Abstract
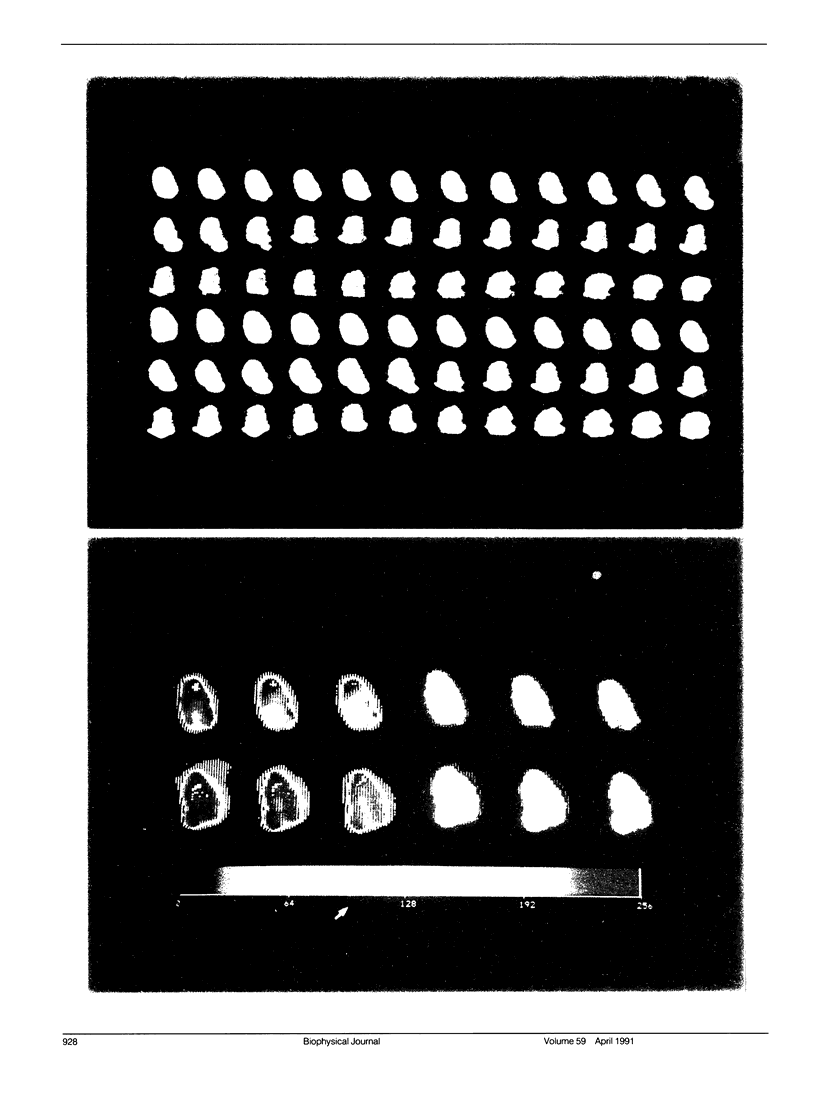
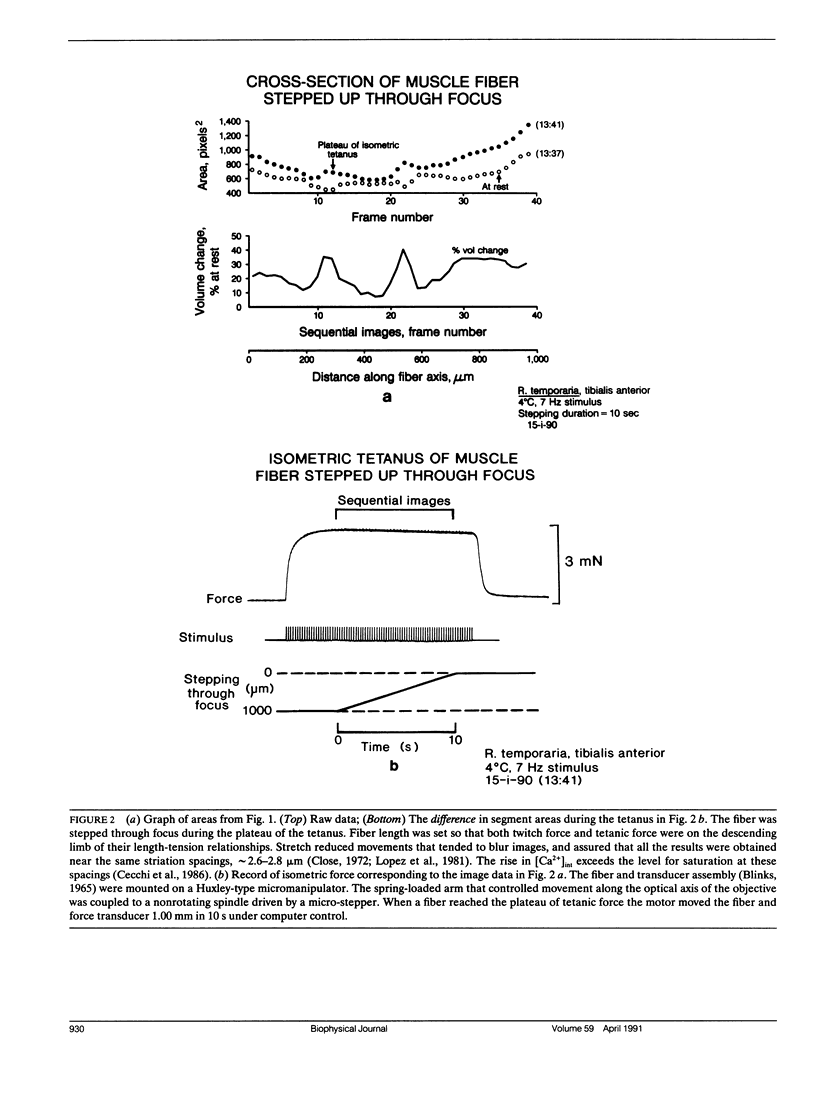
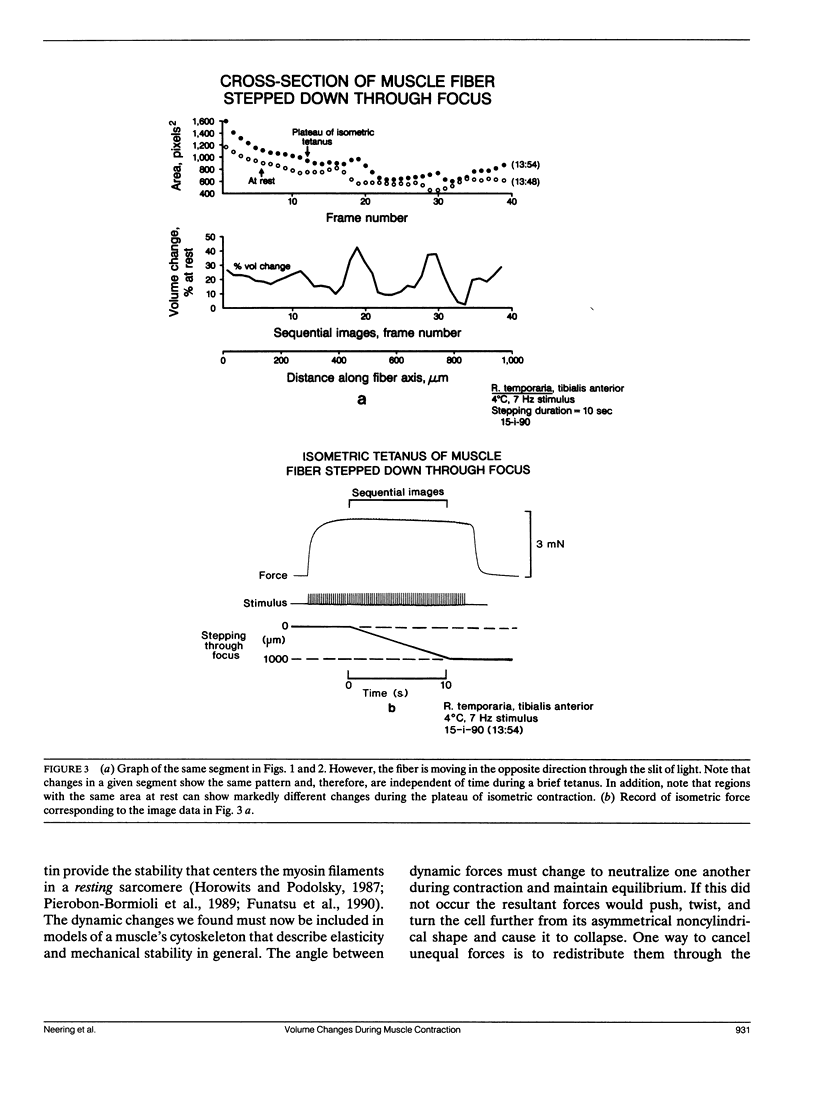
We measured dynamic changes in volume during contraction of live, intact frog skeletal muscle fibers through a high-speed, intensified, digital-imaging microscope. Optical cross-sections along the axis of resting cells were scanned and compared with sections during the plateau of isometric tetanic contractions. Contraction caused an increase in volume of the central third of a cell when axial force was maximum and constant and the central segment was stationary or lengthened slightly. But changes were unequal along a cell and not predicted by a cell's resting area or shape (circularity). Rapid local adjustments in the cytoskeletal evidently keep forces in equilibrium during contraction of living skeletal muscle. These results also show that optical signals may be distorted by nonuniform volume changes during contraction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin R. J., Paolini P. J. Muscle volume changes. J Gen Physiol. 1966 Jan;49(3):387–404. doi: 10.1085/jgp.49.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton K., Zagotta W. N., Baskin R. J. Sarcomere length behaviour along single frog muscle fibres at different lengths during isometric tetani. J Muscle Res Cell Motil. 1989 Feb;10(1):67–84. doi: 10.1007/BF01739857. [DOI] [PubMed] [Google Scholar]
