7, 16). The many experimental advantages associated with the use of the nematode Caenorhabditis elegans (6, 11) led us to investigate its interaction with a number of different bacteria.
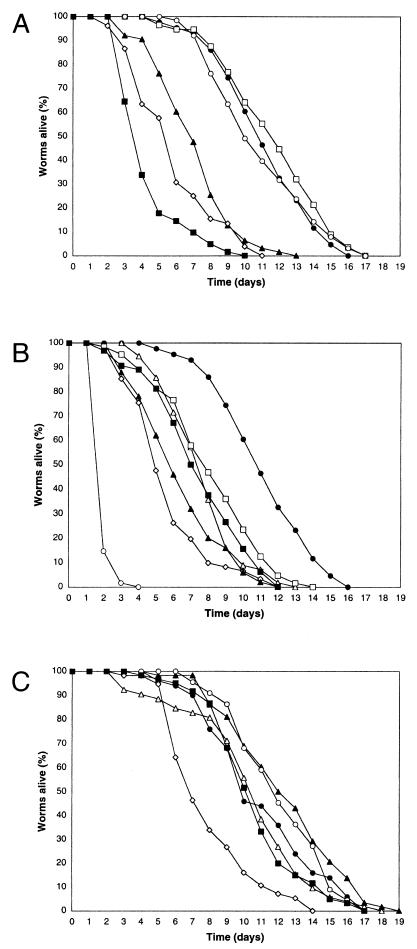
Under standard laboratory conditions, C. elegans is grown on solid nematode growth medium (NGM) in the presence of the Escherichia coli strain OP50 (3) and then can live for up to two and a half weeks at 25°C (Fig. 1A). One can evaluate very simply whether a given bacterium is a pathogen of C. elegans or not. Worms are grown on OP50 cells until they reach the fourth larval stage (L4) and are then transferred to plates seeded with the bacterium of interest. As long as the bacterium in question is an adequate food source, the subsequent life span of the worms then gives an indication of the pathogenicity of the bacterium. The number of worms alive is counted each day as previously described (15). In practical terms, a worm is considered dead when it no longer responds to touch. Any worms that die as a result of getting stuck to the wall of the plate are excluded from the analysis. During the first few days, when the adults lay eggs, the worms are transferred to fresh plates daily, to avoid mixing the generations.
FIG. 1.
Survival of C. elegans on different bacteria. (A) Representative time courses of the survival of wild-type worms in the presence of E. coli OP50 (•), E. coli CFBP 1106 (□), B. megaterium CFBP 486 (○), A. tumefaciens CFBP 2413 (▴), E. chrysanthemi 3937C (⋄), and E. carotovora CFBP 2141 (▪). (B) Representative time courses of the survival of wild-type worms in the presence of E. coli OP50 (•; same data as above), Shewanella massilia strain B (□), S. frigidimarina MR-1 (▪), A. hydrophila AH6 (▵), A. hydrophila AH10 (▴), X. nematophila A24 (⋄), and P. luminescens Hb (○). (C) Representative time courses of the survival of wild-type worms in the presence of heat-killed bacteria: E. coli OP50 (•), A. hydrophila AH6 (▵), A. tumefaciens CFBP 2413 (▴), E. carotovora CFBP 2141 (▪), P. luminescens Hb (○), and X. nematophila A24 (⋄). All tests were repeated at least twice using a minimum of 50 worms for each test. Worms were maintained at 25°C throughout.
When grown on a phytopathogenic strain of E. coli, CFBP 1106, available from the INRA collection of phytopathogenic bacteria (http://brg.prd.fr/brg/ecran2s/rgmBd_Mine_CFBP.htm), the worms' survival was essentially identical to that of worms grown on OP50 (Fig. 1A). This confirms that the worms' survival on OP50 cells can be taken as a baseline for healthy growth on live bacteria. One unnamed strain of Bacillus megaterium that was grown on brain heart infusion (BHI) agar, a rich medium, has been described as being toxic to C. elegans, killing worms in a matter of minutes (1). (In the original report, the bacterium was described as Bacillus megatherium, but this must be assumed to be a typographic error.) We tested the B. megaterium strain CFBP 486. In contrast to the previous report, compared to worms grown on OP50 cells, no difference in survival was observed when the worms were fed bacteria grown on standard NGM agar plates (Fig. 1A) nor when the bacteria were grown on BHI agar (results not shown). For the case of infection of C. elegans by Pseudomonas aeruginosa, strain-specific differences in virulence have already been reported (17). It is interesting to note that worms grown on OP50 cells on BHI agar live significantly shorter than when cultivated on OP50 cells on NGM agar, presumably because the rich BHI medium induces the expression of E. coli factors that are lethal for worms (10). Thus, both the strain and the culture conditions can influence the survival of worms on a given bacterial species.
