Abstract
Monocytes/macrophages are thought to be involved in Acanthamoeba infections. The aim of this work was to study whether soluble metabolites (ADP and other compounds) released by Acanthamoeba castellanii trophozoites could induce morphological and biochemical changes in human monocytic cells in vitro. We demonstrate here that ADP constitutively released in the medium by A. castellanii, interacting with specific P2y2 purinoceptors expressed on the monocytic cell membrane, caused a biphasic rise in [Ca2+]i, morphological changes characteristics of cells undergoing apoptosis, caspase-3 activation, and secretion of tumor necrosis factor alpha (TNF-α). The same results were found in monocytes exposed to purified ADP. Cell damage and TNF-α release induced by amoebic ADP were blocked by the P2y2 inhibitor suramin. Other metabolites contained in amoebic cell-free supernatants, with molecular masses of, respectively, >30 kDa and between 30 and 10 kDa, also caused morphological modifications and activation of intracellular caspase-3, characteristics of programmed cell death. Nevertheless, mechanisms by which these molecules trigger cell damage appeared to differ from that of ADP. In addition, other amoebic thermolable metabolites with molecular masses of <10 kDa caused the secretion of interleukin-1β. These findings suggest that pathogenic free-living A. castellanii by release of ADP and other metabolites lead to human monocytic cell death through apoptosis and stimulate the secretion of proinflammatory cytokines.
Free-living amoebae of the genus Acanthamoeba are ubiquitous in nature. Acanthamoeba species can be opportunistic pathogens for humans, causing granulomatous amoebic encephalitis, skin ulcers, granulomatous sinusitis, and chronic keratitis.
Host resistance mechanisms operative against Acanthamoeba may involve innate and acquired immunity (11, 12, 22, 38, 40). Many studies have indicated that macrophages may play an important role in the control of Acanthamoeba infections (22, 23, 25, 38). In fact, they may be important effectors in the granulomatous reaction which forms around infected tissues (24). In addition, it has been reported that the elimination of conjunctival-corneal macrophages in vivo, with liposomes containing dichloromethylene diphosphonate, resulted in a severe prolonged course of Acanthamoeba keratitis without resolution (42).
A study on amoeba-macrophage interactions has established that activated macrophages are capable of injuring weakly pathogenic species of Acanthamoeba, whereas highly pathogenic species evade killing by these effector cells (22). This study also demonstrated that Acanthamoeba trophozoites can destroy phagocytic cells by contact-dependent cytolysis by using a finger-like projection and apparently insert cytolytic factors (22).
Our in vitro studies, carried out to elucidate the virulence factors responsible for Acanthamoeba infections, have shown that heat-resistant and nonproteinic molecules with low molecular mass (<10 kDa), released by viable A. castellanii trophozoites produce a serious cytopathic effect in human epithelial Wish cells, causing (i) an increase in cytosolic free-calcium ([Ca2+]i), (ii) morphological changes, (iii) cytoskeletal alterations, (iv) a decrease in cell viability, and (v) cell death (26). More recently, we have characterized the chemical nature of these molecules and demonstrated that A. castellanii viable trophozoites release ADP in the medium and in this way they may affect human epithelial cells by a process that begins with a rise of cytosolic free-calcium concentrations and culminates in apoptosis (27).
Since little is known of the cytotoxic action exerted in phagocytes by metabolites released from Acanthamoeba, studies were undertaken to examine morphological and biochemical changes caused in these cells by incubation with conditioned medium obtained from Acanthamoeba castellanii cultures.
Studies with human monocytes/macrophages are liable to some limitations because of the need to have a source of blood donors, the small number of cells that can be recovered, and some contamination with lymphocytes (20). Therefore, as an experimental model, we used the human myelomonocytic cell line THP-1 that represents a nonadherent population of early monoblasts (41, 43) which can acquire the properties of respiratory burst activity and expression of the CR3, FcγRI, and FcγRII receptors (14, 16). The purposes of the present study were as follows: (i) to characterize the action of amoebic released ADP on monocytic cells; (ii) to examine the effect of other amoebic metabolites (contained in conditioned medium); and (iii) to determine whether THP-1 cells, after exposure to amoebic soluble metabolites, could differentiate into adherent cell populations and release cytokines.
In this study, we show that the ADP and some non-heat-resistant compounds (very probably of proteinic nature) secreted by A. castellanii lead to monocyte cell death through apoptosis. ADP-induced apoptosis of monocytes must be distinguished from protein-induced apoptosis and may reflect an additional strategy of this protozooal pathogen to overcome the host defense.
In addition, our results show that amoebic soluble metabolites modify the secretion pattern of several cytokines produced by monocytes. In particular, the ADP released increases the amounts of tumor necrosis factor alpha (TNF-α) secreted from monocytic cells, while an unidentified compound with a mass of <10 kDa, which is non-heat resistant, causes the release of interleukin-1β (IL-1β).
MATERIALS AND METHODS
Amoebas and cell line culture conditions.
Our study was performed by using trophozoites of A. castellanii, isolated from a case of amoebic keratitis (in Ancona, Italy), axenically grown at 25°C in PYG medium (15). The species identification of this isolate was based on cyst morphology and indirect immunofluorescence microscopy.
THP-1 cells were maintained in continuous culture in RPMI 1640 medium (Gibco-BRL/Life Technologies) supplemented with 10% heat-inactivated fetal calf serum (Gibco-BRL/Life Technologies), 100 U of penicillin G, and 100 μg of streptomycin sulfate per ml, and grown in 25-cm2 sterile plastic flasks at 37°C in a humid atmosphere containing 5% CO2. In each experiment cell viability was >95%, as determined by the nigrosin-dye exclusion method.
Preparation and fractionation of amoebic cell-free supernatants.
Amoebas washed twice in phosphate-buffered saline (PBS) solution without Ca2+ and Mg2+ at pH 7.4 were suspended (6 × 106 cells/ml) in the same buffer or in RPMI 1640 medium containing 20 mM HEPES and incubated for 2 h at 25°C. Cell-free supernatants were obtained by centrifugation of these cultures at 500 × g for 15 min. To evaluate the effect of both ADP and other metabolites released by trophozoites, entire conditioned PBS and RPMI (iRPMI and iPBS) were fractionated by using Centriprep-30 (Amicon; cutoff, 30 kDa) and Centriprep-10 microconcentrators (Amicon; cutoff, 10 kDa). After centrifugation (1,500 × g for 15 min), the pellets were removed from the bottom chamber of Centricon-30 microconcentrators, and the volumes were adjusted to the starting volume to obtain the supernatant fractions containing amoebic metabolites of >30 kDa (pRPMI and pPBS). Supernatants removed from the top chamber were again centrifuged by using Centricon-10 microconcentrators (3,000 × g for 20 min) to obtain two fractions containing, respectively, amoebic metabolites of from 30 to 10 kDa (rRPMI and rPBS) and <10 kDa (sRPMI and sPBS). To establish the heat resistance of compounds contained in the conditioned medium, cell-free supernatants, prepared as described above, were treated at 95°C for 10 min, and filtered through Centriprep-10 microconcentrators. After centrifugation (3,000 × g for 20 min), the pellets and supernatants were collected to obtain two fractions containing, respectively, metabolites of >10 kDa (mRPMI and mPBS) and <10 kDa (cRPMI and cPBS). Fractions were used immediately after processing. In particular, samples containing PBS were used only in [Ca2+]i and plasma membrane permeability measurements, since some aromatic RPMI medium components could interfere with these assays.