- Close R. I. The relations between sarcomere length and characteristics of isometric twitch contractions of frog sartorius muscle. J Physiol. 1972 Feb;220(3):745–762. doi: 10.1113/jphysiol.1972.sp009733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Reggiani C. Redistribution of sarcomere length during isometric contraction of frog muscle fibres and its relation to tension creep. J Physiol. 1984 Jun;351:169–198. doi: 10.1113/jphysiol.1984.sp015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. J., Goldstein M. A., Schroeter J. P., Sass R. L. The Z-band lattice in skeletal muscle in rigor. J Ultrastruct Mol Struct Res. 1989 Apr;102(1):59–65. doi: 10.1016/0889-1605(89)90033-5. [DOI] [PubMed] [Google Scholar]
- Elzinga G., Howarth J. V., Rall J. A., Wilson M. G., Woledge R. C. Variation in the normalized tetanic force of single frog muscle fibres. J Physiol. 1989 Mar;410:157–170. doi: 10.1113/jphysiol.1989.sp017526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANZINI-ARMSTRONG C., PORTER K. R. THE Z DISC OF SKELETAL MUSCLE FIBRILS. Z Zellforsch Mikrosk Anat. 1964;61:661–672. doi: 10.1007/BF00342617. [DOI] [PubMed] [Google Scholar]
- Funatsu T., Higuchi H., Ishiwata S. Elastic filaments in skeletal muscle revealed by selective removal of thin filaments with plasma gelsolin. J Cell Biol. 1990 Jan;110(1):53–62. doi: 10.1083/jcb.110.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M. A., Michael L. H., Schroeter J. P., Sass R. L. Structural states in the Z band of skeletal muscle correlate with states of active and passive tension. J Gen Physiol. 1988 Jul;92(1):113–119. doi: 10.1085/jgp.92.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M. A., Michael L. H., Schroeter J. P., Sass R. L. Z band dynamics as a function of sarcomere length and the contractile state of muscle. FASEB J. 1987 Aug;1(2):133–142. doi: 10.1096/fasebj.1.2.3609610. [DOI] [PubMed] [Google Scholar]
