Abstract
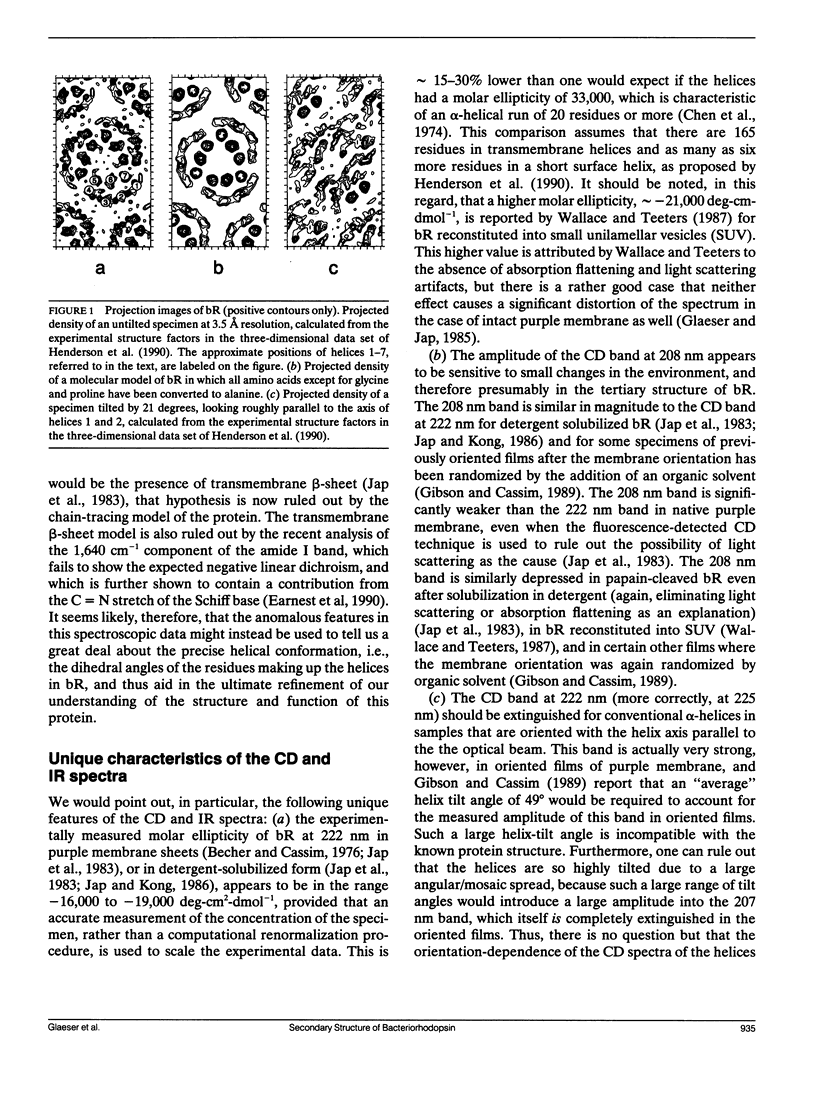
The recently published model of the structure of bacteriorhodopsin (bR), developed by fitting the peptide chain to a high-resolution, three-dimensional density map, rules out the existence of transmembrane beta-sheet and provides an accurate estimate of the helix content. The precise geometry of the dihedral angles in the helical regions of the polypeptide cannot yet be specified from the diffraction data, however. Published data on the circular dichroism (CD) spectrum between 190 and 240 nm, and the infrared (IR) spectrum in the amide I band suggest that the helical conformation in bR may be, for the most part, a rather unusual one. The precise structural model, which specifies the number of residues in transmembrane helices, can now be used as an additional constraint in seeking models of the helical conformation that are in quantitative agreement with the CD and IR spectroscopic data. Further spectroscopic measurements can also be used to determine whether there are changes in the unusual dihedral-angle conformation within the helices during the photocycle.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becher B., Cassim J. Y. Effects of light adaptation on the purple membrane structure of Halobacterium halobium. Biophys J. 1976 Oct;16(10):1183–1200. doi: 10.1016/S0006-3495(76)85767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Dencher N. A., Dresselhaus D., Zaccai G., Büldt G. Structural changes in bacteriorhodopsin during proton translocation revealed by neutron diffraction. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7876–7879. doi: 10.1073/pnas.86.20.7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draheim J. E., Cassim J. Y. Large Scale Global Structural Changes of the Purple Membrane during the Photocycle. Biophys J. 1985 Apr;47(4):497–507. doi: 10.1016/S0006-3495(85)83943-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnest T. N., Herzfeld J., Rothschild K. J. Polarized Fourier transform infrared spectroscopy of bacteriorhodopsin. Transmembrane alpha helices are resistant to hydrogen/deuterium exchange. Biophys J. 1990 Dec;58(6):1539–1546. doi: 10.1016/S0006-3495(90)82498-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnest T. N., Roepe P., Braiman M. S., Gillespie J., Rothschild K. J. Orientation of the bacteriorhodopsin chromophore probed by polarized Fourier transform infrared difference spectroscopy. Biochemistry. 1986 Dec 2;25(24):7793–7798. doi: 10.1021/bi00372a002. [DOI] [PubMed] [Google Scholar]
- Gibson N. J., Cassim J. Y. Nature of forces stabilizing the transmembrane protein bacteriorhodopsin in purple membrane. Biophys J. 1989 Oct;56(4):769–780. doi: 10.1016/S0006-3495(89)82724-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser R. M., Baldwin J., Ceska T. A., Henderson R. Electron diffraction analysis of the M412 intermediate of bacteriorhodopsin. Biophys J. 1986 Nov;50(5):913–920. doi: 10.1016/S0006-3495(86)83532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser R. M., Jap B. K. Absorption flattening in the circular dichroism spectra of small membrane fragments. Biochemistry. 1985 Nov 5;24(23):6398–6401. doi: 10.1021/bi00344a012. [DOI] [PubMed] [Google Scholar]