Analysis of LDH release.
A total of 2 × 106 THP-1 cells per sample were incubated with 2 ml of RPMI-20 mM HEPES or iRPMI at 37°C and with 5% CO2 in a humidified atmosphere. After 24 h, cells were pelleted by centrifugation for 5 min at 400 × g, and the supernatants were removed for subsequent measurement of lactate dehydrogenase (LDH) activity. Enzyme activity in aliquots (100 μl) of the supernatants was determined by spectrophotometry using the Sigma LDH kit (λ = 340 nm). All values are expressed as percentages of LDH release relative to the value obtained after total cell permeabilization with 0.03% Triton X-100.
Cytosolic free-calcium ([Ca2+]i) measurement.
Intracellular calcium in THP-1 cells was monitored by using the fluorescent calcium probe Fura 2-AM (Sigma-Aldrich Srl, Milano, Italy). Dye loading was standardized by incubating cells, suspended in HEPES buffer (137 mM NaCl, 1.2 mM MgSO4, 1.5 mM CaCl2, 5 mM KCl, 15 mM glucose; pH 7.4), with Fura 2-AM for 10 min at room temperature. Loaded cells were washed twice with the same buffer, and the assay was performed on a stirred aliquot (0.5 ml) at 37°C with the use of a Hitachi F-2000 spectrophotometer. The excitation and emission wavelengths were 340 to 380 nm and 510 nm, respectively, detected every 500 ms and stored in separate memories of the F-2000 spectrophotometer. A data manager was used to monitor the fluorescence signal of Fura 2-AM-loaded cells. Basal and stimulated cytosolic calcium were quantified following the Grynkiewicz et al. method (17) by using the ratio technique and a Kd of 224 nM as the dissociation constant of Fura 2-AM; maximal and minimal values of the fluorescence were evaluated after the addition of 0.006% Triton X-100 and 10 mM EGTA, respectively. Hitachi F-2000 software was used for calculation.
Analysis of changes in plasma membrane permeability.
This parameter in THP-1 cells was measured with the fluorescent dye ethidium bromide (Sigma-Aldrich). Cells (7.5 × 105) suspended in PBS (1.2 ml) were incubated in the fluorimeter cuvette (37°C). Ethidium bromide was added to a final concentration of 5 μM. Cells were allowed to equilibrate for 200 s and then stimulated with 300 μl of PBS-20 mM HEPES, with conditioned PBS (entire and fractionated), or with the same volume of PBS-20 mM HEPES containing external ADP (ADP0 at 20 and 150 μM). Fluorescence was monitored at wavelengths of 360 nm (excitation) and 580 nm (emission). In each assay, maximum cell permeabilization-ethidium uptake was defined as the fluorescence value achieved by adding Triton X-100 (0.03%) to the cuvette at the end of each trace. Fluorescence increases were expressed as the percent fraction of maximum fluorescence attained 11 min after stimulation.
Phase-contrast microscopy.
A total of 106 THP-1 cells were suspended in 1 ml of fresh RPMI medium-20 mM HEPES, iRPMI, pRPMI, rRPMI, sRPMI, mRPMI, or cRPMI or the same volume of RPMI-20 mM HEPES containing 20 μM ADP0 and seeded in 24-well tissue culture dishes. Experiments were performed in the presence or in the absence of suramin (20 μM). After incubation at 37°C in 5% CO2 atmosphere, at the selected time intervals (1, 3, 7, 14, and 20 h), the cell morphology was observed with a Zeiss (Tilaval 31) microscope equipped with a ×40 lens.
Assessment of apoptosis by fluorescence microscopy.
THP-1 cells growing under the same experimental conditions as those described above were also examined by microscopy after DAPI (4′,6′-diamidino-2-phenylindole) staining in order to visualize the nuclei. After incubation, cells were washed with PBS, fixed in 70% ethanol at 0°C, seeded on polylysine-coated coverslips, and stained with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) buffer containing 200 ng of DAPI (Sigma-Aldrich) per ml. After being washed with PBS, coverslips were mounted in Gelvatol (Monsanto Corp.) and examined by a Nikon Optiphot microscope.
Assessment of apoptosis by analysis of caspase-3 activation.
Cell suspension aliquots of 0.1 ml containing 2 × 106 THP-1 cells were seeded in 24-well tissue culture dishes and incubated for 14 h at 37°C in a 5% CO2 atmosphere with 2 ml of RPMI medium-20 mM HEPES; the same volume of iRPMI, pRPMI, rRPMI, sRPMI, mRPMI, and cRPMI; or with RPMI-20 mM HEPES containing 20 μM ADP0. Experiments were performed in the presence or in the absence of suramin (20 μM). After centrifugation (400 × g for 10 min), pellets were washed twice with PBS, and lysed. Caspase-3 activation was measured fluorometrically on the cell lysates with the ApoAlert Caspase-3 Fluorescent Assay Kit (Clontech) as indicated by the manufacturer. The fluorescence values were monitored at wavelengths of 400 nm (excitation) and 505 nm (emission).
Analysis of cytokine secretion.
Supernatants obtained from the centrifugation of cell suspensions employed in the caspase experiments were collected, frozen (−80°C) and used to evaluate the cytokine production. IL-1β and IL-12 release induced on THP-1 cells by amoebic metabolites were quantitatively determined with the Human IL-1β ELISA Kit and Human IL-12 ELISA Kit (EuroClone) as, respectively, indicated by the manufacturer. IL-6 and TNF-α production were measured with the [hIL-6] human enzyme-linked immunosorbent assay (ELISA) system and with [hTNF-α] human ELISA system (Biotrak-Amersham Life Science) according to the manufacturer's instructions. The optical density of each well was determined by using a spectrophotometer (SIRIO SSEAC) set to a 450-nm wavelength, and the results are expressed in picograms per milliliter.
Statistics.
Statistical difference between groups was determined by using a two-tailed Student's t test. Differences were considered significant at P < 0.05.
RESULTS
Cytotoxic effect of iRPMI on THP-1 monocytes.