We tested a number of other known plant pathogens. We found that worms grown on strains of Erwinia chrysanthemi 3937c and Agrobacterium tumefaciens CFBP 2413 showed a significantly decreased survival. An even more dramatic effect was observed when worms were cultivated in the presence of Erwinia carotovora carotovora CFBP 2141 (Fig. 1A). The latter bacterium has also been shown to infect Drosophila larvae (2). These results encouraged us to test additional bacteria. A reduced survival was equally observed for worms grown on the marine gram-negative enterobacteria Shewanella frigidimarina (formerly Shewanella putrefaciens) strain MR-1 and Shewanella massilia strain B, as well as on strains AH6 and AH10 of the fish pathogen Aeromonas hydrophila (Fig. 1B). The death of worms on A. hydrophila was followed by the rapid lysis of the cadavers, presumably a consequence of the secretion of proteases by the bacteria.
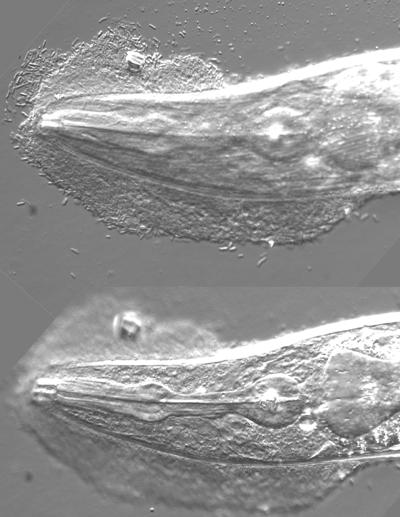
We then turned our attention to two strains of bacteria that are entomopathogenic symbionts of nematodes, Photorhabdus luminescens and Xenorhabdus nematophila. These are normally associated with nematodes of the families Heterorhabditidae and Steinermatidae, respectively (5). Both bacteria exhibited a strong negative effect on the survival of C. elegans (Fig. 1B). In the case of P. luminescens, not only were adults susceptible but early-stage larvae also succumbed. For X. nematophila, the bacteria were found to adhere strongly in clumps to the worms' cuticle, especially at the tail and the head (Fig. 2). Despite the mass of bacteria that made movement progressively difficult, the worms' feeding did not seem to be impaired nor did they appear to be starved. Indeed, it is important to note that no overt symptoms of starvation were observed for worms grown on any of the different bacteria described here.
FIG. 2.
Adhesion of X. nematophila to the head of C. elegans. Photomicrographs were taken at two different focal planes of the head of a worm that had been in contact for 2 days with X. nematophila and that had then been very thoroughly washed before being mounted for observation. In the top image, the adherent rod-like bacteria can be discerned. In the lower image, the two-lobed structure of the pharynx is clearly visible.
To exclude further the possibility that the observed reductions in life span might be due to a dietary problem, for a selected number of the bacterial species the influence of dead bacteria (killed by heating at 75°C for 1 h) on worm survival was measured. While the survival of worms grown on live and heat-killed OP50 cells was not greatly different, with the exception of X. nematophila, for all the bacteria tested, namely A. hydrophila, A. tumefaciens, E. carotovora, and P. luminescens, the worms' survival was close to that of worms grown on OP50 cells. Preliminary results suggest that growing worms on bacteria seeded on plates containing 5-fluoro-2′-deoxyuridine, which blocks DNA synthesis and prevents bacterial replication, similarly eliminates the observed reduction in survival (results not shown). These results indicate that for A. hydrophila, A. tumefaciens, E. carotovora, and P. luminescens, the observed reductions in survival require live bacteria and strongly suggests that it is independent of any dietary deficit. The reduced survival of worms on heat-killed X. nematophila suggests that the bacteria contain a product that is toxic for worms. A heat resistance factor from X. nematophila with hemolytic activity against insect cells has recently been described (4). In the future, it will be interesting to determine whether this same factor is responsible for the observed precocious death of C. elegans. Indeed, for the different bacteria, their interaction with C. elegans will clearly need to be studied in greater detail to demonstrate that each is capable of provoking an infection in the strict sense of the term.
We also tested the Brucella sp. strain 96-566 (which was isolated from Parafilaroides lungworms infesting a Pacific harbor seal [9]), Mycobacterium fortuitum ATCC 6841 (a reference strain), and M. marinum BTB 03/98 (a clinical isolate). These all supported the growth and reproduction of C. elegans and caused no obvious sickness. Practical limitations associated with the confinement of these bacteria meant that the survival of the worms could not be followed as closely as was the case for the other bacteria, and we cannot exclude the possibility that they too have a deleterious effect.
Until relatively recently, it was believed that animal and plant pathogens did not share virulence factors, the idea being that the differences in their respective hosts required distinct mechanisms of virulence. It has become clear, however, that some, if not many, of the molecular mechanisms that underlie a given bacterium's infectious potential are independent of the host (reviewed in reference 8). The existence of universal virulence factors has been clearly demonstrated in the case of P. aeruginosa. Of eight bacterial mutants isolated on the basis of a reduced pathogenicity against C. elegans, six showed an attenuated virulence in a plant model of infection, six showed an attenuated virulence in an insect model, and seven showed an attenuated virulence in a mouse model (13). The concept of universal mechanisms of interaction between bacteria and higher organisms can indeed be extended further to include symbionts, since in some cases they depend on analogous mechanisms to interact with their hosts (12, 14). Thus, the finding that diverse bacteria are pathogenic to C. elegans opens the prospect of using this experimentally simple model to identify genes that are necessary not only for pathogenesis in the nematode but also for virulence or symbiosis in other hosts.
