Abstract
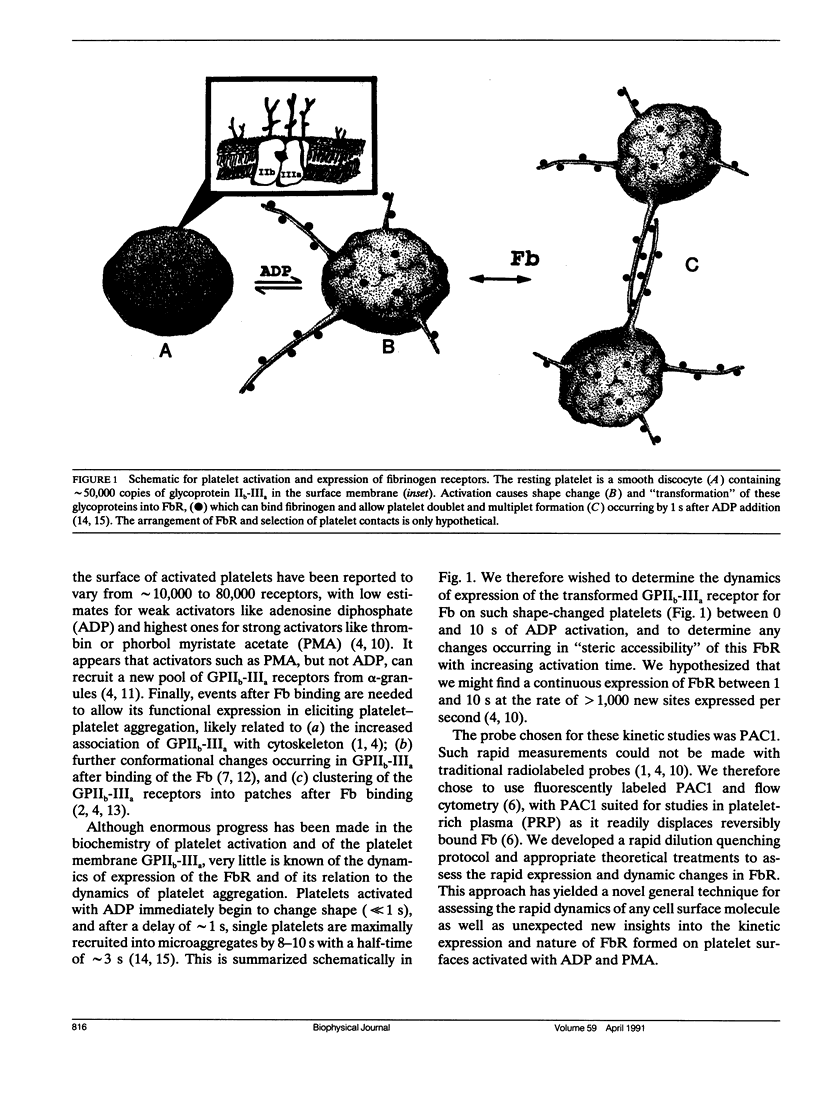
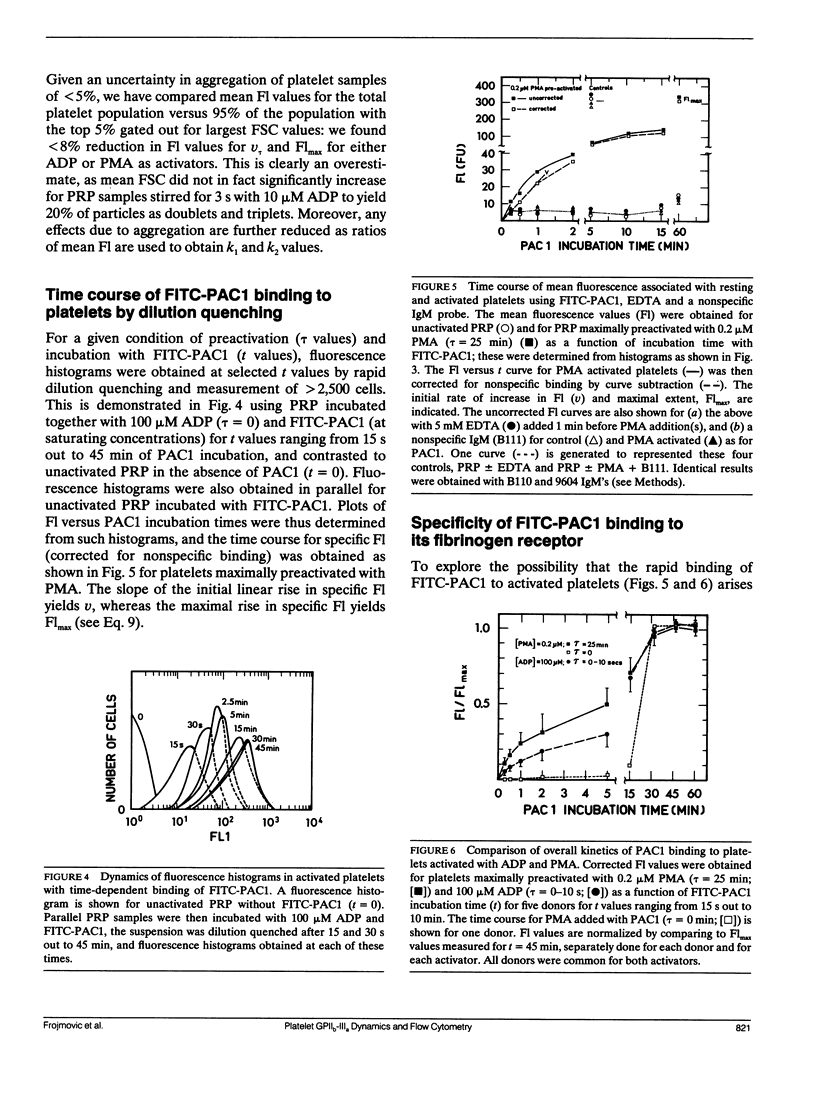
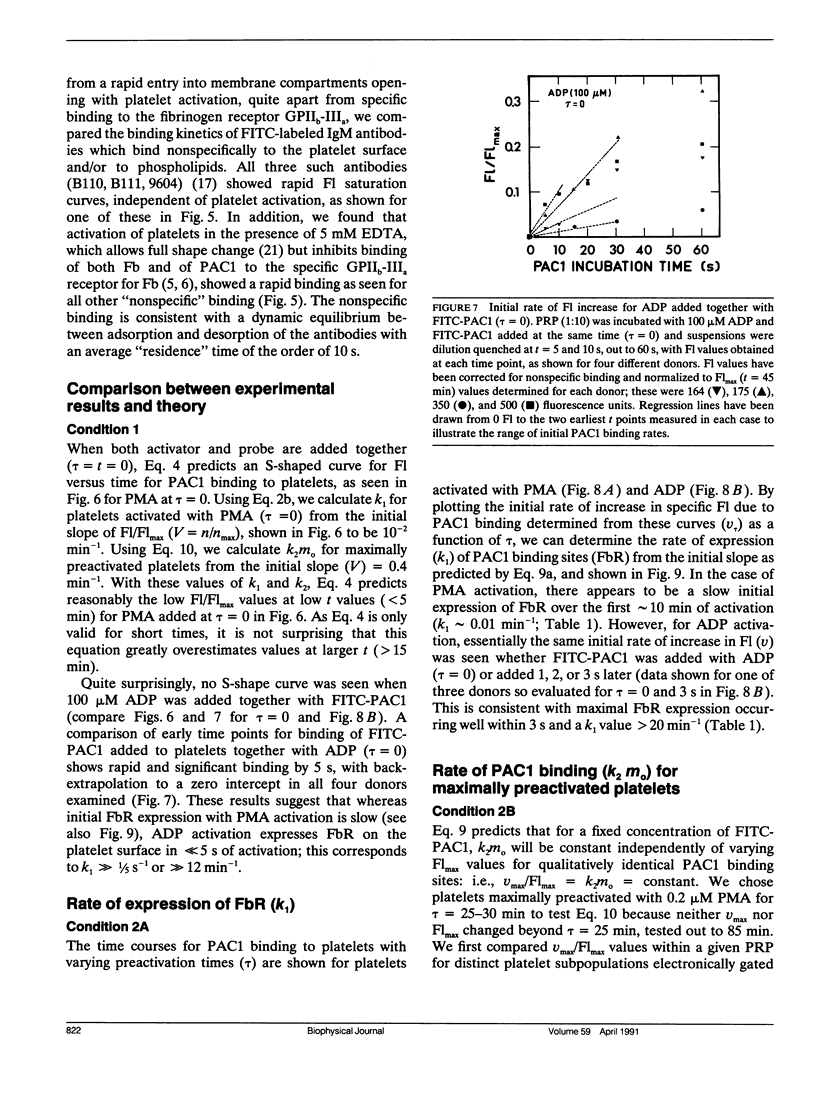
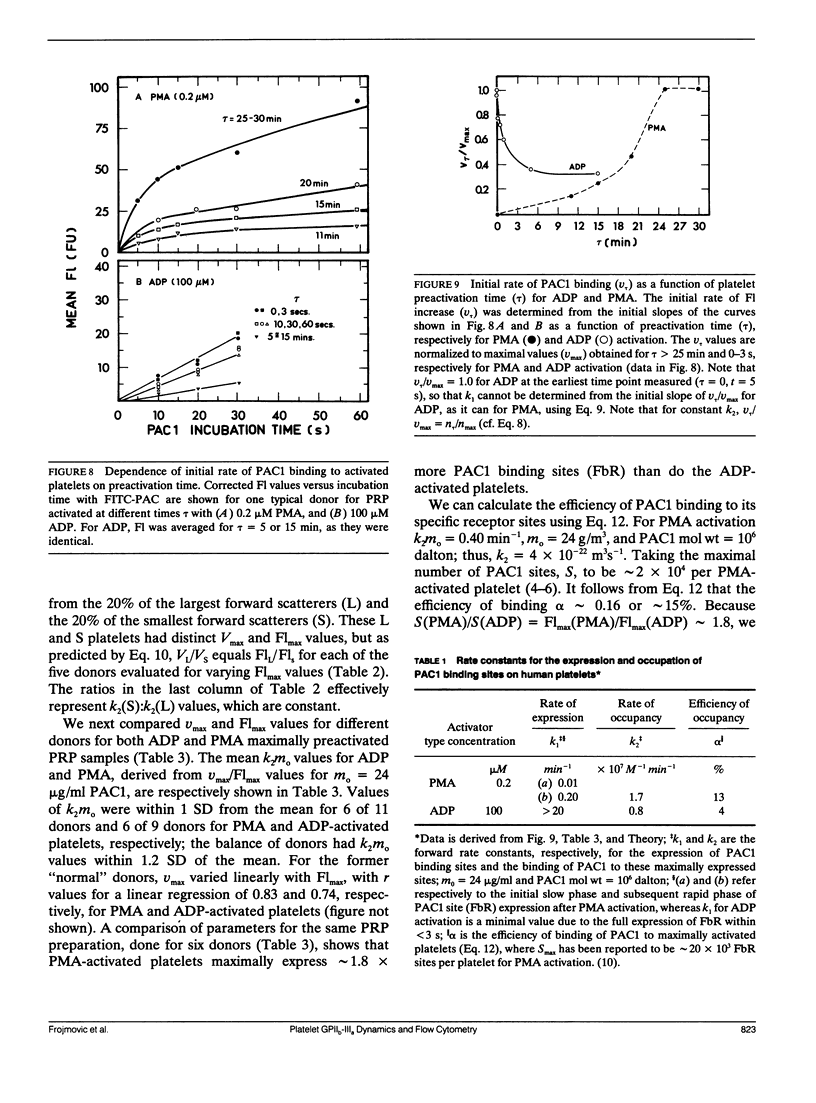
Platelet aggregation, which occurs within seconds of activation, is generally considered to be mediated by fibrinogen binding to glycoprotein IIb-IIIa which becomes expressed as a fibrinogen receptor (FbR) on the activated platelet surface. This receptor expression has, however, only been measured to date at relatively long activation times (greater than 15 min). We have therefore developed a theoretical and experimental approach for determining FbR expression within seconds of platelet activation using flow cytometry. The fluorescently labeled IgM monoclonal antibody FITC-PAC1, was used to report on the GPIIb-IIIa receptor for Fb (FbR). Human citrated platelet-rich plasma (PRP; diluted 1:10) was incubated with adenosine diphosphate (ADP) or phorbol myristate acetate (PMA) for varying times (tau = 0-10 s, out to 60 min), followed by incubation with fluorescein isothiocyanate (FITC)-PAC1 antibody at saturating concentrations. The time course of FITC-PAC1 binding was then measured for these variously preactivated samples (different tau) from the mean platelet-bound fluorescence (Fl), determined for greater than or equal to 5 s of PAC1 addition by dilution quenching and determination of fluorescence intensity histograms with the FACSTAR or FACSCAN (Becton-Dickinson Canada, Mississauga, Ontario) flow cytometers. Both rapid, initial rate of increase in Fl (nu) (related to PAC1 on-rates) and maximal extent of increase (Flmax) were thus determined for different tau values. These measurements yield the rate of formation of FbR (k1), and both the rate (k2) and efficiency (alpha) of binding of PAC1 to FbR as a function of activator type and time of action. We have found that ADP appears to cause rapid, maximal expression of FbR within 1-3 s (k1 greater than 20 min-1), whereas PMA expresses FbR in a slow, biphasic manner (k1 - 0.01 and 0.2 min-1). However, k2 and alpha for maximal PMA activation are about two and three times greater, respectively, than for maximal ADP-activation. Moreover, k2 decreases with post ADP activation time. These differences are discussed in terms of altered FbR organization and accessibility. This kinetic approach can be widely used to analyze the dynamics and organization of molecules on cell surfaces by flow cytometry, including studies of size-dependent subpopulations (see Part II, Frojmovic, M., and T. Wong. 1991. Biophys. J. 59:828-837).
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch A. S., Leung L. L., Polley M. J., Nachman R. L. Platelet membrane topography: colocalization of thrombospondin and fibrinogen with the glycoprotein IIb-IIIa complex. Blood. 1985 Oct;66(4):926–934. [PubMed] [Google Scholar]
- Coller B. S. A new murine monoclonal antibody reports an activation-dependent change in the conformation and/or microenvironment of the platelet glycoprotein IIb/IIIa complex. J Clin Invest. 1985 Jul;76(1):101–108. doi: 10.1172/JCI111931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelinger A. L., 3rd, Lam S. C., Plow E. F., Smith M. A., Loftus J. C., Ginsberg M. H. Occupancy of an adhesive glycoprotein receptor modulates expression of an antigenic site involved in cell adhesion. J Biol Chem. 1988 Sep 5;263(25):12397–12402. [PubMed] [Google Scholar]