To test the cytopathic activity, the human monocyte cell line THP-1 was incubated with the cell-free supernatant obtained from A. castellanii cultures. After 24 h, the culture medium was removed and analyzed by photometry to assess the LDH released from cells; this result was used as an index of cell damage. Compared to the controls, treated cells showed a significant LDH release (P < 0.01) corresponding to 80% ± 6.2% (n = 6) of total cellular LDH content (216 U/ml). Moreover, nigrosin exclusion revealed that ca. 50% of THP-1 cells exposed to conditioned medium were already dead after 24 h of incubation.
Characterization of metabolites contained in conditioned medium.
Our previous sodium dodecyl sulfate-polyacrylamide gel electrophoresis experiments of conditioned medium, obtained from the same Acanthamoeba isolate and in the same experimental conditions, showed 10 bands corresponding to 97, 53.7, 48.9, 45.1, 40.2, 32.7, 28.8, 22.6, 19.2, and 16.4 kDa (26). In addition, capillary electrophoresis showed the presence of ADP in significant micromolar concentrations (27). To further characterize the cytopathic action of ADP and proteinic molecules released from A. castellanii, we fractionated the medium conditioned by trophozoites as described in Materials and Methods and then examined the effect of each fraction on monocytic cells.
Cytosolic free-calcium measurement.
In order to establish the role of amoebic released-ADP on monocyte calcium concentration, we first compared the effect exerted by both cPBS and external ADP (ADP0). In single isolated THP-1 cells, the resting level of [Ca2+]i was calculated to be 133.55 ± 3.58 nM (n = 45), and the addition of PBS alone did not cause variation ([Ca2+]i = 136.22 ± 5.12 nM; n = 18). THP-1 stimulation with 80 μl of 20 μM ADP0 or with 80 μl of cPBS (that, as previously reported, contained the same concentration of amoebic released ADP) led to a rapid biphasic increase of [Ca2+]i, which consisted of an initial transient elevation, maintained for up to 100 s, followed by a sustained elevation at levels higher than the basal value (Fig. 1A and B). THP-1 response to external ADP or cPBS showed very similar shapes and time courses. These experiments resulted in a peak increase in [Ca2+]i of 198.83 ± 13.82 nM (n = 9) or 189.27 ± 14.19 nM (n = 10), respectively, and between these conditions any statistical difference was shown by a two-tailed Student's t test calculation. The cellular responses induced by extracellular nucleotides were mediated by P2 nucleotide receptors that include two distinct subtypes classified as P2x and P2y receptors. P2x are ligand-gated ion channels permeable to Na+ and K+, whereas P2y are G protein-coupled receptors (9). THP-1 monocytes are known to express several P2x (P2x1 and P2x7/P2z), as well as P2y (P2y1, P2y2, P2y4, and P2y6), receptors (3, 5, 19). Upon testing the effect of two specific antagonists, we identified the nucleotide receptor expressed in THP-1 cells on which ADP0 and cPBS acted. Suramin (P2x and P2y2 receptor antagonist), nearly abolished the effect of both ADP0 and cPBS on a [Ca2+]i, whereas the purinergic P2x and P2y3 receptor antagonist PPADS (pyridoxal phosphate-6-azophenil-2′,4′-disulfonic acid) (44) was ineffective (Table 1). This clearly indicated that cPBS, like external ADP, acted on P2y2 purinergic receptors expressed on THP-1 cell membranes inducing calcium overload. Phosphatidylinositol is known to be hydrolyzed upon metabotropic purinoceptor activation and calcium is released from intracellular stores; depletion of the intracellular stores in turn induces calcium influx across the plasma membrane (9).
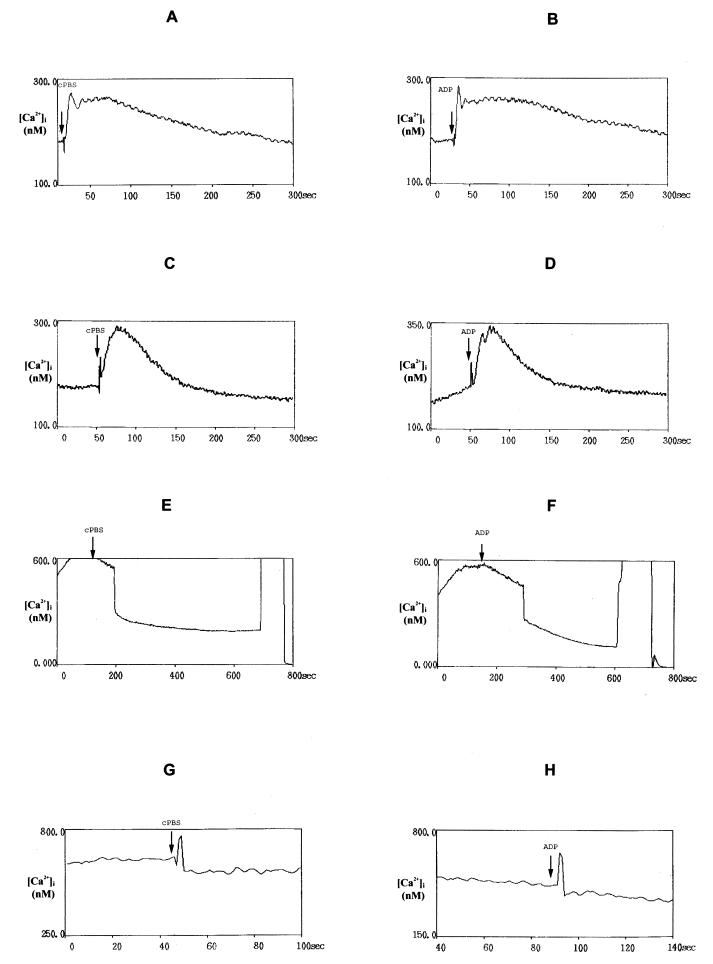
FIG. 1.
Time courses of the [Ca2+]i evoked in THP-1 cells, loaded with Fura 2-AM (3 μM) by 80 μl of heat-treated filtered cell-free PBS (cPBS) conditioned for 2 h by the addition of 6 × 106 A. castellanii trophozoites/ml (A), by the same volume of PBS containing 20 μM ADP0 (B), by cPBS (C) or 20 μM ADP0 (D) after chelation of extracellular Ca2+ with 5 mM EGTA, by cPBS (E) or 20 μM ADP0 (F) after exposition to 10 μM thapsigargin, or by cPBS (G) or 20 μM ADP0 (H) after 20 min of loaded THP-1 cells exposure to 10 μM ryanodine.
TABLE 1.
