Abstract
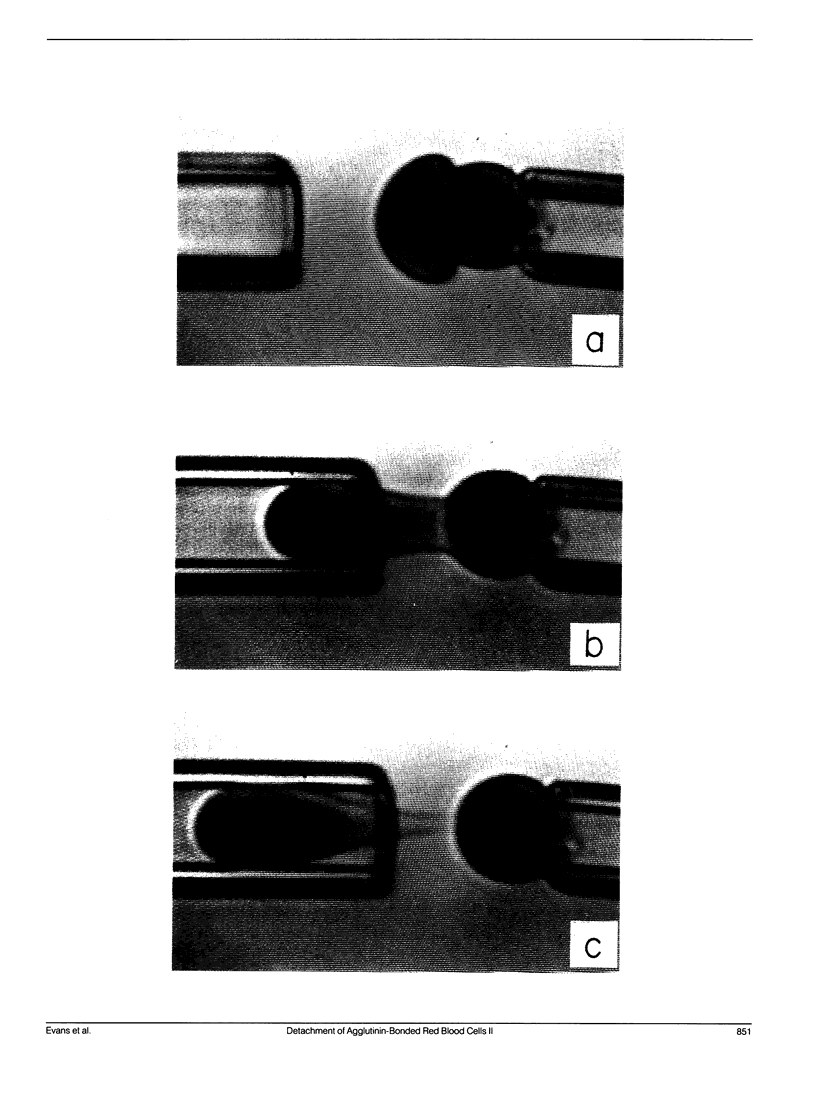
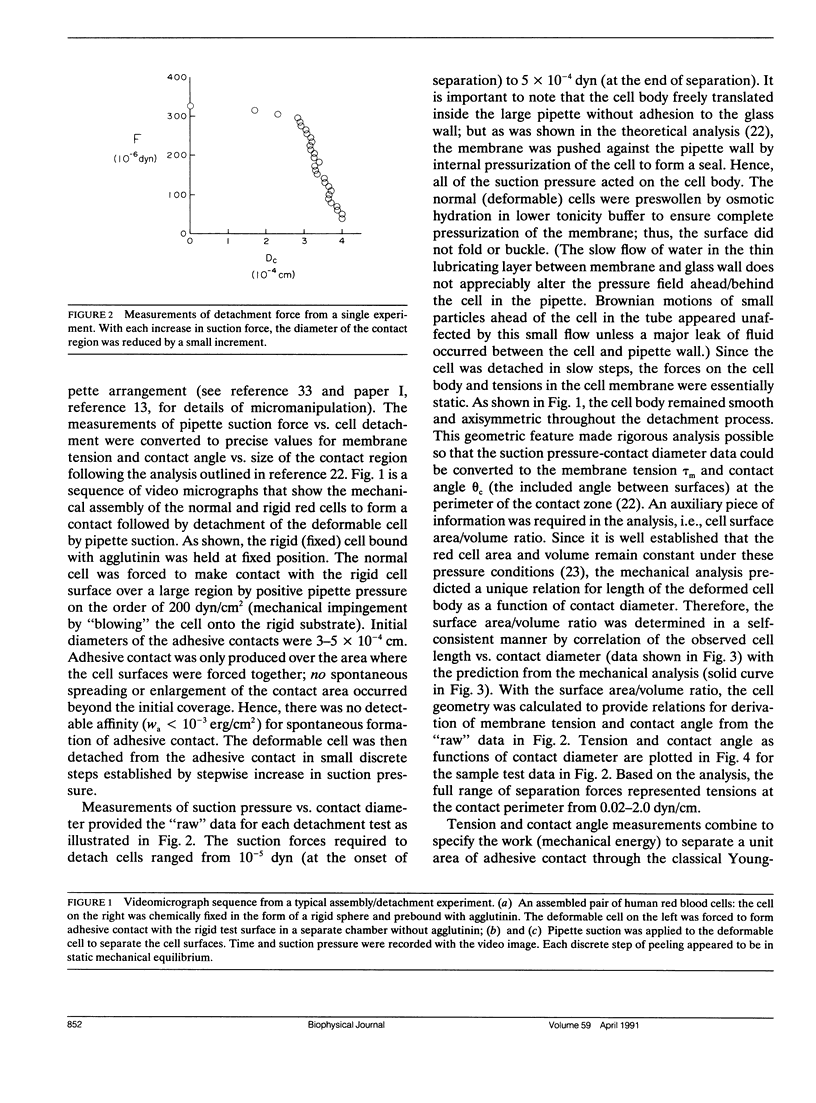
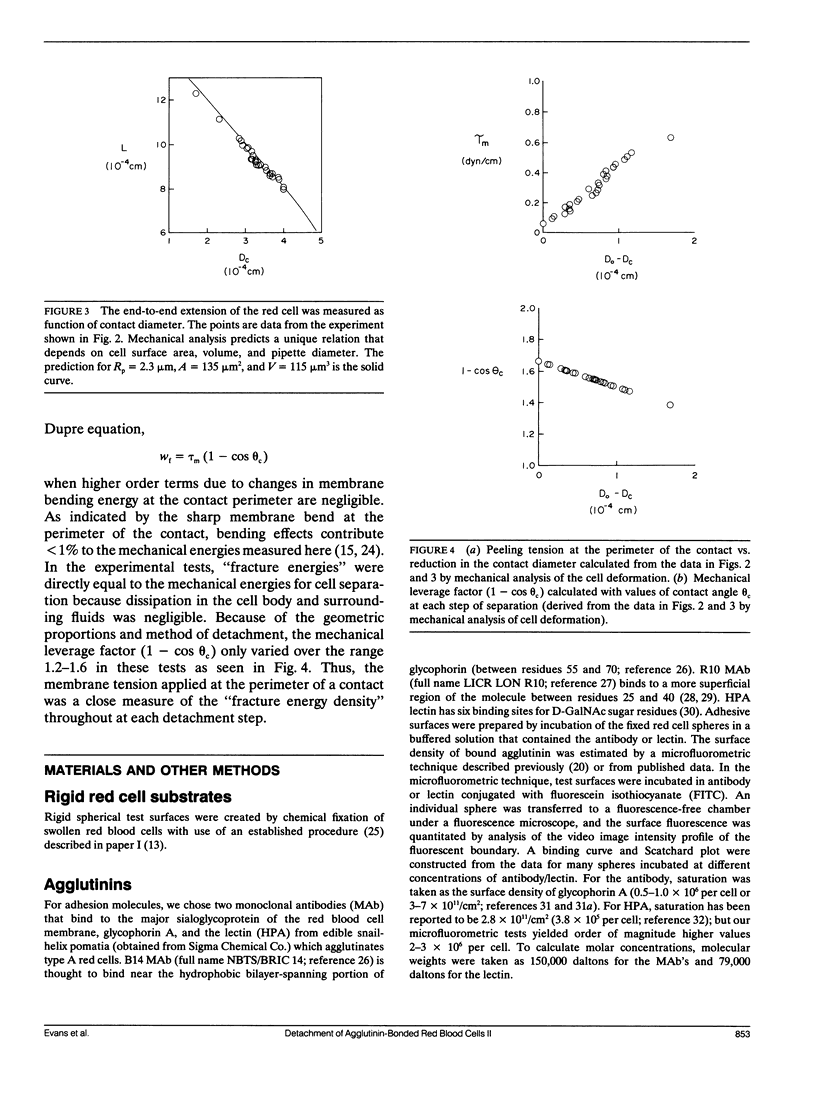
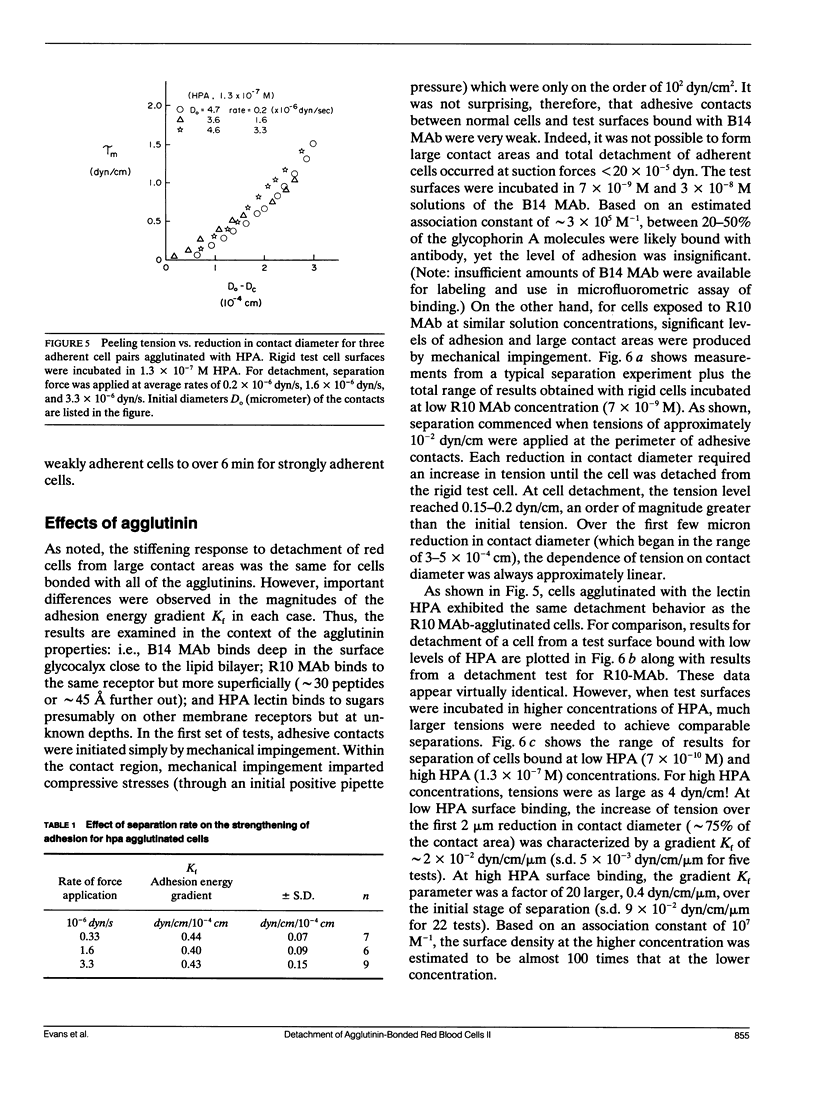
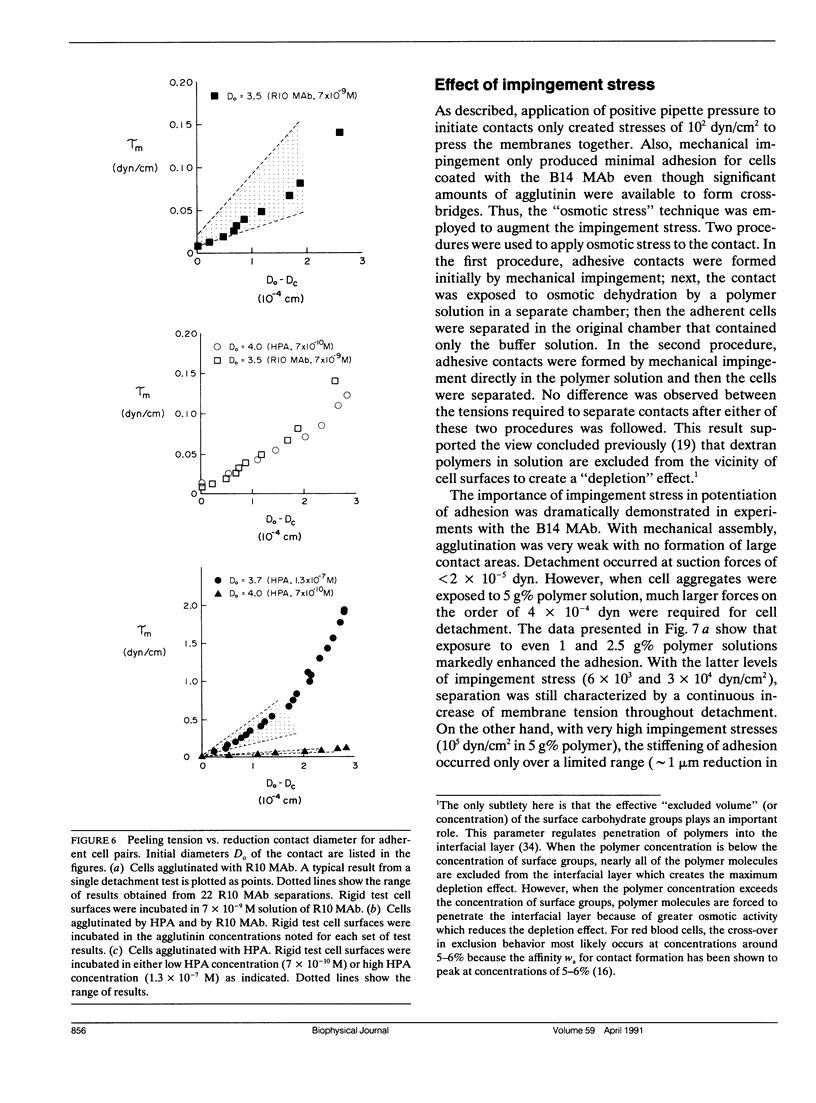
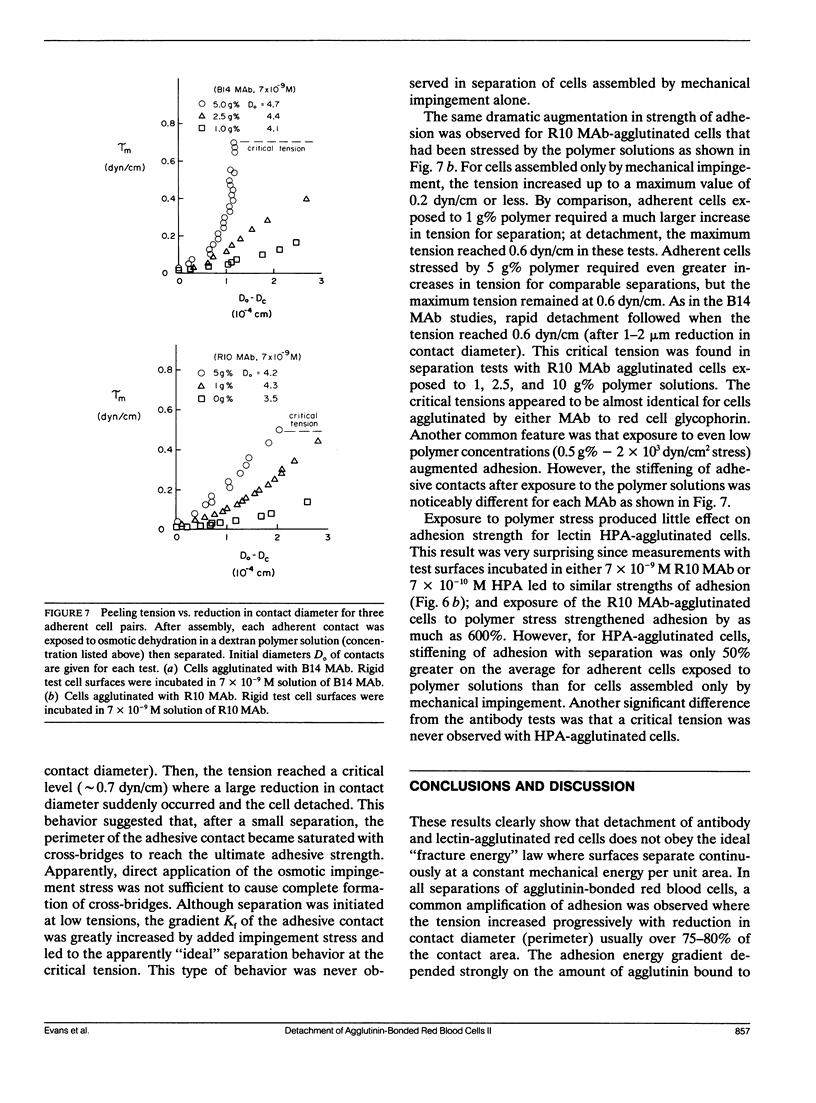
As detailed in a companion paper (Berk, D., and E. Evans. 1991. Biophys. J. 59:861-872), a method was developed to quantitate the strength of adhesion between agglutinin-bonded membranes without ambiguity due to mechanical compliance of the cell body. The experimental method and analysis were formulated around controlled assembly and detachment of a pair of macroscopically smooth red blood cell surfaces. The approach provides precise measurement of the membrane tension applied at the perimeter of an adhesive contact and the contact angle theta c between membrane surfaces which defines the mechanical leverage factor (1-cos theta c) important in the definition of the work to separate a unit area of contact. Here, the method was applied to adhesion and detachment of red cells bound together by different monoclonal antibodies to red cell membrane glycophorin and the snail-helix pomatia-lectin. For these tests, one of the two red cells was chemically prefixed in the form of a smooth sphere then equilibrated with the agglutinin before the adhesion-detachment procedure. The other cell was not exposed to the agglutinin until it was forced into contact with the rigid cell surface by mechanical impingement. Large regions of agglutinin bonding were produced by impingement but no spontaneous