Abstract
Infection with Helicobacter pylori is chronic despite a vigorous cellular and humoral immune response and causes severe pathology in some patients. In this study, phage display was used as a new approach in order to investigate the role of the host's humoral immune response in the pathogenesis of H. pylori gastritis. Human monoclonal single-chain Fv (scFv) antibody fragments against H. pylori cell lysate and the H. pylori urease were isolated from an immune phage display library, constructed from peripheral blood lymphocytes of an H. pylori-infected patient. After affinity selection, 23% of the clones tested showed binding activity against a lysate of the H. pylori Sydney strain in enzyme-linked immunosorbent assay (ELISA) and 9% bound the H. pylori urease. Further characterization by PCR-fingerprint analysis and sequencing revealed that two closely related H. pylori binders and one antiurease scFv could be isolated. The selected scFvs were highly specific as analyzed by ELISA and immunoblots using various bacterial lysates and recombinant proteins. Analysis of the humoral immune response following H. pylori infection using human monoclonal antibodies might contribute to a better understanding of the pathogenesis of the disease. Moreover, using immune phage display libraries, it might be possible for relevant epitopes of H. pylori antigens to be determined, which might be of use for vaccine development.
Infection with Helicobacter pylori is the major cause of chronic gastritis in humans and is associated with several gastro-duodenal diseases, such as gastric and duodenal ulceration, gastric atrophy, gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue (MALT)-type lymphoma.
During the past few years it became evident that it is not only certain bacterial virulence factors, such as the vacuolating cytotoxin VacA and some of the products of the Cag pathogenicity island (Cag PAI), that determine the pathogenesis of the infection. It has been shown that differences in the hosts' immune responses are responsible for the various inflammatory patterns in the gastric mucosa and for the development of certain clinical complications of the infection. There is increasing evidence that a predominant T-helper-1 response seems to lead to a more aggressive course of infection, while a predominant T-helper-2 response might be protective for the gastric mucosa (12, 26). Furthermore, about 30% of H. pylori-infected patients develop autoantibodies that are reactive with human gastric antigens, such as the H+,K+-ATPase. This H. pylori-associated antigastric autoimmunity has been shown to correlate with atrophy of gastric mucosa (6, 10, 24). Therefore, further analysis of the humoral immune response following H. pylori infection might give new insight into the pathogenesis of H. pylori gastritis. So far, investigation of the humoral immune response has focused on the analysis of the polyclonal repertoire in human sera and/or mucosa. A more detailed insight into humoral immunity in H. pylori infection could be achieved if monoclonal antibodies against H. pylori antigens could be generated and characterized.
However, to the best of our knowledge human monoclonal antibodies against H. pylori antigens have been established only by one research group (16, 30). This might be due to the labor-intensive and time-consuming hybridoma technique. However, during the last few years a new method for the generation of human single-chain Fv (scFv) antibody fragments in bacteria has been developed and optimized (3, 20, 21). Instead of immortalizing human B cells for the production of monoclonal antibodies, the genes coding for the variable regions of the heavy and light chains of human immunoglobulins (V genes) are amplified by reverse transcription-PCR and cloned into Escherichia coli (XL1-blue). For the generation of antibody fragments of a desired specificity, the amplified V-genes are expressed on the surface of filamentous phage M13, and antigen-binding scFvs are isolated by affinity selection.
Human scFvs against several relevant antigens have already been generated and have brought new insight into the pathogenesis of various disorders, such as neoplastic (14, 25), infectious (2), and autoimmune diseases (7, 13). So far, only one human scFv against H. pylori has been described (17). However, the V-gene repertoire used in that study was derived from uninfected donors (29). Therefore, the aim of our study was to construct an immune V-gene library using peripheral blood lymphocytes (PBLs) of an H. pylori-infected patient and to prove that this immune V-gene repertoire is suitable for the generation of human scFvs against H. pylori antigens. Two different antigens were used: first, a lysate of the H. pylori Sydney strain (19), and second, the recombinant H. pylori urease, which is known to be a major immunogen and was used in several vaccination trials (22).
MATERIALS AND METHODS
Assay of donor serum for presence of anti-H. pylori antibodies.
Sera of 21 patients with upper abdominal complaints were screened for antibodies against H. pylori using a standard enzyme-linked immunosorbent assay (ELISA) test (Pyloriset; Orion, Espoo, Finland) according to the manufacturer's instructions. Additionally, H. pylori infection was tested by routine histological analysis of gastric biopsy specimens using hematoxylin-eosin (H&E) and Warthin-Starry stains.
cDNA synthesis and PCR amplification of human V genes.
Peripheral blood mononuclear cells were purified from 10 ml of peripheral blood that was taken from the H. pylori-infected patient with the highest anti-H. pylori antibody titer. This 86-year-old female patient had chronic active and antrum-predominant gastritis. Poly(A)+ RNA was isolated from the isolated cells using the Oligotex Direct mRNA Kit (Qiagen, Hilden, Germany). Eluted RNA (10-μl samples) was used for oligo(dT)12-18-primed first-strand cDNA synthesis with the reverse transcriptase Superscript II (Life Technologies, Karlsruhe, Germany) according to the supplier's protocol. The variable parts of the heavy chains (VH) and light chains (VL) of human immunoglobulin genes (V genes) were then amplified by PCR using a set of 15 primers that was previously described (31). Reaction mixtures (50 μl) were prepared containing 1 μl of cDNA, 20 pmol each of forward and back primers, 200 μM concentrations of deoxynucleoside triphosphates, 5 μl of reaction buffer (10×), 1 mM MgCl2 and 0.2 μl (1 U) of DNA polymerase (HotGoldstar; Eurogentec, Cologne, Germany). The reactions were overlaid with mineral oil and subjected to 30 cycles of 94°C for 1 min (denaturation), 55°C for 1 min (annealing), and 72°C for 1 min (extension). Amplified products (1 μl) were added to a second PCR mixture using homologous oligonucleotides with appended restriction sites. The second PCR was performed for 20 cycles with an annealing temperature of 57°C. After amplification, the VH, Vκ, and Vλ products were purified from 2% (wt/vol) agarose gels with the Gel Extraction kit (Qiagen).