Effect of two purinergic antagonists on the cytosolic-free-calcium peak increase (Δ[Ca2+]i) induced in THP-1 cells stimulated with heat-treated filtered cell-free PBS-20 mM HEPES conditioned for 2 h by the addition of A. castellanii trophozoites (6 × 106/ml) (cPBS) or PBS-20 mM HEPES containing 20 mM ADP0a
| Sample | Mean Δ[Ca2+]i (nM) ± SE with:
|
|||||
|---|---|---|---|---|---|---|
| No treatment | Suramin (P2x-P2y2 antagonist) at:
|
PPADS (P2x-P2y1,4,6 antagonist) at:
|
||||
| 10 μM | 15 μM | 20 μM | 5 μM | 10 μM | ||
| cPBS | 189.27 ± 14.19 | 48.15 ± 5.22∗ | 25.37 ± 3.34∗ | 0 ± 0∗ | 211.19 ± 26,56§ | 207.38 ± 14.96§ |
| PBS-ADP0 (20 μm) | 198.83 ± 13.82 | 36.89 ± 2.18∗ | 15.08 ± 2.02∗ | 0 ± 0∗ | 232.47 ± 35.20§ | 224.44 ± 46.19§ |
Cell stimulation was obtained with 80 μl of the samples indicated. Values are means ± the standard error of at least six experiments. ∗, P < 0.01 versus the respective controls; §, not significant. The basal value of the [Ca2+]i in THP-1 cells is 133.55 ± 3.58 nM (n = 45).
To further investigate the Ca2+ signaling pathway coupled to the action exerted by cPBS on THP-1 P2y3 receptors, we removed extracellular Ca2+ by chelation with 5 mM EGTA. In this condition both cPBS and ADP0 still evoked an initial rise in [Ca2+]i, but the response was now transient, and no sustained plateau phase could be observed (Fig. 1C and D). To further explain whether the rise of [Ca2+]i could be dependent on ion release from intracellular calcium stores, target cells were exposed to thapsigargin and ryanodine (both from Sigma-Aldrich) that, respectively, inhibit the sarco-endoplasmic reticulum Ca2+ ATPase and activate the Ca2+ release channels (called “feet”) responsible for calcium release from internal stores (28, 33). THP-1 cell stimulation with 10 μM thapsigargin induced a prolonged elevation of cytosolic free-calcium concentrations, and the additional stimulation with cPBS and ADP0 in the presence of thapsigargin failed to elicit any further increase in [Ca2+]i (Fig. 1E and F). Ryanodine treatment of THP-1 cells for 20 min abolished the [Ca2+]i induced by cPBS or ADP0 (Fig. 1G and H).
These findings suggest that, in response to cPBS and ADP0, the initial peak of the cytosolic free-calcium increase was caused by depletion of intracellular calcium stores, whereas the plateau phase depended on the transmembraneous influx of Ca2+, and confirmed that the cytosolic free-calcium increase observed was mediated by P2y2 purinergic receptors. To demonstrate whether iPBS and the fraction containing the amoebic ADP not exposed to heat treatment induced the same action, THP-1 cells were stimulated, respectively, with 80 μl of iPBS or sPBS. Both of these samples produced a biphasic change in [Ca2+]i; iPBS, however, showed a significantly lower effect than either sPBS and cPBS (P < 0.05; Table 2). Finally, to examine the effect of amoebic compounds with molecular masses of >10 kDa exposed and not exposed to heat treatment, monocytes were stimulated with the same volume of mPBS, pPBS, or rPBS. In all of these cases any increase of calcium concentration was detected in THP-1 cells (Table 2).
TABLE 2.
Cytosolic-free-calcium peak increase (Δ[Ca2+]i) induced on THP-1 cells stimulated with cell-free PBS-20 mM HEPES conditioned for 2 h by the addition of A. castellanii trophozoites (6 × 106/ml) (iPBS) or with its fractionsa
| Sample | Mean Δ[Ca2+]i (nM) ± SE |
|---|---|
| iPBS | 158.36 ± 12.50* |
| pPBS (amoebic metabolites, >30 kDa) | 2.66 ± 1.23§ |
| rPBS (amoebic metabolites, 10-30 kDa) | 4.80 ± 1.98§ |
| sPBS (amoebic metabolites, <10 kDa) | 172.28 ± 8.24** |
| mPBS (amoebic metabolites, >10 kDa, heat inactivated) | 2.54 ± 1.52§ |
Cell stimulation was obtained with 80 μl of the samples indicated. Values are means ± the standard error of at least three experiments. ∗, P < 0.05 versus the control; ∗∗, P < 0.01 versus the control; §, not significant. The basal value of the [Ca2+]i in THP-1 cells is 133.55 ± 3.58 nM (n = 45).
Changes in plasma membrane permeability.
To further exclude the involvement of the monocyte surface-associated P2z (P2x7) receptors during monocyte interaction with amoebic ADP, we investigated its ability to open large pores in the THP-1 cell membrane. In fact, it is known that the activation of these purinergic receptors causes a rapid increase in [Ca2+]i, followed in time by a progressive increase in permeability of the membrane to molecules with molecular mass of up to 900 Da (9, 10, 31). Ethidium, a cation with molecular mass of 314 Da, is normally excluded from the cytoplasm. However, if ethidium gains access to the cytosol, a large fluorescence signal will be generated on the binding to cellular DNA and RNA. Thus, ethidium fluorescence can be used as an index of permeabilization of the plasmalemma. The uptake of ethidium was determined after cell stimulation with entire conditioned PBS or with its fractions. There was no significant increase in ethidium bromide permeability with the various conditioned media or ADP (Table 3).
TABLE 3.
Ethidium bromide uptake induced on THP-1 cells stimulated with PBS-20 mM HEPES (control) or cell-free PBS-20 mM HEPES conditioned for 2 h by the addition of A. castellanii trophozoites (6 × 106/ml) or its fractions or with PBS-20 mM HEPES containing ADP0a
| Sample | Mean percent fraction of maximum fluorescence attained 11 min after stimulation ± SE |
|---|---|
| PBS (control) | 2.71 ± 0.59 |
| iPBS (entire conditioned PBS) | 3.55 ± 0.68* |
| pPBS (amoebic metabolites, >30 kDa) | 2.93 ± 0.46* |
| rPBS (amoebic metabolites, 10-30 kDa) | 2.39 ± 0.52* |
| sPBS (amoebic metabolites, <10 kDa) | 3.01 ± 0.54* |
| cPBS (amoebic metabolites, <10 kDa, heat inactivated) | 3.26 ± 0.49* |
| mPBS (amoebic metabolites, >10 kDa, heat inactivated) | 3.18 ± 0.48* |
| PBS-ADP0 (20 μM) | 2.98 ± 0.56* |
| PBS-ADP0 (150 μM) | 3.76 ± 0.80* |
Cell stimulation was obtained with 300 μl of the sample indicated. Maximum fluorescence values were achieved by adding Triton X-100 (0.03%). Values are means ± the standard error of at least three experiments. *, Not significant versus the control.
Morphological changes of THP-1 cells upon Acanthamoeba metabolite exposition.