spreading was observed beyond the forced contact. Measurements of suction force to detach the deformable cell yielded consistent behavior for all of the agglutinins: i.e., the strength of adhesion increased progressively with reduction in contact diameter throughout detachment. This tension-contact diameter behavior was not altered over a ten-fold range of separation rates. In special cases, contacts separated smoothly after critical tensions were reached; these were the highest values attained for tension. Based on measurements reported in another paper (Evans et al. 1991. Biophys. J. 59:838-848) of the forces required to rupture molecular-point attachments, the density of cross-bridges was estimated with the assumption that the tension was proportional to the discrete rupture force x the number of attachments per unit length. These estimates showed that only a small fraction of agglutinin formed cross-bridges at initial assembly and increased progressively with separation. When critical tension levels were reached, it appeared that nearly all local agglutinin was involved as cross-bridges. Because one cell surface was chemically fixed, receptor accumulation was unlikely; thus, microscopic "roughness" and steric repulsion probably modulated formation of cross-bridges on initial contact. To counter the steric repulsion, adhesive contacts were exposed to solutions of a high molecular weight polymer to draw the surfaces together by osmotic dehydration of the adhesion gap. These stresses exceeded initial mechanical assembly stresses by up to three orders of magnitude. As expected, the strength of adhesion was greatly enhanced by the added impingement stress.