Cloning of the scFv gene repertoire.
The purified light chains (Vκ and Vλ) were digested with MluI and NotI (MBI Fermentas, St. Leon-Rot, Germany). After 4 h of incubation at 37°C, the enzymes were inactivated at 65°C (20 min), followed by purification of digested DNA with the PCR Purification kit (Qiagen). Approximately 15 ng of digested Vκ or Vλ (respectively) was then ligated into 65 ng of phagemid surface expression vector pSEX81 (4) in 20-μl ligation mixtures with 1 U of phage T4 DNA ligase (MBI Fermentas) by an overnight incubation at 16°C. The ligated DNA was purified by ethanol precipitation, resuspended in 10 μl of water, and electroporated into 40 μl of E. coli XL1-blue (Stratagene, Amsterdam, The Netherlands) according to the supplier's instructions (2,500 V, 201 Ω, 25 μF, 5 ms). After 1 h of incubation in SOC medium (20 g of tryptone, 5 g of yeast extract, 10 mM NaCl, and 2.5 mM KCl per liter; after autoclaving, 10 ml of 1 M MgCl2 and 10 ml of 1 M MgSO4 are added before use) containing 20 mM glucose at 37°C and 260 rpm, transformed bacteria were plated on three, 145-mm-diameter SOB-GA agar plates (SOB medium contains, per liter: 20 g of tryptone, 5 g of yeast extract, and 0.5 g of NaCl; after autoclaving, 10 ml of 1 M MgCl2 is added before use) containing 100 mM glucose and 100 μg of ampicillin/ml. After overnight growth at 30°C, the bacteria were scraped off the agar plates into YT-GA medium (17 g of tryptone, 10 g of yeast extract, and 5 g of NaCl per liter) containing 100 mM glucose and 100 μg of ampicillin/ml and phagemid DNA containing the (κ or λ) light chains was prepared using the Qiafilter Plasmid Maxi kit (Qiagen). For cloning of the heavy chains, the purified phagemid DNA and VH products were digested with NcoI and HindIII (MBI Fermentas, St. Leon-Rot, Germany), ligated, and electroporated into XL1-blue as described. Bacteria were then plated on SOB-GA agar plates and harvested after overnight growth at 30°C, representing two phage display sublibraries (κ-sub and λ-sub) of human scFv antibody fragments.
Phage rescue.
For preparation of recombinant, antibody-presenting phages from the phagemid library, harvested bacteria were used to inoculate 400 ml of SB-GAT medium (30 g of tryptone, 20 g of yeast extract, and 10 g of morpholinepropanesulfonic acid per liter) containing 100 mM glucose, 100 μg of ampicillin/ml, and 10 μg of tetracycline/ml to an optical density at 600 nm (OD600) of ≈0.03 and grown for approximately 2 h. After reaching an OD600 of ≈0.1, M13 KO7 helper phage were added at a multiplicity of infection of 20 and the infected bacteria were incubated for 15 to 30 min at 37°C without agitation, followed by a 30- to 45-min incubation at 37°C with shaking at 260 rpm. After infection, the bacteria were pelleted, resuspended in the same volume of prewarmed SB-AKT medium (containing 100 μg of ampicillin/ml, 50 μg of kanamycin/ml, and 10 μg of tetracycline/ml) and grown overnight at 34°C. Finally, phage particles were purified and concentrated from the bacterial supernatant by precipitation with 1/5 volume polyethylene glycol solution (20% PEG 6000, 2.5 M NaCl) for 1 h on ice. The precipitated phagemids were centrifuged (20 min, 7,000 × g, 4°C) and resuspended in 4 ml of phage dilution buffer (10 mM Tris-HCl [pH 7.5], 20 mM NaCl, 2 mM EDTA). Following another brief centrifugation (5 min, 13,000 × g, 4°C) to remove cellular debris, the phage stocks were stored at 4°C until use.
Panning.
Immunotubes (Nunc, Wiesbaden, Germany) were coated with a lysate prepared from the Sydney strain of H. pylori (50 μg of total protein) or with 50 μg of recombinant H. pylori urease (Acambis, Cambridge, Mass.) in 2.5 ml of 50 mM Na2CO3 (pH 9.6) overnight at 4°C by rotating end over end. Immunotubes were also coated with buffer alone as a negative control. In the case of the H. pylori lysate, panning was performed separately with phages of either the κ or the λ sublibrary. For the H. pylori urease, a mix of phages from both sublibraries (κ-sub and λ-sub) was used. After three washes with phosphate-buffered saline (PBS), the coated tubes were blocked with 2% (wt/vol) dried skimmed milk in PBS (M-PBS) for 1 h at room temperature (RT). Rescued phage particles were also blocked in M-PBS (1012 PFU/5 ml) for 15 min at RT before being added to the immunotubes (2.5 ml/tube). Binding of the phages was carried out by end-over-end rotation for 30 min, and then the tubes were left undisturbed for another 1.5 h. Subsequently the tubes were washed 20 times with PBS containing 0.1% Tween 20 and 20 times with PBS before elution of bound phages by addition of 1 ml of 100 mM triethylamine (pH 11) for 5 min with gentle agitation. Eluted phages were neutralized immediately with 1 ml of 1 M Tris-HCl, pH 7.4, and stored at 4°C or used directly for reinfection of logarithmically growing E. coli XL1-blue (OD600, ≈0.4) in 20 ml of YT medium. This represents a 50- to 50,000-fold excess of bacteria depending on the number of panning rounds performed. Infected bacteria were pelleted, resuspended in 1 ml of medium, and plated on three YT-GA agar plates (145-mm diameter). After overnight growth at 37°C, bacteria were harvested as described and a phage rescue was performed in a 100-ml scale to produce phagemids for the next selection cycle. Five (lysate) or three (urease) consecutive rounds of panning were performed, and rescued phage particles of each round were finally tested for binding activity in a phage ELISA.