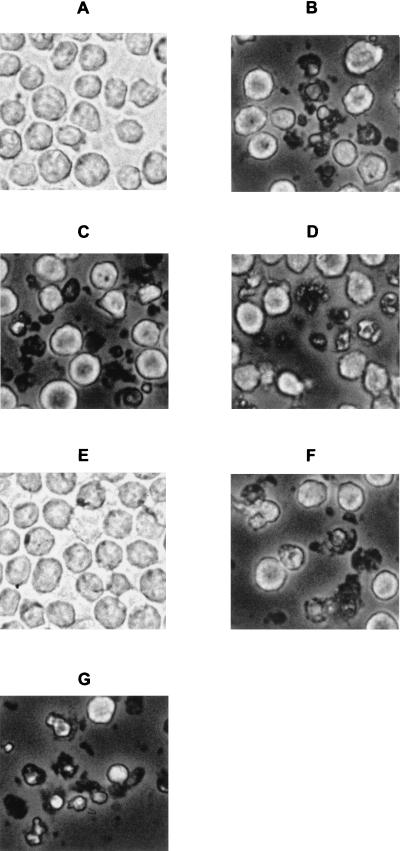
To further characterize the cytopathic effect due to amoebic ADP and other compounds released from Acanthamoeba, monocytes were incubated with each fraction obtained from conditioned amoebic-cell-free RPMI. At selected time intervals cells morphology was analyzed by phase-contrast microscopy. Compared to untreated cells (Fig. 2A), after 7 h of exposition to the various conditioned media or to external ADP0 the monocytes showed a heterogeneous picture of morphological changes characteristic of cells undergoing apoptosis. Some cells were remarkably shrunken and condensed, other cells had acquired an intense granular appearance (Fig. 2). Morphological alterations in THP-1 cells exposed to the fractions that contained the amoebic products with a molecular mass of >10 kDa (pRPMI and rRPMI) were prevented by previous heat treatment (Fig. 2E). The presence of 20 μM suramin during incubation, prevented for up to 24 h only the cell damage induced by cRPMI, sRPMI, or RPMI-20 μM ADP0 (data not shown).
FIG. 2.
Phase-contrast microscopy of THP-1 cells incubated for 7 h at 37°C in 5% CO2 atmosphere with RPMI-20 mM HEPES alone (A) and with fractions of conditioned medium containing amoebic metabolites (>30 kDa; pRPMI) (B), amoebic metabolites (30 to 10 kDa; rRPMI) (C), amoebic ADP (sRPMI) (D), heat-inactivated amoebic metabolites (>10 kDa; mRPMI) (E), heat-treated amoebic ADP (cRPMI) (F), or containing 20 μM ADP0 (G).
Assessment of apoptosis.
To find out whether THP-1 cells injured by amoebic ADP and other metabolites undergo apoptotic cell death, we investigated the nuclear morphology after DAPI staining. The fluorescence microscopy of nuclei showed different forms of chromatin aggregation and nuclear fragmentation that increased in proportion to the exposure time to each fraction of amoebic supernatant. After 3 h of incubation, all control cells and those incubated with pRPMI, rRPMI, or mRPMI showed normal nuclear morphology, while ca. 10% of nuclei were apoptotic in cells exposed to iRPMI, sRPMI, cRPMI, and RPMI containing 20 μM ADP0. After 7 h of exposure to cRPMI, pRPMI, or rRPMI, apoptotic nuclei were observed with more than 37, 20, and 25% of monocytes, respectively.
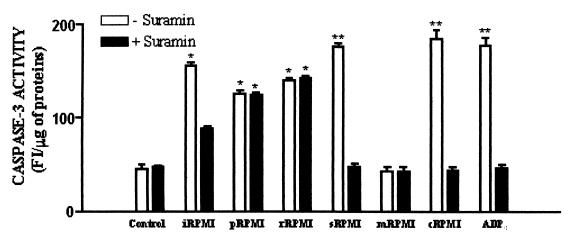
To further investigate the apoptotic effect of amoebic ADP and other metabolites on monocytes and to determine differences in the proportion of apoptotic cells, we analyzed in THP-1 cells the activation of intracellular caspase-3. This is an active cell death protease involved in the execution phase of apoptosis, where cells undergo morphological changes such as DNA fragmentation, chromatin condensation, and apoptotic body formation (6). These experiments showed that, in contrast to control cells, a significant increase of active caspase-3 occurred in monocytes exposed for 14 h to entire conditioned RPMI medium and to its fractions containing released ADP or amoebic metabolites with higher molecular masses (Fig. 3). No statistical difference was found in the caspase-3 activation induced by sRPMI and cRPMI with respect that induced by ADP0, whereas significant differences (P < 0.05) were found between these and those caused by iRPMI, pRPMI, and rRPMI. The results also indicated that amoebic metabolites >10-kDa heat-inactivated (mRPMI) were unable to cause the activation of caspase-3 in THP-1 cells (Fig. 3). The presence of suramin during incubation totally inhibited caspase-3 activation induced by sRPMI, cRPMI, ADP0, and partly inhibited that induced by iRPMI, whereas it did not affect the action of pRPMI and rRPMI (Fig. 3).
FIG. 3.
Activation of intracellular caspase-3 on THP-1 cells exposed, both in the presence or in the absence of 20 μM suramin, for 14 h at 37°C in 5% CO2 atmosphere to RPMI medium (Control), entire conditioned medium (iRPMI), or fractions of conditioned medium containing, respectively, amoebic metabolites of >30 kDa (pRPMI), amoebic metabolites of from 30 to 10 kDa (rRPMI), amoebic ADP (sRPMI) or heat-treated amoebic ADP (cRPMI), amoebic metabolites of >10 kDa heat inactivated (mRPMI), or to RPMI containing 20 μM ADP0. Values are means ± the standard error of at least four experiments. ✽, P < 0.05 versus the control; ✽✽, P < 0.01 versus control.
Cytokine production.