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anstee D. J., Edwards P. A. Monoclonal antibodies to human erythrocytes. Eur J Immunol. 1982 Mar;12(3):228–232. doi: 10.1002/eji.1830120311. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Dembo M., Bongrand P. Cell adhesion. Competition between nonspecific repulsion and specific bonding. Biophys J. 1984 Jun;45(6):1051–1064. doi: 10.1016/S0006-3495(84)84252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I. Models for the specific adhesion of cells to cells. Science. 1978 May 12;200(4342):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Berk D., Evans E. Detachment of agglutinin-bonded red blood cells. III. Mechanical analysis for large contact areas. Biophys J. 1991 Apr;59(4):861–872. doi: 10.1016/S0006-3495(91)82298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigbee W. L., Vanderlaan M., Fong S. S., Jensen R. H. Monoclonal antibodies specific for the M- and N-forms of human glycophorin A. Mol Immunol. 1983 Dec;20(12):1353–1362. doi: 10.1016/0161-5890(83)90166-9. [DOI] [PubMed] [Google Scholar]
- Buxbaum K., Evans E., Brooks D. E. Quantitation of surface affinities of red blood cells in dextran solutions and plasma. Biochemistry. 1982 Jun 22;21(13):3235–3239. doi: 10.1021/bi00256a032. [DOI] [PubMed] [Google Scholar]
- Coakley W. T., Hewison L. A., Tilley D. Interfacial instability and the agglutination of erythrocytes by polylysine. Eur Biophys J. 1985;13(2):123–130. doi: 10.1007/BF00256532. [DOI] [PubMed] [Google Scholar]