Phage ELISA.
Analysis of phage for binding to H. pylori antigens was performed on phage preparations (phage rescue) or bacterial supernatants containing phage from deep-well plates (small-scale phage rescue). Microtiter plates (Nunc) were coated with antigen in coating buffer (0.2 M Na2CO3-NaHCO3 [pH 9.6]) at a concentration of 1 μg per well by an overnight incubation at 4°C. After three washes with PBS, 1011 phages (or 100 μl of bacterial supernatant) per well were added and incubated for 2 h at RT. Binding of the phage particles to antigen was detected with the anti-M13 antibody B62-FE2 (Progen, Heidelberg, Germany), a mouse monoclonal antibody specific for the major coat protein pVIII, 10% (vol/vol) in M-PBS. After 1 h incubation at RT, the ELISA was developed with horseradish peroxidase-conjugated rabbit anti-mouse antibody (DAKO, Hamburg, Germany) in M-PBS for 1 h at RT and stained with o-phenylenediamine and hydrogen peroxide in citrate buffer (34 mM citric acid, 67 mM Na2HPO4). The peroxidase reaction was stopped with 10% H2SO4 and the optical density was measured at 490 nm. To assess the specificity of individual binders, plates were coated with various bacterial lysates (Campylobacter jejuni, Staphylococcus aureus, Salmonella enterica serovar Enteritidis, Escherichia coli, and eight clinical isolates of H. pylori) or recombinant H. pylori antigens (urease, cagA, flagellin A/B, alpA) and bovine serum albumin and phOx-bovine serum albumin as controls.
Small-scale phage rescue.
To identify binding scFvs from selected clones after the panning procedure, individual colonies resulting from reinfection of XL1-blue bacteria with eluted phages of the fifth round (Hp-SS lysate) or the third round (urease) were picked and cultivated overnight at 37°C in 500 μl of SB-GAT medium/well in deep-well plates (Beckman-Coulter, Unterschleissheim, Germany). The next day, cultures were diluted 1:100 into 500 μl of SB-GAT medium, grown for 3 h, and infected with helper phage (1010 CFU/well) as already described. After infection, the bacteria were resuspended in SB-AKT medium and grown overnight at 34°C. Finally, bacteria were pelleted and the supernatants of the cultures were used directly in a phage ELISA.
Western blot.
For Western blot analysis, antigens (1 μg of proteins or 30 μg [total protein] of bacterial lysates) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% polyacrylamide gels (18). The separated proteins were transferred to a nitrocellulose membrane and blocked for 1 h in M-TTBS (100 mM Tris-HCl [pH 7.5], 0.9% [wt/vol] NaCl) containing 4% (wt/vol) dried skimmed milk and 0.1% Tween 20 at RT. HB2151 bacteria transformed with the vector pOPE101 (28) containing the respective scFv cassette were induced to produce soluble scFvs containing a c-myc tag. Sonicates of these bacteria were used as primary antibody after a brief centrifugation (5 min, 13,000 × g) to remove cellular debris (dilution, 1:2 in M-TTBS). For detection of bound scFvs, the anti-c-myc antibody 9E10 (Ab-1; Calbiochem-Novabiochem, Bad Soden, Germany) and horseradish peroxidase-conjugated rabbit anti-mouse antibody were used. Finally, Western blots were developed with the SuperSignal substrate kit (Pierce, Bonn, Germany), according to the manufacturer's protocol. In addition, Western blots with proteinase K-digested lysates of H. pylori and E. coli were performed to examine whether bacterial lipopolysaccharide is recognized by the two isolated anti-H. pylori scFvs. For digestion of the proteins, 100 μg of proteinase K was added to the lysates (total protein, 90 μg) and incubated at 56°C for 2 h.
DNA fingerprinting and sequencing of clones.
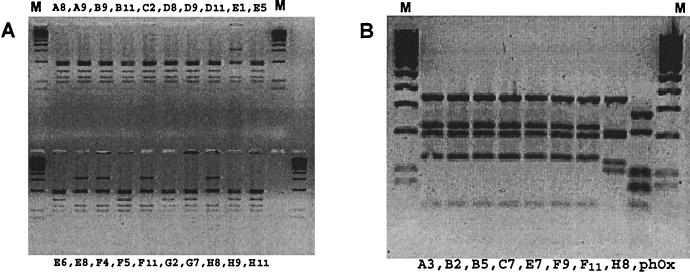
The diversity of the original library and of selected clones after panning was estimated by PCR screening. The scFv insert of individual clones was reamplified using primers lying 57 bp upstream (5′-ATT AAA GAG GAG AAA TTA ACC A-3′) and 65 bp downstream (5′-CTT TCC AGA CGT TAG TAA ATG-3′) of the scFv insert. After amplification, the 1,000-bp scFv-cassette was digested with the frequent cutting enzyme BstNI (MvaI; MBI Fermentas). The heavy and light chains of selected clones representing different restriction patterns (anti-H. pylori scFvs A9 and E8; antiurease scFv F11) (see Fig. 4) were sequenced in sense and antisense directions by the dideoxy chain termination method (27) with the BigDye Terminator Cycle Sequencing kit (Applied Biosystems, Weiterstadt, Germany) using a semiautomated sequencer, ABI 310 (Applied Biosystems).