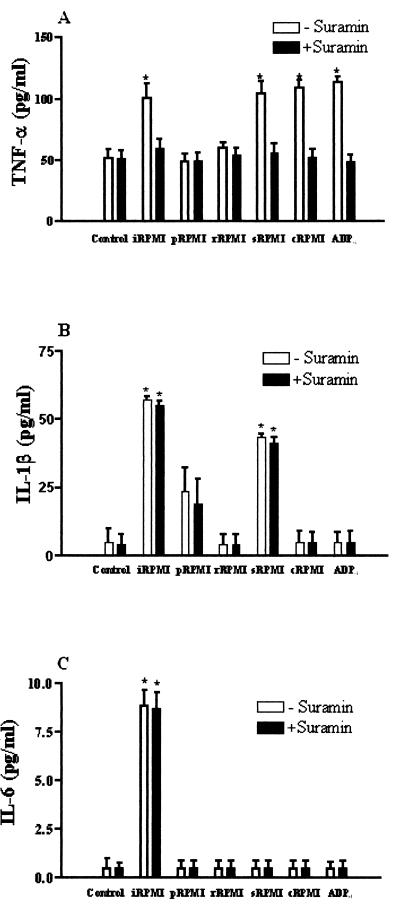
The ability to produce potent cytokines that induce inflammation and recruit other immune cells is included in the antimicrobial arsenal of macrophages. Over the past decade, data have accumulated to suggest that pathogen-induced apoptosis of macrophages triggers severe inflammation via the production and release of proinflammatory cytokines (46). In other instances, however, macrophage apoptosis may occur as a result of mechanisms employed by the pathogen to stop the inflammatory cytokine response (1). We have determined, therefore, the secretion of four kinds of cytokines (TNF-α, IL-1β, IL-6, and IL-12) from THP-1 cells in response to soluble metabolites released by A. castellanii. The amounts of TNF-α secreted from cells incubated for 14 h with iRPMI or with the fractions containing the amoebic ADP (sRPMI and cRPMI) or ADP0 increased significantly (Fig. 4A). In all of these cases the presence of suramin during incubation prevented the TNF secretion. At the same time, the release of IL-1β increased significantly only in THP-1 cells incubated with iRPMI or with the fraction sRPMI (Fig. 4B), while a small but significant rise of secreted IL-6 was observed only after exposition to iRPMI (Fig. 4C). Finally, the amounts of IL-12 secreted from THP-1 cells were not affected by incubation with amoebic metabolites (data not shown).
FIG. 4.
Release of TNF-α (A), IL-1β (B), and IL-6 (C) from THP-1 cells exposed, both in presence or in absence of 20 μM suramin, for 14 h at 37°C in 5% CO2 atmosphere to RPMI medium (Control), entire conditioned medium (iRPMI), or fractions of conditioned medium containing amoebic metabolites of >30 kDa (pRPMI), amoebic metabolites included of from 30 to 10 kDa (rRPMI), amoebic ADP (sRPMI) or heat-treated amoebic ADP (cRPMI), amoebic metabolites of >10 kDa heat inactivated (mRPMI), or to RPMI containing 20 μM ADP0. Values are means ± the standard error of at least four experiments. ✽, P < 0.01 versus the control.
DISCUSSION
Monocytes and macrophages are important effector cells in Acanthamoeba infections. Previous reports show that A. castellanii can destroy phagocytic cells by contact-dependent cytolysis (22).
Our data indicate that A. castellanii cytotoxicity against human monocytes does not necessarily require ameba-host cell contact, in that cell-free supernatants obtained from trophozoite cultures can also induce cell damage.
An important finding of the present study is that several soluble compounds released from these amoebas can affect the human monocytic cell line THP-1 through the induction of apoptosis.
We demonstrate here that ADP is involved in the cytotoxic action exerted on these cells by Acanthamoeba cell-free supernatants. Monocytes exposed to amoebic ADP showed an immediate biphasic rise in [Ca2+]i and rapidly exhibited characteristic features of cells undergoing programmed cell death, such as extensive cell membrane blebbing, chromatin condensation, breakdown of nuclei. Many of the morphological and biochemical changes of apoptosis result from the cleavage of specific cellular proteins by a family of caspases. These are synthesized in the cytosol of mammalian cells as inactive zymogens, which become active through intracellular caspase cascades. One of the key executioners of apoptosis is the intracellular caspase-3, and the exposure of monocytes to amoebic ADP, actually, caused a significant activation of this intracellular protease. The ADP effect was mediated by specific P2y2 purinoceptors expressed on the THP-1 cell membrane. The P2y2 inhibitor suramin, in fact, blocked the cytosolic free-calcium increase and, also, inhibited morphological changes and caspase-3 ADP-induced activation. These data suggest that the substantial elevation of cytosolic free calcium plays a fundamental role in the initial commitment phase of programmed cell death induced by amoebic ADP. Although monocytes and macrophages, like other immune system cells (mast cells and microglia), express the pore-forming P2x7/P2z receptor that mediates ATP-induced cell death (8, 10, 13, 39), we present here evidence that this purinergic receptor is not involved in cytosolic-free-calcium increase and cell death induced by amoebic ADP. Comparing the effects observed on human monocytes with the events previously triggered in human epithelial cells by amoebic ADP, it clearly appears that these different cell types, via P2y2, respond in the same way to ADP exposition. These results confirm that A. castellanii viable trophozoites constitutively release ADP in the medium by which they affect different human cells, by a process that begins with a rise of cytosolic-free-calcium concentrations and culminates in apoptosis.
Other metabolites, released from A. castellanii, contribute to induce cell damage on monocytes. Several compounds with molecular masses of, respectively, >30 kDa and from 30 to 10 kDa also cause morphological modifications and the activation of intracellular caspase-3 characteristics of programmed cell death. Nevertheless, the mechanisms by which these molecules trigger apoptosis appear to differ from those of ADP-mediated apoptosis. In fact, they are unable to cause calcium overload in THP-1 cells, require more time to induce morphological changes, determine a lower caspase-3 activation, and are not affected by suramin. We still do not know the chemical nature of the amoebic compounds with high molecular masses that elicit apoptosis in monocytes, but this study demonstrates that they can be inactivated by heat treatment. This finding leads us to suppose that some proteins, among those released from A. castellanii trophozoites, might be implicated in this phenomenon. Further studies are necessary to identify the exact nature of these molecules and to characterize their mechanisms of action on monocyte apoptosis induction. Previous reports suggest the role of secreted proteinases as important virulence factors in Acanthamoeba keratitis (4, 18, 21, 29, 32). Up to now, the literature has not discriminated between Acanthamoeba proteinase-induced morphological alterations related to cell necrosis and those related to programmed cell death; both were considered “cytotoxic” effects. However, several works indicate that microbial proteases can induce programmed cell death in target cells (7, 30). In addition, our results confirm previous observations on microglial cells that originate from the monocyte/macrophage lineage and are involved in the protective immune response of the central nervous system, functioning as inflammatory or immunoregulatory cells. In fact, a recent report demonstrates that primary-culture rat microglial cells incubated with the pathogenic A. culbertsoni lysate underwent apoptotic processes, followed by DNA fragmentation (34).
Cells of the monocyte/macrophage lineage can orchestrate the immune response to microbes and microbial products by both the induction of membrane-associated signaling molecules and the synthesis and secretion of soluble cytokines. This study demonstrates that A. castellanii trophozoites by soluble compound release stimulates the secretion of proinflammatory cytokines (TNF-α and IL-1β) and IL-6 in human monocytic cells. Furthermore, it shows that amoebic-released ADP can induce TNF-α secretion. This event was prevented by suramin, and it indicates an induction via P2y2 purinoreceptors. Previous studies have shown that nucleotides activate various pathways in monocytic cells. For instance, extracellular ATP regulates proinflammatory signaling pathways, including caspase-1 activation, IL-1β release, and nitric oxide synthase via P2x7 (2, 36, 37), whereas UDP induces IL-8 release via P2y6 receptors (45). Now our findings suggest that also ADP, via P2y2 receptors, might play an important role in inflammation and innate immune defenses. In addition, they support a recent study of H. J. Shin et al. (35), which showed that the coincubation of microglial cells with A. culbertsoni trophozoites or lysate increased the amounts of TNF-α secreted.