- Frojmovic M. M., Milton J. G., Duchastel A. Microscopic measurements of platelet aggregation reveal a low ADP-dependent process distinct from turbidometrically measured aggregation. J Lab Clin Med. 1983 Jun;101(6):964–976. [PubMed] [Google Scholar]
- Frojmovic M. M., Milton J. G., Gear A. L. Platelet aggregation measured in vitro by microscopic and electronic particle counting. Methods Enzymol. 1989;169:134–149. doi: 10.1016/0076-6879(89)69055-6. [DOI] [PubMed] [Google Scholar]
- Frojmovic M. M., Milton J. G. Human platelet size, shape, and related functions in health and disease. Physiol Rev. 1982 Jan;62(1):185–261. doi: 10.1152/physrev.1982.62.1.185. [DOI] [PubMed] [Google Scholar]
- Frojmovic M., Wong T. Dynamic measurements of the platelet membrane glycoprotein IIb-IIIa receptor for fibrinogen by flow cytometry. II. Platelet size-dependent subpopulations. Biophys J. 1991 Apr;59(4):828–837. doi: 10.1016/S0006-3495(91)82295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J. N., Pickett E. B., Saucerman S., McEver R. P., Kunicki T. J., Kieffer N., Newman P. J. Platelet surface glycoproteins. Studies on resting and activated platelets and platelet membrane microparticles in normal subjects, and observations in patients during adult respiratory distress syndrome and cardiac surgery. J Clin Invest. 1986 Aug;78(2):340–348. doi: 10.1172/JCI112582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M. H., Loftus J. C., Plow E. F. Cytoadhesins, integrins, and platelets. Thromb Haemost. 1988 Feb 25;59(1):1–6. [PubMed] [Google Scholar]
- Isenberg W. M., McEver R. P., Phillips D. R., Shuman M. A., Bainton D. F. The platelet fibrinogen receptor: an immunogold-surface replica study of agonist-induced ligand binding and receptor clustering. J Cell Biol. 1987 Jun;104(6):1655–1663. doi: 10.1083/jcb.104.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakaiya R. M., Kiraly T. L., Cable R. G. Concanavalin A induces patching/capping of the platelet membrane glycoprotein IIb/IIIa complex. Thromb Haemost. 1988 Apr 8;59(2):281–283. [PubMed] [Google Scholar]