FIG. 4.
Diversity of the isolated monoclonal scFvs. The scFv inserts of the isolated binders were screened for diversity by PCR-fingerprint analysis. All selected clones carried a 1,000-bp insert, and the inserts were digested with BstNI. The 20 H. pylori binders showed two different restriction patterns (A), and the eight anti-urease scFvs also had two distinct restriction patterns (B), indicating two genetically different clones in both cases. M, 100-bp molecular size marker; phOx, control with scFv against phenyloxazol-5-1-one.
RESULTS
Donor serum and generation of human V-gene repertoires.
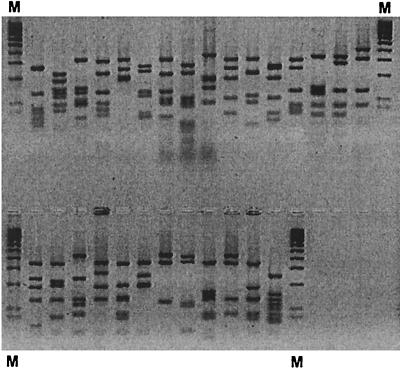
Antibody genes were derived from peripheral mononuclear cells of the patient with the highest anti-H. pylori antibody titer. Histologically, this patient had antrum-predominant, chronic active H. pylori gastritis. PCR products of the correct size of 400 bp (VH and Vλ) and 360 bp (Vκ) were obtained after amplification from first-strand cDNA. After cloning of the light-chain PCR products into the phagemid surface expression vector pSEX81, cDNA libraries of about 1 × 106 (κ) and 1.4 × 106 (λ) individual colonies were obtained. Subsequently, the VH products were subcloned into the purified phagemids containing the κ or λ repertoires, respectively. Titration and PCR screening indicated that two sublibraries (κ-sub and λ-sub) with approximately 106 members each were achieved. PCR-fingerprint analysis of 20 clones from each unselected sublibrary indicated that about 80% (κ-sub) and 60% (λ-sub) of the clones carried an insert of the correct size (1,000 bp), and the libraries appeared to be quite diverse as judged by the BstN1 restriction patterns (Fig. 1).
FIG. 1.
Characterization of the generated antibody library. The scFv inserts of 20 individual clones of each sublibrary (κ-sub and λ-sub) were reamplified, and PCR products of the correct size (1,000 bp) were digested with BstNI and analyzed on a 4% agarose gel. Sixteen of 20 clones (κ-sub) and 12 of 20 clones (λ-sub) carried an insert. The 16 upper lanes are digests of clones from the κ-sublibrary, and the 12 lower lanes are digests of clones from the λ-sublibrary. M, 100-bp molecular size marker.
Panning.
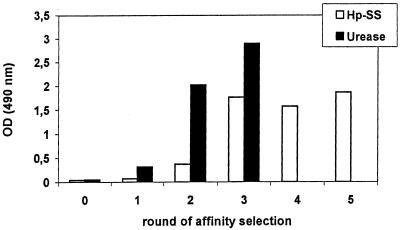
For selection of H. pylori-specific scFvs, phage particles were rescued from the κ sublibrary and subjected to five consecutive selection rounds on immobilized H. pylori lysate in immunotubes. There was a steady rise in the number of eluted phages after each round of selection. To analyze whether enrichment of H. pylori binding phages was achieved, phage preparations from the unselected library and from reinfected bacteria of each selection round were tested in a phage ELISA on the lysate of the H. pylori Sydney strain. A marked increase in anti-H. pylori binding activity was found after the third round of panning (Fig. 2).
FIG. 2.
Enrichment of binding phages during affinity selection. Five (H. pylori lysate) or three (urease) rounds of panning were performed with phages of the κ-sublibrary (lysate) or with phages of both sublibraries (urease). Subsequently phage particles of the unselected library (round 0) and of each selection round were tested for binding activity in a phage ELISA. A marked increase in binding affinity occurred after the third round (lysate) and after the second round (urease).
For selection of antiurease scFvs, three rounds of affinity selection were performed using phage particles of both sublibraries (κ-sub and λ-sub). Phagemids of the third round showed the highest level of reactivity against the H. pylori urease in a phage ELISA (Fig. 2).
Finally, our library was also screened for scFvs against the H. pylori antigens AlpA and HSP60. However, after five rounds of panning we could not find a significant increase in anti-AlpA or anti-HSP60 reactivity.
Isolation and characterization of monoclonal scFvs.
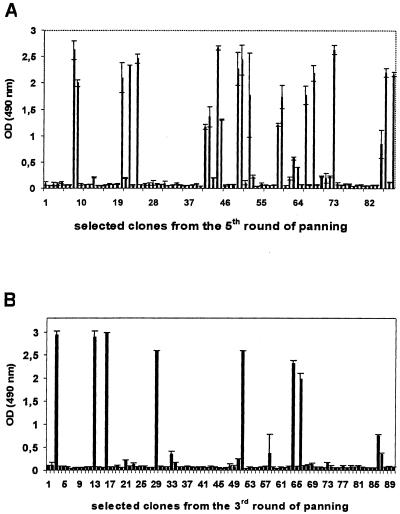
For isolation of monoclonal H. pylori-specific scFvs, 88 (lysate) or 90 (urease) single clones were selected from bacteria infected with phages from the fifth (lysate) or third (urease) round, respectively, of panning and tested for binding activity in ELISA after a small-scale phage rescue. Twenty of the eighty-eight clones (23%) could be identified which bound to the H. pylori lysate (Fig. 3A). Similar results were achieved with the λ-sublibrary (results not shown). Eight of the 90 clones (9%) bound to the H. pylori urease (Fig. 3B).