Acknowledgments
This work was supported by institutional grants from the CNRS and INSERM and a CNRS ATIPE to J.J.E.
We thank Fréderic Barras, Frédéric Boccard, Noël Boemare, Dominique Ferrandon, Sven Hoffner, Anne Lanois, Vincent Méjean, Janet Payeur, and Gisou van der Goot for their generous gifts of bacteria.
Editor: B. B. Finlay
REFERENCES
- 1.Andrew, P. A., and W. L. Nicholas. 1976. Effect of bacteria on dispersal of Caenorhabditis elegans (Rhabditidae). Nematologica 22:451-461. [Google Scholar]
- 2.Basset, A., R. S. Khush, A. Braun, L. Gardan, F. Boccard, J. A. Hoffmann, and B. Lemaitre. 2000. The phytopathogenic bacteria Erwinia carotovora infects Drosophila and activates an immune response. Proc. Natl. Acad. Sci. USA 97:3376-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner, S. 1974. The genetics of Caenorhabditis elegans. Genetics 77:71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brillard, J., C. Ribeiro, N. Boemare, M. Brehelin, and A. Givaudan. 2001. Two distinct hemolytic activities in Xenorhabdus nematophila are active against immunocompetent insect cells. Appl. Environ. Microbiol. 67:2515-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunel, B., A. Givaudan, A. Lanois, R. J. Akhurst, and N. Boemare. 1997. Fast and accurate identification of Xenorhabdus and Photorhabdus species by restriction analysis of PCR-amplified 16S rRNA genes. Appl. Environ. Microbiol. 63:574-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chauvet, S., and J. J. Ewbank. 2000. Caenorhabditis elegans: un modèle d'étude des interactions hôte-organisme pathogène. Médecine/Sciences 16:912-916. [Google Scholar]
- 7.Ewbank, J. J. 2002. Tackling both sides of the host-pathogen equation with Caenorhabditis elegans. Microbes Infect. 4:247-256. [DOI] [PubMed] [Google Scholar]
- 8.Finlay, B. B. 1999. Bacterial disease in diverse hosts. Cell 96:315-318. [DOI] [PubMed] [Google Scholar]
- 9.Garner, M. M., D. M. Lambourn, S. J. Jeffries, P. B. Hall, J. C. Rhyan, D. R. Ewalt, L. M. Polzin, and N. F. Cheville. 1997. Evidence of Brucella infection in Parafilaroides lungworms in a Pacific harbor seal (Phoca vitulina richardsi). J. Vet. Diagn. Investig. 9:298-303. [DOI] [PubMed] [Google Scholar]
- 10.Garsin, D. A., C. D. Sifri, E. Mylonakis, X. Qin, K. V. Singh, B. E. Murray, S. B. Calderwood, and F. M. Ausubel. 2001. A simple model host for identifying Gram-positive virulence factors. Proc. Natl. Acad. Sci. USA 98:10892-10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurz, C. L., and J. J. Ewbank. 2000. Caenorhabditis elegans for the study of host-pathogen interactions. Trends Microbiol. 8:142-144. [DOI] [PubMed] [Google Scholar]
- 12.LeVier, K., R. W. Phillips, V. K. Grippe, R. M. Roop 2nd, and G. C. Walker. 2000. Similar requirements of a plant symbiont and a mammalian pathogen for prolonged intracellular survival. Science 287:2492-2493. [DOI] [PubMed] [Google Scholar]
- 13.Mahajan-Miklos, S., L. G. Rahme, and F. M. Ausubel. 2000. Elucidating the molecular mechanisms of bacterial virulence using non-mammalian hosts. Mol. Microbiol. 37:981-988. [DOI] [PubMed] [Google Scholar]
- 14.Ochman, H., and N. A. Moran. 2001. Genes lost and genes found: evolution of bacterial pathogenesis and symbiosis. Science 292:1096-1099. [DOI] [PubMed] [Google Scholar]
- 15.Pujol, N., E. M. Link, L. X. Liu, L. C. Kurz, G. Alloing, M. W. Tan, K. P. Ray, R. Solari, C. D. Johnson, and J. J. Ewbank. 2001. A reverse genetic analysis of components of the Toll signalling pathway in Caenorhabditis elegans. Curr. Biol. 11:809-821. [DOI] [PubMed] [Google Scholar]
- 16.Tan, M. W., and F. M. Ausubel. 2000. Caenorhabditis elegans: a model genetic host to study Pseudomonas aeruginosa pathogenesis. Curr. Opin. Microbiol. 3:29-34. [DOI] [PubMed] [Google Scholar]
- 17.Tan, M. W., S. Mahajan-Miklos, and F. M. Ausubel. 1999. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. USA 96:715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]