Abstract
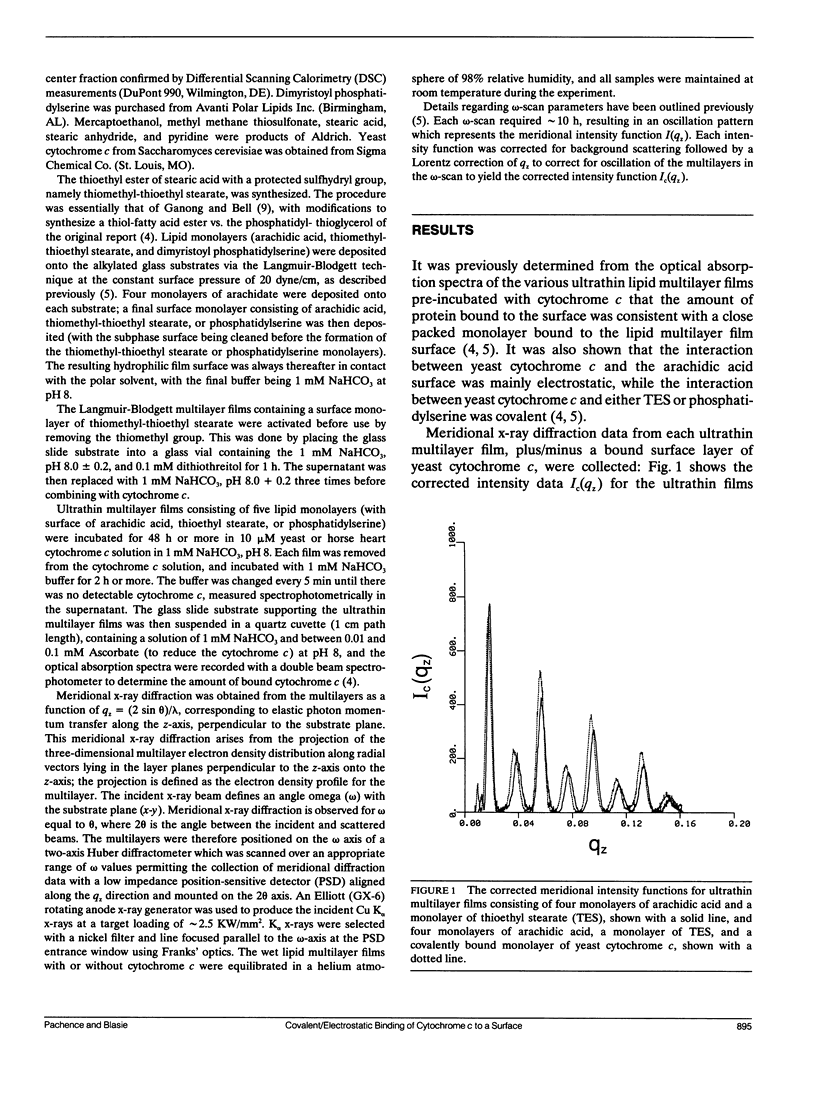
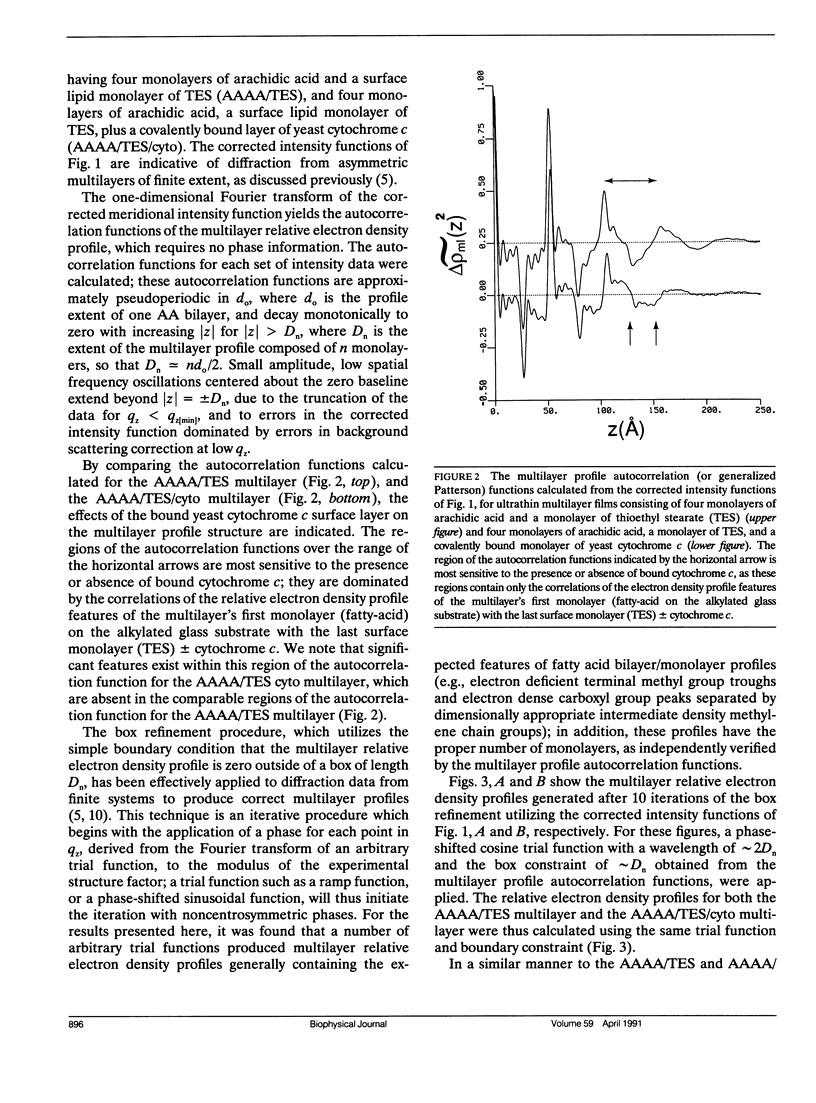
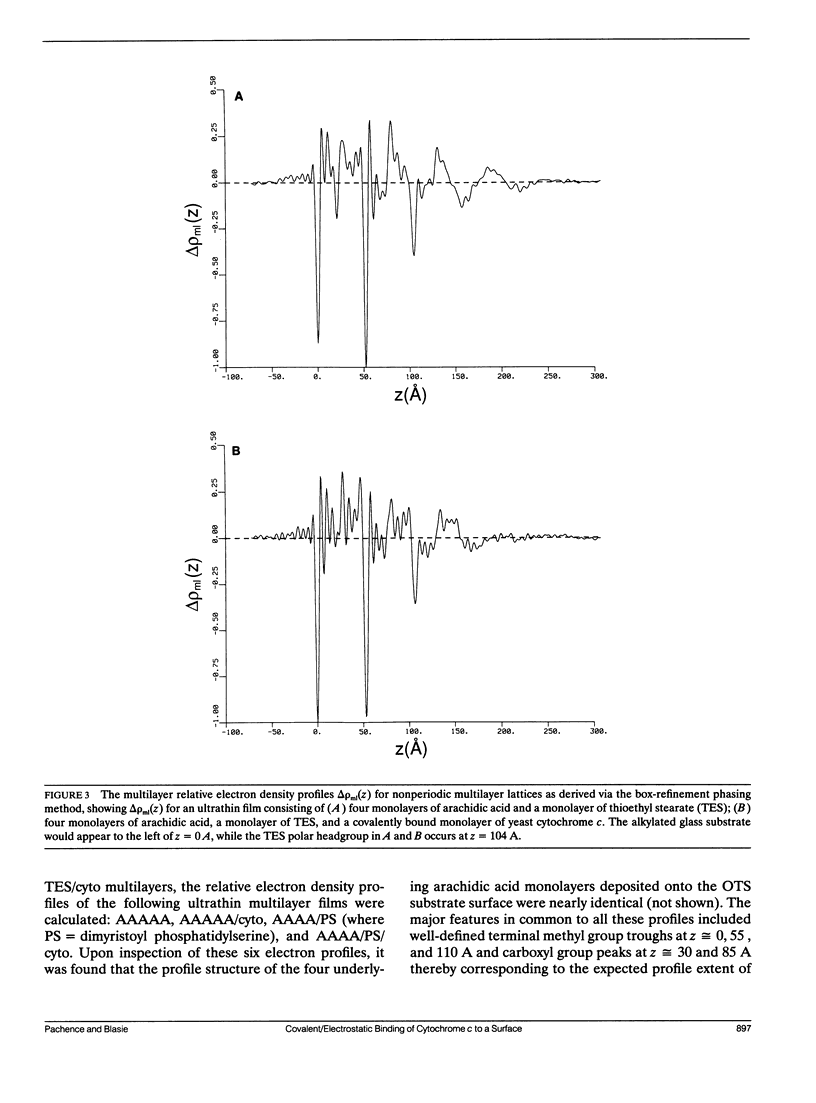
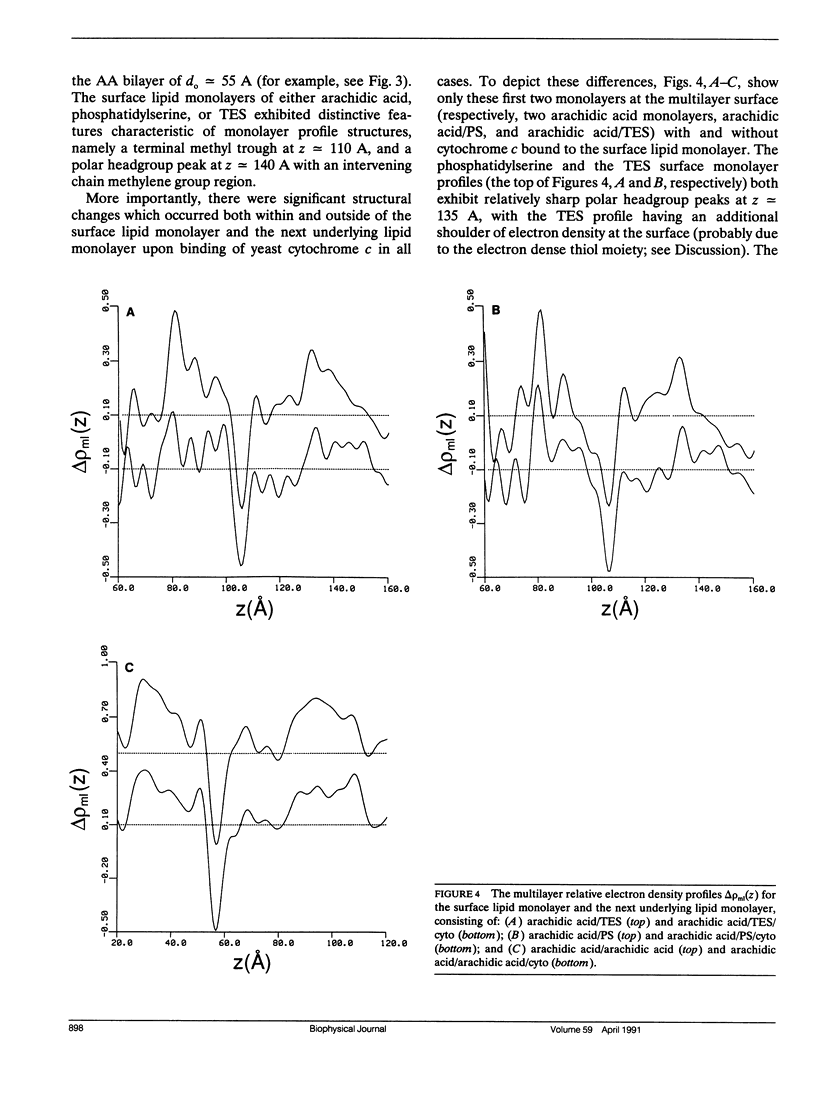
X-Ray diffraction was used to characterize the profile structures of ultrathin lipid multilayers having a bound surface layer of cytochrome c. The lipid multilayers were formed on an alkylated glass surface, using the Langmuir-Blodgett method. The ultrathin lipid multilayers of this study were: five monolayers of arachidic acid, four monolayers of arachidic acid with a surface monolayer of dimyristoyl phosphatidylserine, and four monolayers of arachidic acid acid with a surface monolayer of thioethyl stearate. Both the phosphatidylserine and the thioethyl stearate surfaces were found previously to covalently bind yeast cytochrome c, while the arachidic acid surface electrostatically binds yeast cytochrome c. Meridional x-ray diffraction data were collected from these lipid multilayer