FIG. 3.
Isolation of monoclonal scFvs from enriched phages after affinity selection. Individual clones of the fifth (H. pylori lysate) or the third (urease) selection round were screened for binding activity in ELISA. Twenty of 88 clones (23%) bound to the H. pylori lysate (A), and 8 of 90 clones (9%) reacted with the H. pylori urease (B).
Fingerprint analysis of the 20 H. pylori-binding clones showed the presence of two different restriction patterns. Fifteen clones (80%) belonged to one restriction pattern, and five clones (20%) belonged to the other (Fig. 4A). PCR-fingerprinting of the eight urease binders also revealed two different restriction patterns. Here, seven clones belonged to one restriction pattern while only one clone belonged to the other (Fig. 4B). For further characterization of the isolated scFvs, two clones representing the different restriction patterns (A9 and E8) were randomly chosen from the 20 H. pylori binders.
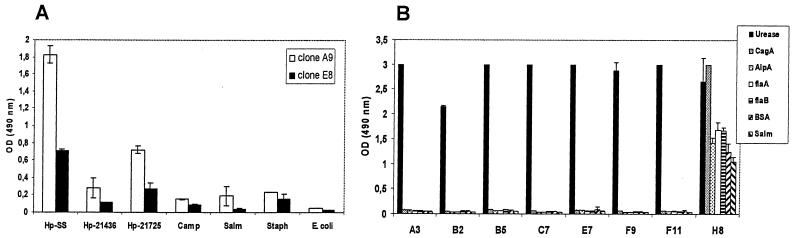
The specificity of the two clones A9 and E8 and of all eight antiurease scFvs was assessed in an ELISA using various bacterial lysates and recombinant H. pylori antigens (Fig. 5). The isolated binders were found to be highly specific for their respective antigens (Fig. 5). Only antiurease scFv H8, which had a different restriction pattern, showed considerable reactivity to all antigens tested (Fig. 5B) and was therefore not further characterized.
FIG. 5.
Specificity of the isolated binders. Clones representing the different restriction patterns were screened for specificity in an ELISA using various bacterial lysates and recombinant H. pylori antigens. (A) Both anti-H. pylori scFvs were highly specific, with clone E8 binding with a much lower affinity. (B) The urease binder H8, which showed a different restriction pattern, showed significant binding to all antigens tested and therefore was not further characterized by sequencing.
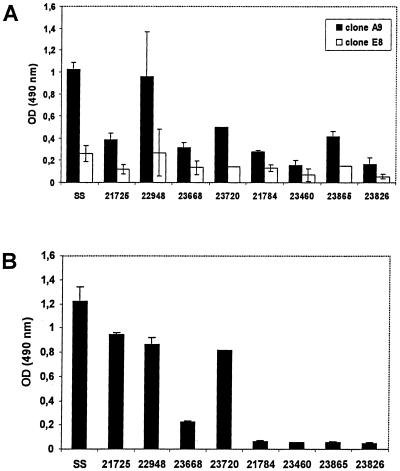
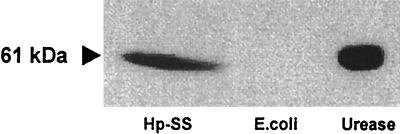
Interestingly, the isolated anti-H. pylori and antiurease clones demonstrated quite different immunoreactivities against various H. pylori strains (clinical isolates) in the ELISA (Fig. 6). The two clones A9 and E8 did not show a significant binding with several recombinant H. pylori antigens, such as HSP60, FlaA, FlaB, AlpA, and CagA. Also, Western blot analysis revealed no distinct bands for these two anti-H. pylori scFvs. To analyze whether these two clones react against the bacterial lipopolysaccharide, blotting experiments with proteinase K-digested lysates were performed. But no distinct bands were visible in these assays (data not shown). In contrast, the antiurease scFv F11 recognized specifically not only the large subunit of the recombinant H. pylori urease but also a distinct band of the same molecular weight in the H. pylori lysate. No reactivity was observed with a lysate of E. coli (Fig. 7).
FIG. 6.
Binding activity against different H. pylori strains. The anti-H. pylori and antiurease scFvs were screened for binding activity against different H. pylori strains (clinical isolates) in ELISA. Both H. pylori binders (A) and the antiurease scFv F11 (B) showed quite different immunoreactivities against the different H. pylori strains.
FIG. 7.
Western blot analysis. Antiurease scFv F11 not only bound to the recombinant H. pylori urease but also recognized a distinct band of the expected molecular mass (61 kDa) in the H. pylori lysate. No reactivity against a lysate of E. coli was observed.
Sequencing of selected clones.
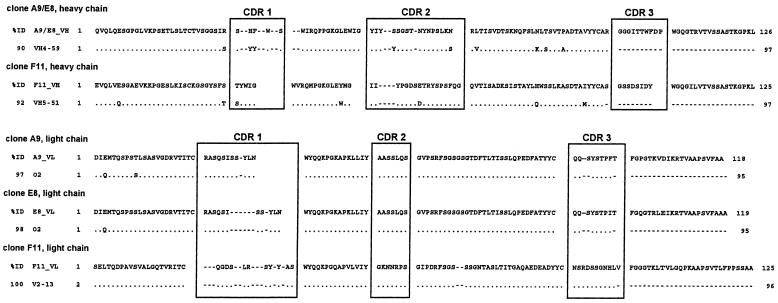
Sequencing of the H. pylori-binding clones representing the different restriction patterns (A9 and E8, respectively) indicated that the clones were closely related, with heavy and light chains derived from the same germ line V genes. The heavy chains belonged to the VH4 gene family, and the light chains belonged to the Vκ1 gene family. Sequence alignment using the Clustal software showed that the heavy chains of clones A9 and E8 were identical, whereas the light chains showed some mutations in framework 1, complementarity-determining region (CDR) 3, and especially framework region 4.