- Dembo M., Torney D. C., Saxman K., Hammer D. The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc R Soc Lond B Biol Sci. 1988 Jun 22;234(1274):55–83. doi: 10.1098/rspb.1988.0038. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules. Science. 1983 Feb 4;219(4584):450–457. doi: 10.1126/science.6823544. [DOI] [PubMed] [Google Scholar]
- Edwards P. A. Monoclonal antibodies that bind to the human erythrocyte-membrane glycoproteins glycophorin A and Band 3 [proceedings]. Biochem Soc Trans. 1980 Jun;8(3):334–335. doi: 10.1042/bst0080334. [DOI] [PubMed] [Google Scholar]
- Evans E. A. Detailed mechanics of membrane-membrane adhesion and separation. I. Continuum of molecular cross-bridges. Biophys J. 1985 Jul;48(1):175–183. doi: 10.1016/S0006-3495(85)83770-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A. Detailed mechanics of membrane-membrane adhesion and separation. II. Discrete kinetically trapped molecular cross-bridges. Biophys J. 1985 Jul;48(1):185–192. doi: 10.1016/S0006-3495(85)83771-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A. Minimum energy analysis of membrane deformation applied to pipet aspiration and surface adhesion of red blood cells. Biophys J. 1980 May;30(2):265–284. doi: 10.1016/S0006-3495(80)85093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Berk D., Leung A. Detachment of agglutinin-bonded red blood cells. I. Forces to rupture molecular-point attachments. Biophys J. 1991 Apr;59(4):838–848. doi: 