- Horowits R., Podolsky R. J. The positional stability of thick filaments in activated skeletal muscle depends on sarcomere length: evidence for the role of titin filaments. J Cell Biol. 1987 Nov;105(5):2217–2223. doi: 10.1083/jcb.105.5.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J. A., Zećevic D., Cohen L. B. Simultaneous monitoring of activity of many neurons from invertebrate ganglia using a multielement detecting system. Soc Gen Physiol Ser. 1986;40:115–131. [PubMed] [Google Scholar]
- Lopez J. R., Wanek L. A., Taylor S. R. Skeletal muscle: length-dependent effects of potentiating agents. Science. 1981 Oct 2;214(4516):79–82. doi: 10.1126/science.6974399. [DOI] [PubMed] [Google Scholar]
- Lännergren J. Contractile properties and myosin isoenzymes of various kinds of Xenopus twitch muscle fibres. J Muscle Res Cell Motil. 1987 Jun;8(3):260–273. doi: 10.1007/BF01574594. [DOI] [PubMed] [Google Scholar]
- Magid A., Reedy M. K. X-ray diffraction observations of chemically skinned frog skeletal muscle processed by an improved method. Biophys J. 1980 Apr;30(1):27–40. doi: 10.1016/S0006-3495(80)85074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magid A., Ting-Beall H. P., Carvell M., Kontis T., Lucaveche C. Connecting filaments, core filaments, and side-struts: a proposal to add three new load-bearing structures to the sliding filament model. Adv Exp Med Biol. 1984;170:307–328. doi: 10.1007/978-1-4684-4703-3_26. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Goldman Y. E., Simmons R. M. Changes in the lateral filament spacing of skinned muscle fibres when cross-bridges attach. J Mol Biol. 1984 Feb 15;173(1):15–33. doi: 10.1016/0022-2836(84)90401-7. [DOI] [PubMed] [Google Scholar]