The increase of both IL-1β and IL-6 release shown in this work are not induced by amoebic ADP, and further studies are necessary to identify products secreted from Acanthamoeba which are able to stimulate their release. Nevertheless, as a possible candidate for changes in levels of secretion of IL-1β, our experiments suggest compound(s) with a molecular mass of <10 kDa that is susceptible to heat inactivation.
In summary, this study demonstrates that several compounds produced by pathogenic Acanthamoeba can induce cell death in human monocytes that occurs by apoptosis, and release of proinflammatory cytokines. It also indicates that, by undergoing apoptosis, monocytic cells may be able to expose these amoebas to more potent cytocidal cells, such as neutrophils, and to the humoral arm of the immune system. It has been shown that Acanthamoeba spp. are not susceptible to killing by TNF-α or IL-1β, either alone or in combination, and that these cytolytic factors, readily inducing trophozoite encystment, may paradoxically offer protection from macrophage phagocytosis (22). On the basis of all of these data, Acanthamoeba appears to provide an example in which pathogen-induced monocyte apoptosis and the resulting inflammation benefit both the pathogen and the host.
Acknowledgments
This work was supported by grants (ex 60%) from the University of Sassari, Sassari, Italy, and PRIN-2001 (MIUR [Italy]).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aepfelbacher, M., R. Zumbihl, K. Ruckdeschel, C. A. Jacobi, C. Barz, and J. Heesemann. 1999. The tranquilizing injection of Yersinia proteins: a pathogen's strategy to resist host defence. J. Biol. Chem. 380:795-802. [DOI] [PubMed] [Google Scholar]
- 2.Balboa, M. A., J. Balsinde, C. A. Johnson, and A. Dennis. 1999. Regulation of arachidonic acid mobilization in lipopolysaccharide-activated P388D(1) macrophages by adenosine triphosphate. J. Biol. Chem. 274:36764-36768. [DOI] [PubMed] [Google Scholar]
- 3.Berchtold, S., A. L. J. Ogilvie, C. Bogdan, P. Mulh-Zurbes, A. Ogilvie, G. Schuler, and A. Steinkasserer. 1999. Human monocyte derived dendritic cells express functional P2x and P2y receptors as well as ecto-nucleotidase. FEBS Lett. 458:424-428. [DOI] [PubMed] [Google Scholar]
- 4.Byoung-Kuk, N., K. Jae-Chan, and S. Chul-Yong. 2001. Characterization and pathogenetic role of proteinase from Acanthamoeba castellanii. Microb. Pathog. 30:39-48. [DOI] [PubMed] [Google Scholar]
- 5.Clifford, E. E., K. A. Martin, P. Dalal, R. Thomas, and G. Dubyak. 1997. Stage-specific expression of P2y receptors, ecto-apyrase, and ecto-5′-nucleotidase in myeloid leukocytes. Am. J. Physiol. 273:C973-C987. [DOI] [PubMed] [Google Scholar]
- 6.Cohen G. M. 1997. Caspases: the executioners of apoptosis. Biochem. J. 326:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denecker, G., W. Declercq, C. A. Geuijen, A. Boland, R. Benabdillah, M. van Gurp, M. P. Sory, P. Vandenabeele, and G. R. Cornelis. 2001. Yersinia enterocolitica YopP-induced apoptosis of macrophages involved signaling cascade upstream of bid. J. Biol. Chem. 276:19706-19714. [DOI] [PubMed] [Google Scholar]
- 8.DiVirgilio, F. 1996. The P2z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol. Today 16:524-528. [DOI] [PubMed] [Google Scholar]
- 9.Dubyak, G. R., and C. El-Moatassim. 1993. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am. J. Physiol. 265(Pt. 1):C577-C606. [DOI] [PubMed] [Google Scholar]
- 10.Falzoni, S., M. Munerati, D. Ferrari, S. Spisani, S. Moretti, and F. DiVirgilio. 1995. The purinergic P2z receptor of human macrophage cell: characterization and possible physiological role. J. Clin. Investig. 95:1207-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrante, A., and T. J. Abell. 1986. Conditioned medium from stimulated mononuclear leukocytes augments human neutrophil-mediated killing of a virulent Acanthamoeba spp. Infect. Immun. 51:607-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrante, A. 1991. Immunity to Acanthamoeba. Rev. Infect. Dis. 13:S403-S409. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari, D., P. Chiozzi, S. Falzoni, M. Dal Susino, G. Collo, G. Buell, and F. DiVirgilio. 1997. ATP-mediated cytotoxicity in microglial cells. Neuropharmacology 36:1295-1301. [DOI] [PubMed] [Google Scholar]
- 14.Fleit, H. B., and C. D. Kobasiuk. 1991. The human monocyte-like cell line THP-1 express Fc gamma RI and Fc gamma RII. J. Leukoc. Biol. 49:556-565. [DOI] [PubMed] [Google Scholar]
- 15.Garcia, L. S., and D. A. Bruckner. 1993. Diagnostic medical parasitology, 2nd ed., p. 601-605. American Society for Microbiology, Washington, D.C.