10.1016/S0006-3495(91)82296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Buxbaum K. Affinity of red blood cell membrane for particle surfaces measured by the extent of particle encapsulation. Biophys J. 1981 Apr;34(1):1–12. doi: 10.1016/S0006-3495(81)84834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Leung A. Adhesivity and rigidity of erythrocyte membrane in relation to wheat germ agglutinin binding. J Cell Biol. 1984 Apr;98(4):1201–1208. doi: 10.1083/jcb.98.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Metcalfe M. Free energy potential for aggregation of mixed phosphatidylcholine/phosphatidylserine lipid vesicles in glucose polymer (dextran) solutions. Biophys J. 1984 Apr;45(4):715–720. doi: 10.1016/S0006-3495(84)84213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Jokinen M., Andersson L. C. Expression of the major red cell sialoglycoprotein, glycophorin A, in the human leukemic cell line K562. J Biol Chem. 1979 Aug 10;254(15):7442–7448. [PubMed] [Google Scholar]
- Greig R. G., Brooks D. E. Shear-induced concanavalin A agglutination of human erythrocytes. Nature. 1979 Dec 13;282(5740):738–739. doi: 10.1038/282738a0. [DOI] [PubMed] [Google Scholar]
- Hammarström S., Kabat E. A. Studies on specificity and binding properties of the blood group A reactive hemagglutinin from Helix pomatia. Biochemistry. 1971 Apr 27;10(9):1684–1692. doi: 10.1021/bi00785a028. [DOI] [PubMed] [Google Scholar]