- Loftus J. C., Albrecht R. M. Redistribution of the fibrinogen receptor of human platelets after surface activation. J Cell Biol. 1984 Sep;99(3):822–829. doi: 10.1083/jcb.99.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver R. P., Bennett E. M., Martin M. N. Identification of two structurally and functionally distinct sites on human platelet membrane glycoprotein IIb-IIIa using monoclonal antibodies. J Biol Chem. 1983 Apr 25;258(8):5269–5275. [PubMed] [Google Scholar]
- Meng Q. H., Rauch J. Differences between human hybridoma platelet-binding antibodies derived from systemic lupus erythematosus patients and normal individuals. Autoimmunity. 1990;5(3):151–167. doi: 10.3109/08916939009002974. [DOI] [PubMed] [Google Scholar]
- Milton J. G., Frojmovic M. M. Turbidometric evaluations of platelet activation: relative contributions of measured shape change, volume, and early aggregation. J Pharmacol Methods. 1983 Apr;9(2):101–115. doi: 10.1016/0160-5402(83)90002-5. [DOI] [PubMed] [Google Scholar]
- Peerschke E. I. Decreased accessibility of platelet-bound fibrinogen to antibody and enzyme probes. Blood. 1989 Aug 1;74(2):682–689. [PubMed] [Google Scholar]
- Peerschke E. I. The platelet fibrinogen receptor. Semin Hematol. 1985 Oct;22(4):241–259. [PubMed] [Google Scholar]
- Phillips D. R., Baughan A. K. Fibrinogen binding to human platelet plasma membranes. Identification of two steps requiring divalent cations. J Biol Chem. 1983 Sep 10;258(17):10240–10246. [PubMed] [Google Scholar]