A sequence comparison with human germ line V genes was performed using the Ig-BLAST (http://www.ncbi.nlm.nih.gov/blast/index.html) and V-BASE (http://www.mrc-cpe.cam.ac.uk) databases. The heavy chains showed 94% identity to the germ line gene DP-71 (VH4-59; 90% identity for the deduced amino acid sequences), and the light chain sequences were most closely related to the germ line gene DPK9 (O2/O12), with 97 and 98% (A9 and E8) identity, respectively.
The antiurease scFv F11 used a heavy chain of the VH5 family and a lambda light chain of the VL3 family. Here, the most closely related human germ line V genes were DP-73 (VH5-51) for the heavy chain and DPL-16 (V2-13) for the lambda light chain (Fig. 8).
FIG. 8.
Sequence comparison with human germ line V genes. Sequence comparison of the deduced amino acid sequences of the isolated H. pylori binders (A9 and E8) and the antiurease scFv F11 revealed close homologies (between 90 and 100%) of all scFvs to their germ line V genes. The anti-H. pylori scFvs had identical heavy chains of the VH4 family which were most closely related to germ line gene DP-71 (VH4-59) and closely related kappa light chains with the highest homology to germ line gene DPK9 (O2/O12). The heavy chain of antiurease scFv F11 belonged to the VH5 family and was most closely related to germ line gene DP-73 (VH5-51). The lambda light chain (VL3 family) showed the highest homology (100%) to germ line gene DPL-16 (V2-13). % ID, identity to the germ line gene; dots denote sequence identity, and dashes denote gaps in the sequence.
The ratio of replacement mutations to silent mutations (R/S ratio) of the framework regions and the CDRs is commonly used as an indicator for affinity maturation of antibodies. The calculated R/S ratios of our scFvs (between 1 and 2) indicated that the isolated antibody fragments were not affinity matured by somatic hypermutation.
DISCUSSION
In this study we describe the construction of an immune cDNA library of human scFv antibody fragments from PBLs of an H. pylori-infected patient. Using phage display technology, we isolated human, monoclonal scFvs against H. pylori (Sydney strain) and against the H. pylori urease from this library. As analyzed by PCR-fingerprint restriction patterns and sequencing, two closely related H. pylori binders and one antiurease scFv could be isolated. These three scFvs showed specific patterns of reaction to their respective antigens when a variety of other bacterial strains and different H. pylori antigens were used. Thus, we have demonstrated the applicability of our immune antibody library for the generation of human scFvs against H. pylori antigens.
The only other human monoclonal antibodies against H. pylori (16, 30) were isolated from human spleen cells, immortalized by fusion to the heteromyeloma cell line HAB-1. The spleen cells were derived from stomach cancer patients in order to generate mitogenic antibodies against cancer cells. Each isolated antibody was found to be cross-reactive with H. pylori and showed a broad reactivity against H. pylori antigens in Western blot analysis. The molecular weight of the H. pylori antigens recognized by a single antibody lay between 30 and 40 kDa and 70 and 100 kDa, but the antigens were not further characterized in that study. The nature of the antigens recognized by our anti-Sydney strain, scFvs A9 and E8, could also not be precisely characterized. It was not possible to determine the size of the antigen in a Western blot, probably because the scFvs only recognize native antigen. As judged from the R/S ratios of the framework regions and the CDRs, and as indicated by the high homology (90 to 100%) to the germ line genes, our anti-H. pylori scFvs were also not affinity matured by somatic hypermutation. This might be due to our type of library, since PBLs are a poor source of blast cells (20).
In this study a scFv specific for the H. pylori urease (clone F11), which was also isolated from our immune scFv library, demonstrated specific binding in ELISA and Western blot analysis, not only to the recombinant protein but also to the urease in the H. pylori lysate of the Sydney strain. Human scFvs against the H. pylori urease have also been generated by Houimel et al. (17). Their four scFvs were selected from a large nonimmune phage display library (29). Interestingly, all of the four urease-specific scFvs used the same lambda light chain of the VL3 subgroup (DPL-16), which was also used by our antiurease scFv F11. The heavy chains used by the nonimmune antiurease scFvs were mainly of the VH3 family (seven of nine clones sequenced) or of the VH1 family (two of nine clones), while scFv F11 used a heavy chain of the VH5 family (VH5-51, DP-73). Unlike our scFv F11, which recognizes the large subunit (ureB; 61 kDa), all of the four other antiurease scFvs described by Houimel et al. reacted with the ureA subunit (26 kDa).
It is obvious that the scFvs generated in our study primarily represent the immune repertoire of the patient investigated. To prove whether our data are of general interest or apply only to our single patient, more experiments are needed, such as generation and screening of antibody libraries from more patients or competition experiments with sera from different patients.
Although we used an immune library, our specifically binding scFvs are not affinity matured. However, Finnern et al. has also described non-affinity-matured scFvs from an immune library which bound the target antigen (11). Furthermore, Hensel et al. used immunized spleen cells as the primary source for their anti-H. pylori monoclonal antibodies. But sequence analysis of the antibodies showed that they also were not affinity matured (16). Although the scFvs isolated from our library are not hypermutated, they might be of immunological value, particularly for the analysis of the natural immune response against H. pylori.