- Haris P. I., Chapman D. Fourier transform infrared spectra of the polypeptide alamethicin and a possible structural similarity with bacteriorhodopsin. Biochim Biophys Acta. 1988 Aug 18;943(2):375–380. doi: 10.1016/0005-2736(88)90571-8. [DOI] [PubMed] [Google Scholar]
- Hayward S. B., Stroud R. M. Projected structure of purple membrane determined to 3.7 A resolution by low temperature electron microscopy. J Mol Biol. 1981 Sep 25;151(3):491–517. doi: 10.1016/0022-2836(81)90007-3. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Holloway P. W., Mantsch H. H. Structure of cytochrome b5 in solution by Fourier-transform infrared spectroscopy. Biochemistry. 1989 Feb 7;28(3):931–935. doi: 10.1021/bi00429a002. [DOI] [PubMed] [Google Scholar]
- Jap B. K., Kong S. H. Secondary structure of halorhodopsin. Biochemistry. 1986 Jan 28;25(2):502–505. doi: 10.1021/bi00350a034. [DOI] [PubMed] [Google Scholar]
- Jap B. K., Maestre M. F., Hayward S. B., Glaeser R. M. Peptide-chain secondary structure of bacteriorhodopsin. Biophys J. 1983 Jul;43(1):81–89. doi: 10.1016/S0006-3495(83)84326-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimm S., Dwivedi A. M. Infrared spectrum of the purple membrane: clue to a proton conduction mechanism? Science. 1982 Apr 23;216(4544):407–408. doi: 10.1126/science.6280277. [DOI] [PubMed] [Google Scholar]
- Leifer D., Henderson R. Three-dimensional structure of orthorhombic purple membrane at 6.5 A resolution. J Mol Biol. 1983 Jan 25;163(3):451–466. doi: 10.1016/0022-2836(83)90068-2. [DOI] [PubMed] [Google Scholar]
- Ormos P. Infrared spectroscopic demonstration of a conformational change in bacteriorhodopsin involved in proton pumping. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):473–477. doi: 10.1073/pnas.88.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande C., Callender R., Henderson R., Pande A. Purple membrane: color, crystallinity, and the effect of dimethyl sulfoxide. Biochemistry. 1989 Jul 11;28(14):5971–5978. doi: 10.1021/bi00440a038. [DOI] [PubMed] [Google Scholar]
- Parrish J. R., Jr, Blout E. R. The conformation of poly-L-alanine in hexafluoroisopropanol. Biopolymers. 1972;11(5):1001–1020. doi: 10.1002/bip.1972.360110506. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Clark N. A. Anomalous amide I infrared absorption of purple membrane. Science. 1979 Apr 20;204(4390):311–312. doi: 10.1126/science.432645. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Clark N. A. Polarized infrared spectroscopy of oriented purple membrane. Biophys J. 1979 Mar;25(3):473–487. doi: 10.1016/S0006-3495(79)85317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. A., Teeters C. L. Differential absorption flattening optical effects are significant in the circular dichroism spectra of large membrane fragments. Biochemistry. 1987 Jan 13;26(1):65–70. doi: 10.1021/bi00375a010. [DOI] [PubMed] [Google Scholar]
- Wu Y., Huang H. W., Olah G. A. Method of oriented circular dichroism. Biophys J. 1990 Apr;57(4):797–806. doi: 10.1016/S0006-3495(90)82599-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