- Phillips D. R., Charo I. F., Parise L. V., Fitzgerald L. A. The platelet membrane glycoprotein IIb-IIIa complex. Blood. 1988 Apr;71(4):831–843. [PubMed] [Google Scholar]
- Santoso S., Zimmermann U., Neppert J., Mueller-Eckhardt C. Receptor patching and capping of platelet membranes induced by monoclonal antibodies. Blood. 1986 Feb;67(2):343–349. [PubMed] [Google Scholar]
- Shattil S. J., Budzynski A., Scrutton M. C. Epinephrine induces platelet fibrinogen receptor expression, fibrinogen binding, and aggregation in whole blood in the absence of other excitatory agonists. Blood. 1989 Jan;73(1):150–158. [PubMed] [Google Scholar]
- Shattil S. J., Cunningham M., Hoxie J. A. Detection of activated platelets in whole blood using activation-dependent monoclonal antibodies and flow cytometry. Blood. 1987 Jul;70(1):307–315. [PubMed] [Google Scholar]
- Shattil S. J., Hoxie J. A., Cunningham M., Brass L. F. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem. 1985 Sep 15;260(20):11107–11114. [PubMed] [Google Scholar]
- Wencel-Drake J. D., Plow E. F., Kunicki T. J., Woods V. L., Keller D. M., Ginsberg M. H. Localization of internal pools of membrane glycoproteins involved in platelet adhesive responses. Am J Pathol. 1986 Aug;124(2):324–334. [PMC free article] [PubMed] [Google Scholar]
- Wong T., Pedvis L., Frojmovic M. Platelet size affects both micro- and macro-aggregation: contributions of platelet number, volume fraction and cell surface. Thromb Haemost. 1989 Sep 29;62(2):733–741. [PubMed] [Google Scholar]
- Woods V. L., Jr, Wolff L. E., Keller D. M. Resting platelets contain a substantial centrally located pool of glycoprotein IIb-IIIa complex which may be accessible to some but not other extracellular proteins. J Biol Chem. 1986 Nov 15;261(32):15242–15251. [PubMed] [Google Scholar]