- Hammer D. A., Lauffenburger D. A. A dynamical model for receptor-mediated cell adhesion to surfaces. Biophys J. 1987 Sep;52(3):475–487. doi: 10.1016/S0006-3495(87)83236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgwell K., Tanner M. J., Anstee D. J. The Wrb antigen, a receptor for Plasmodium falciparum malaria, is located on a helical region of the major membrane sialoglycoprotein of human red blood cells. Biochem J. 1983 Jan 1;209(1):273–276. doi: 10.1042/bj2090273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalak R., Zarda P. R., Jan K. M., Chien S. Mechanics of Rouleau formation. Biophys J. 1981 Sep;35(3):771–781. doi: 10.1016/S0006-3495(81)84826-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung K. L., Sung L. A., Crimmins M., Burakoff S. J., Chien S. Determination of junction avidity of cytolytic T cell and target cell. Science. 1986 Dec 12;234(4782):1405–1408. doi: 10.1126/science.3491426. [DOI] [PubMed] [Google Scholar]
- Sung L. A., Kabat E. A., Chien S. Interaction of lectins with membrane receptors on erythrocyte surfaces. J Cell Biol. 1985 Aug;101(2):646–651. doi: 10.1083/jcb.101.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tha S. P., Shuster J., Goldsmith H. L. Interaction forces between red cells agglutinated by antibody. II. Measurement of hydrodynamic force of breakup. Biophys J. 1986 Dec;50(6):1117–1126. doi: 10.1016/S0006-3495(86)83556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozeren A., Sung K. L., Chien S. Theoretical and experimental studies on cross-bridge migration during cell disaggregation. Biophys J. 1989 Mar;55(3):479–487. doi: 10.1016/S0006-3495(89)82841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]