- 16.Ghazizadeh, S., and H. B. Fleit. 1994. Tyrosine phosphorylation provides an obligatory early signal for FcγRII-mediated endocytosis in the monocytic cell line THP-1. J. Immunol. 152:30-41. [PubMed] [Google Scholar]
- 17.Grynkiewicz, G., M. Poenie, and R. J. Tsien. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440-3451. [PubMed] [Google Scholar]
- 18.He, Y. J., J. Y. Niederkorn, J. P. McCullay, G. L. Stewart, D. R. Meyer, R. Silvany, and J. Dougherty. 1990. In vivo and in vitro collagenolytic activity of Acanthamoeba castellanii. Investig. Ophthalmol. Visual Sci. 31:2235-2240. [PubMed] [Google Scholar]
- 19.Jin, J., V. R. Dasari, F. D. Sistare, and S. P. Kunapuli. 1998. Distribution of P2y receptor subtypes on haematopoietic cells. Br. J. Pharmacol. 123:789-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumaratilake, L. M., and A. Ferrante. 1998. Purification of human monocyte/macrophages by adherence to cytodex microcarriers. J. Immunol. Methods 112:183-190. [DOI] [PubMed] [Google Scholar]
- 21.Leher, H., R. Silvany, H. Alizadeh, J. Huang, and J. Y. Niererkorn. 1998. Mannose induces the release of cytopathic factors from Acanthamoeba castellanii. Infect. Immun. 66:5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marciano-Cabral, F., and D. M. Toney. 1998. The interaction of Acanthamoeba spp. with activated macrophages and with macrophage cell lines. J. Eukaryot. Microbiol. 45:452-458. [DOI] [PubMed] [Google Scholar]
- 23.Marciano-Cabral, F., R. Puffenbarger, and G. A. Cabral. 2000. The increasing importance of Acanthamoeba infections. J. Eukaryot. Microbiol. 47:29-36. [DOI] [PubMed] [Google Scholar]
- 24.Martinez, A. J., and G. S. Visvesvara. 1997. Free-living, amphizoic and opportunistic amoebas. Brain Pathol. 7:583-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masihi, K. N., C. Ranjan-Bhaduri, H. Werner, K. Janitschke, and W. Lange. 1986. Effects of muramyl dipeptide and trehalose dimycolate on resistance of mice to Toxoplasma gondii and Acanthamoeba culbertsoni infections. Int. Arch. Allergy Appl. Immunol. 81:112-117. [DOI] [PubMed] [Google Scholar]
- 26.Mattana, A., F. Bennardini, S. Usai, P. L. Fiori, F. Franconi, and P. Cappuccinelli. 1997. Acanthamoeba castellanii metabolites increase the intracellular calcium level and cause cytotoxicity in Wish cells. Microb. Pathog. 23:85-93. [DOI] [PubMed] [Google Scholar]
- 27.Mattana, A., M. G. Tozzi, M. Costa, G. Delogu, P. L. Fiori, and P. Cappuccinelli. 2001. By releasing ADP, Acanthamoeba castellanii causes an increase in the cytosolic free-calcium concentration and apoptosis in Wish cells. Infect. Immun. 69:4134-4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McPherson, P. S., and K. P. Campbell. 1993. The ryanodine receptor/Ca2+ release channel. J. Biol. Chem. 268:13765-13768. [PubMed] [Google Scholar]
- 29.Mitro, K., A. Bhagavathiammai, O. M. Zhou, G. Bobbet, J. H. McKerrow, R. Chokski, and B. Chokski. 1994. Partial characterization of the proteolytic secretions of Acanthamoeba polyphaga. Exp. Parasitol. 78:377-385. [DOI] [PubMed] [Google Scholar]
- 30.Naim, R., I. Yanagihara, T. Iida, and T. Honda. 2001. Vibrio parahaemolyticus thermostable direct haemolysin can induce an apoptotic cell death in Rat-1 cells from inside and outside of the cells. FEMS Microbiol. Lett. 195:237-244. [DOI] [PubMed] [Google Scholar]
- 31.Nuttle, L. C., and G. R. Dubyak. 1994. Differential activation of cation channels and non-selective pores by macrophage P2z purinergic receptors expressed in Xenopus oocytes. J. Biol. Chem. 269:13988-13996. [PubMed] [Google Scholar]
- 32.Park, K. V., and C. Y. Song. 1996. Purification and characterization of a serine proteinase from Acanthamoeba culbertsoni. J. Biochem. Mol. Biol. 29:455-461. [Google Scholar]
- 33.Pozzan, T., R. Rizzato, P. Volpe, and J. Meldolesi. 1994. Molecular and cellular physiology of intracellular calcium stores. Physiol. Rev. 74:595-636. [DOI] [PubMed] [Google Scholar]
- 34.Shin, H. J., M. S. Cho, H. I. Kim, M. Lee, S. Park, S. Sohn, and K. I. Im. 2000. Apoptosis of primary-culture rat microglial cells induced by pathogenic Acanthamoeba spp. Clin. Diagn. Lab. Immunol. 7:510-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin, H. J., M. S. Cho, S. Y. Jung, H. I. Kim, S. Park, J. H. Seo, J. C. Yoo, and K. I. Im. 2001. Cytopathic changes in rat microglial cells induced by pathogenic Acanthamoeba culbertsoni: morphology and cytokine release. Clin. Diagn. Lab. Immunol. 8:837-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solle, M., J. Labasi, D. G. Perregaux, E. Stam, N. Petrushova, B. H. Koller, R. J. Griffiths, and C. A. Gabel. 2001. Altered cytokine production in mice lacking P2x7 receptors. J. Biol. Chem. 276:125-132. [DOI] [PubMed] [Google Scholar]
- 37.Sperlagh, B., G. Hasko, Z. Nemeth, and E. S. Vizi. 1998. ATP released by LPS increases nitric oxide production in Raw 264.7 macrophage cell line via P2z/P2x7 receptors. Neurochem. Int. 33:209-215. [DOI] [PubMed] [Google Scholar]
- 38.Stewart, G. L., I. Kim, K. Shupe, H. Alizadeh, R. Silvany, A. P. McCulley, and J. Y. Niederkorn. 1992. Chemotactic response of macrophages to Acanthamoeba castellanii antigen and antibody-dependent macrophage-mediated killing of the parasite. J. Parasitol. 78:849-855. [PubMed] [Google Scholar]
- 39.Surprenant, A., F. Rassendren, E. Kawashima, R. A. North, and G. Buell. 1996. The cytolytic P2z receptor for extracellular ATP identified as a P2x receptor (P2x7). Science 272:735-740. [DOI] [PubMed] [Google Scholar]
- 40.Toney, D. M., and F. Marciano-Cabral. 1998. Resistance of Acanthamoeba species to complement lysis. J. Parasitol. 84:338-344. [PubMed] [Google Scholar]
- 41.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic cell line (THP-1). Int. J. Cancer 26:171-176. [DOI] [PubMed] [Google Scholar]
- 42.Van Klink, F., W. M. Taylor, H. Alizadeh, M. J. Jager, M. Van Rooijen, and J. Y. Niederkorn. 1996. The role of macrophages in Acanthamoeba keratitis. Investig. Ophthalmol. Vis. Sci. 37:1271-1281. [PubMed] [Google Scholar]
- 43.Vey, E., J. Z. Zhang, and J. M. Dayer. 1992. IFN-γ and 1,25(OH)2D3 induce on THP-1 cells distinct patterns of cell surface antigen expression, cytokine production, and responsiveness to contact with activated T cells. J. Immunol. 149:2040-2046. [PubMed] [Google Scholar]
- 44.Von Kugelgen, I., and A. Wetter. 2000. Molecular pharmacology of P2y receptors. Arch. Pharmacol. 362:310-323. [DOI] [PubMed] [Google Scholar]
- 45.Warny, M., S. Aboudola, S. C. Robson, J. Sévigny, D. Communi, S. P. Soltoff, and P. K. Ciaran. 2001. P2y6 nucleotide receptor mediates monocyte interleukin-8 production in response to UDP or lipopolysaccharide. J. Biol. Chem. 276:26051-26056. [DOI] [PubMed] [Google Scholar]
- 46.Zychlinsky, A., and P. J. Sansonetti. 1997. Apoptosis as a pro-inflammatory event or, what we can learn from bacterial induced cell death. Trends Microbiol. 5:201-204. [DOI] [PubMed] [Google Scholar]