The development of vaccines against H. pylori becomes more and more important with the increasing problem of antibiotic resistance. H. pylori antigens, such as urease, have already been shown to be protective in vaccination studies (1). By the approach used in this study, it might be possible to specify the relevant epitopes on H. pylori urease which might be useful for vaccine development. Furthermore, the generation of anti-idiotype antibodies reactive against H. pylori-specific human scFvs might also represent an approach for further vaccination trials (17).
Additionally, anti-H. pylori scFvs might also be used in other treatment strategies. Cao et al. have described a murine anti-H. pylori scFv that was cloned from mouse hybridomas and recognized a 30-kDa surface protein (5). Their murine anti-H. pylori scFv inhibited growth of H. pylori in vitro and reduced colonization of the mouse stomach significantly.
Our earlier studies have shown that a considerable proportion of H. pylori-infected patients develop autoantibodies against gastric epitopes. The presence of these autoantibodies is associated with histological and clinical parameters of gastric atrophy (8). However, the pathogenesis of these autoantibodies is still controversial (9, 15, 23). Furthermore, the epitope specificity was determined for only 50% of these antigastric autoantibodies (6). Therefore, the generation of monoclonal antibodies would help to give more insight into the pathogenesis and specificity of these autoimmune responses during H. pylori infection.
In conclusion, phage display technology might be a promising approach for further analysis of the pathogenesis and treatment of H. pylori gastritis.
Acknowledgments
This study was financially supported by the Interdisciplinary Center for Clinical research at the University of Erlangen-Nuremberg (A5).
We thank Melvyn Little, Stefan Dübel (German Cancer Research Center, Heidelberg, Germany), and Peter Terness (Institute of Immunology, University of Heidelberg, Heidelberg, Germany) for providing the vectors pSEX81 and pOPE101. Roland Kontermann (Institute of Molecular Biology and Tumor Research, University of Marburg, Marburg, Germany) kindly provided HB2151 bacteria. We further thank Sebastian Suerbaum (Institut fur Hygiene und Mikrobiologie, University of Würzburg, Würzburg, Germany) for the recombinant H. pylori flagellins (flaA and flaB) and Matthias Peipp for helper phage M13KO7. Our special thanks go to Acambis (Cambridge, Mass.) for generously supplying us with recombinant H. pylori antigens (urease, AlpA, and CagA). Finally, we appreciate the excellent technical assistance of Sabine Dörr and Manuela Häfner.
Editor: R. N. Moore
REFERENCES
- 1.Banerjee, P., and P. Michetti. 2001. Development of a Helicobacter pylori vaccine. Horizon Scientific Press, Norfolk, Va.
- 2.Boel, E., H. Bootsma, J. de Kruif, M. Jansze, K. Klingman, H. van Dijk, and T. Logtenberg. 1998. Phage antibodies obtained by competitive selection on complement-resistant Moraxella (Branhamella) catarrhalis recognize the high-molecular-weight outer membrane protein. Infect. Immun. 66:83-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breitling, F., and S. Dübel. 1997. Rekombinante Antikörper. Spektrum, Akademischer Verlag, Berlin, Germany.
- 4.Breitling, F., S. Dubel, T. Seehaus, I. Klewinghaus, and M. Little. 1991. A surface expression vector for antibody screening. Gene 104:147-153. [DOI] [PubMed] [Google Scholar]
- 5.Cao, J., Y. Sun, T. Berglindh, B. Mellgard, Z. Li, B. Mardh, and S. Mardh. 2000. Helicobacter pylori-antigen-binding fragments expressed on the filamentous M13 phage prevent bacterial growth. Biochim. Biophys. Acta 1474:107-113. [DOI] [PubMed] [Google Scholar]
- 6.Claeys, D., G. Faller, B. J. Appelmelk, R. Negrini, and T. Kirchner. 1998. The gastric H+,K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology 115:340-347. [DOI] [PubMed] [Google Scholar]
- 7.de Wildt, R. M., R. Finnern, W. H. Ouwehand, A. D. Griffiths, W. J. van Venrooij, and R. M. Hoet. 1996. Characterization of human variable domain antibody fragments against the U1 RNA-associated A protein, selected from a synthetic and patient-derived combinatorial V gene library. Eur. J. Immunol. 26:629-639. [DOI] [PubMed] [Google Scholar]
- 8.Faller, G., and T. Kirchner. 2000. Role of antigastric autoantibodies in chronic Helicobacter pylori infection. Microsc. Res. Tech. 48:321-326. [DOI] [PubMed] [Google Scholar]