- Maughan D. W., Godt R. E. Stretch and radial compression studies on relaxed skinned muscle fibers of the frog. Biophys J. 1979 Dec;28(3):391–402. doi: 10.1016/S0006-3495(79)85188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierobon-Bormioli S., Betto R., Salviati G. The organization of titin (connectin) and nebulin in the sarcomeres: an immunocytolocalization study. J Muscle Res Cell Motil. 1989 Dec;10(6):446–456. doi: 10.1007/BF01771820. [DOI] [PubMed] [Google Scholar]
- Rowe R. W. Ultrastructure of the Z line of skeletal muscle fibers. J Cell Biol. 1971 Dec;51(3):674–685. doi: 10.1083/jcb.51.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. S., Ovalle W. K., Jr Varieties of fast and slow extrafusal muscle fibres in amphibian hind limb muscles. J Anat. 1973 Oct;116(Pt 1):1–24. [PMC free article] [PubMed] [Google Scholar]
- Street S. F. Lateral transmission of tension in frog myofibers: a myofibrillar network and transverse cytoskeletal connections are possible transmitters. J Cell Physiol. 1983 Mar;114(3):346–364. doi: 10.1002/jcp.1041140314. [DOI] [PubMed] [Google Scholar]
- Taylor S. R., Rüdel R., Blinks J. R. Calcium transients in amphibian muscle. Fed Proc. 1975 Apr;34(5):1379–1381. [PubMed] [Google Scholar]
- Wang K. Cytoskeletal matrix in striated muscle: the role of titin, nebulin and intermediate filaments. Adv Exp Med Biol. 1984;170:285–305. doi: 10.1007/978-1-4684-4703-3_25. [DOI] [PubMed] [Google Scholar]