films with and without a bound yeast cytochrome c surface layer. A box refinement technique, previously shown to be effective in deriving the profile structures of ultrathin multilayer lipid films with and without electrostatically bound cytochrome c, was used to determine the multilayer electron density profiles. The surface monolayer of bound cytochrome c was readily apparent upon comparison of the multilayer electron density profiles for the various pairs of ultrathin multilayer films plus/minus cytochrome c for all cases. In addition, cytochrome c binding to the multilayer surface significantly perturbs the underlying lipid monolayers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ganong B. R., Bell R. M. Transmembrane movement of phosphatidylglycerol and diacylglycerol sulfhydryl analogues. Biochemistry. 1984 Oct 9;23(21):4977–4983. doi: 10.1021/bi00316a023. [DOI] [PubMed] [Google Scholar]
- Louie G. V., Hutcheon W. L., Brayer G. D. Yeast iso-1-cytochrome c. A 2.8 A resolution three-dimensional structure determination. J Mol Biol. 1988 Jan 20;199(2):295–314. doi: 10.1016/0022-2836(88)90315-4. [DOI] [PubMed] [Google Scholar]
- Nicholls P. Cytochrome c binding to enzymes and membranes. Biochim Biophys Acta. 1974 Dec 30;346(3-4):261–310. doi: 10.1016/0304-4173(74)90003-2. [DOI] [PubMed] [Google Scholar]
- Pachence J. M., Amador S., Maniara G., Vanderkooi J., Dutton P. L., Blasie J. K. Orientation and lateral mobility of cytochrome c on the surface of ultrathin lipid multilayer films. Biophys J. 1990 Aug;58(2):379–389. doi: 10.1016/S0006-3495(90)82384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachence J. M., Blasie J. K. The location of cytochrome c on the surface of ultrathin lipid multilayer films using x-ray diffraction. Biophys J. 1987 Nov;52(5):735–747. doi: 10.1016/S0006-3495(87)83268-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachence J. M., Fischetti R. F., Blasie J. K. Location of the heme-Fe atoms within the profile structure of a monolayer of cytochrome c bound to the surface of an ultrathin lipid multilayer film. Biophys J. 1989 Aug;56(2):327–337. doi: 10.1016/S0006-3495(89)82679-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P. J., Dawson R. M. Interactions of cytochrome c and [14C]. Biochem J. 1969 Oct;115(1):65–75. doi: 10.1042/bj1150065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley G. G., Leslie R. B., Chapman D. X-ray diffraction study of the interaction of phospholipids with cytochrome c in the aqueous phase. Nature. 1969 May 10;222(5193):561–562. doi: 10.1038/222561a0. [DOI] [PubMed] [Google Scholar]
- Skita V, V, Filipkowski M, Garito AF, Blasie JK. Profile structures of very thin multilayers by x-ray diffraction using direct and refinement methods of analysis. Phys Rev B Condens Matter. 1986 Oct 15;34(8):5826–5837. doi: 10.1103/physrevb.34.5826. [DOI] [PubMed] [Google Scholar]