- 9.Faller, G., H. Steininger, B. Appelmelk, and T. Kirchner. 1998. Evidence of novel pathogenic pathways for the formation of antigastric autoantibodies in Helicobacter pylori gastritis. J. Clin. Pathol. 51:244-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faller, G., H. Steininger, J. Kranzlein, H. Maul, T. Kerkau, J. Hensen, E. Hahn, and T. Kirchner. 1997. Antigastric autoantibodies in Helicobacter pylori infection: implications of histological and clinical parameters of gastritis. Gut 41:619-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finnern, R., E. Pedrollo, I. Fisch, J. Wieslander, J. D. Marks, C. M. Lockwood, and W. H. Ouwehand. 1997. Human autoimmune anti-proteinase 3 scFv from a phage display library. Clin. Exp. Immunol. 107:269-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox, J. G., P. Beck, C. A. Dangler, M. T. Whary, T. C. Wang, H. N. Shi, and C. Nagler-Anderson. 2000. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat. Med. 6:536-542. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths, A. D., M. Malmqvist, J. D. Marks, J. M. Bye, M. J. Embleton, J. McCafferty, M. Baier, K. P. Holliger, B. D. Gorick, N. C. Hughes-Jones, et al. 1993. Human anti-self antibodies with high specificity from phage display libraries. EMBO J. 12:725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderikx, P., M. Kandilogiannaki, C. Petrarca, S. von Mensdorff-Pouilly, J. H. Hilgers, E. Krambovitis, J. W. Arends, and H. R. Hoogenboom. 1998. Human single-chain Fv antibodies to MUC1 core peptide selected from phage display libraries recognize unique epitopes and predominantly bind adenocarcinoma. Cancer Res. 58:4324-4332. [PubMed] [Google Scholar]
- 15.Heneghan, M. A., C. F. McCarthy, D. Janulaityte, and A. P. Moran. 2001. Relationship of anti-Lewis x and anti-Lewis y antibodies in serum samples from gastric cancer and chronic gastritis patients to Helicobacter pylori-mediated autoimmunity. Infect. Immun. 69:4774-4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hensel, F., C. Knorr, R. Hermann, V. Krenn, H. K. Muller-Hermelink, and H. P. Vollmers. 1999. Mitogenic autoantibodies in Helicobacter pylori-associated stomach cancerogenesis. Int. J. Cancer. 81:229-235. [DOI] [PubMed] [Google Scholar]
- 17.Houimel, M., I. Corthesy-Theulaz, I. Fisch, C. Wong, B. Corthesy, J. Mach, and R. Finnern. 2001. Selection of human single chain Fv antibody fragments binding and inhibiting Helicobacter pylori urease. Tumour Biol. 22:36-44. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 20.Marks, J. D., H. R. Hoogenboom, T. P. Bonnert, J. McCafferty, A. D. Griffiths, and G. Winter. 1991. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J. Mol. Biol. 222:581-597. [DOI] [PubMed] [Google Scholar]
- 21.McCafferty, J., A. D. Griffiths, G. Winter, and D. J. Chiswell. 1990. Phage antibodies: filamentous phage displaying antibody variable domains. Nature 348:552-554. [DOI] [PubMed] [Google Scholar]
- 22.Michetti, P., C. Kreiss, K. L. Kotloff, N. Porta, J. L. Blanco, D. Bachmann, M. Herranz, P. F. Saldinger, I. Corthesy-Theulaz, G. Losonsky, R. Nichols, J. Simon, M. Stolte, S. Ackerman, T. P. Monath, and A. L. Blum. 1999. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology 116:804-812. [DOI] [PubMed] [Google Scholar]
- 23.Negrini, R., L. Lisato, I. Zanella, L. Cavazzini, S. Gullini, V. Villanacci, C. Poiesi, A. Albertini, and S. Ghielmi. 1991. Helicobacter pylori infection induces antibodies cross-reacting with human gastric mucosa. Gastroenterology 101:437-445. [DOI] [PubMed] [Google Scholar]
- 24.Negrini, R., A. Savio, C. Poiesi, B. J. Appelmelk, F. Buffoli, A. Paterlini, P. Cesari, M. Graffeo, D. Vaira, and G. Franzin. 1996. Antigenic mimicry between Helicobacter pylori and gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology 111:655-665. [DOI] [PubMed] [Google Scholar]
- 25.Roovers, R. C., P. Henderikx, W. Helfrich, E. van der Linden, A. Reurs, A. P. de Bruine, J. W. Arends, L. de Leij, and H. R. Hoogenboom. 1998. High-affinity recombinant phage antibodies to the pan-carcinoma marker epithelial glycoprotein-2 for tumour targeting. Br. J. Cancer 78:1407-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saldinger, P. F., N. Porta, P. Launois, J. A. Louis, G. A. Waanders, H. Bouzourene, P. Michetti, A. L. Blum, and I. E. Corthesy-Theulaz. 1998. Immunization of BALB/c mice with Helicobacter urease B induces a T helper 2 response absent in Helicobacter infection. Gastroenterology 115:891-897. [DOI] [PubMed] [Google Scholar]
- 27.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmiedl, A., F. Breitling, C. H. Winter, I. Queitsch, and S. Dubel. 2000. Effects of unpaired cysteines on yield, solubility and activity of different recombinant antibody constructs expressed in E. coli. J. Immunol. Methods 242:101-114. [DOI] [PubMed] [Google Scholar]
- 29.Sheets, M., P. Amersdorfer, R. Finnern, P. Sargent, E. Lindquist, R. Schier, G. Hemingsen, C. Wong, J. Gerhart, J. Marks, and E. Lindqvist. 1998. Efficient construction of a large nonimmune phage antibody library: the production of high-affinity human single-chain antibodies to protein antigens. Proc. Natl. Acad. Sci. USA 95:6157-6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vollmers, H. P., J. Dammrich, H. Ribbert, S. Grassel, S. Debus, J. Heesemannn, and H. K. Muller-Hermelink. 1994. Human monoclonal antibodies from stomach carcinoma patients react with Helicobacter pylori and stimulate stomach cancer cells in vitro. Cancer 74:1525-1532. [DOI] [PubMed] [Google Scholar]
- 31.Welschof, M., P. Terness, F. Kolbinger, M. Zewe, S. Dubel, H. Dorsam, C. Hain, M. Finger, M. Jung, G. Moldenhauer, et al. 1995. Amino acid sequence based PCR primers for amplification of rearranged human heavy and light chain immunoglobulin variable region genes. J. Immunol. Methods 179:203-214. [DOI] [PubMed] [Google Scholar]