Abstract
Polychlorinated biphenyls (PCBs) are thought to cause numerous adverse health effects, but their impact on estrogen signaling is still not fully understood. In the present study, we used the ER-CALUX bioassay to determine estrogenic/antiestrogenic activities of the prevalent PCB congeners and PCB mixtures isolated from human male serum. The samples were collected from residents of an area with an extensive environmental contamination from a former PCB production site as well as from a neighboring background region in eastern Slovakia. We found that the lower-chlorinated PCBs were estrogenic, whereas the prevalent higher-chlorinated PCB congeners 138, 153, 170, 180, 187, 194, 199, and 203, as well as major PCB metabolites, behaved as anti-estrogens. Coplanar PCBs had no direct effect on estrogen receptor (ER) activation in this in vitro model. In human male serum samples, high levels of PCBs were associated with a decreased ER-mediated activity and an increased dioxin-like activity, as determined by the DR-CALUX assay. 17β-Estradiol (E2) was responsible for a major part of estrogenic activity identified in total serum extracts. Significant negative correlations were found between dioxin-like activity, as well as mRNA levels of cytochromes P450 1A1 and 1B1 in lymphocytes, and total estrogenic activity. For sample fractions containing only persistent organic pollutants (POPs), the increased frequency of anti-estrogenic samples was associated with a higher sum of PCBs. This suggests that the prevalent non-dioxin-like PCBs were responsible for the weak antiestrogenic activity of some POPs fractions. Our data also suggest that it might be important to pay attention to direct effects of PCBs on steroid hormone levels in heavily exposed subjects.
Keywords: CYP1A1, CYP1B1, dioxin-like activity, estradiol, estrogenicity, human serum, polychlorinated biphenyls
Polychlorinated biphenyls (PCBs) are a group of structurally diverse and persistent environmental pollutants, widely distributed as complex mixtures. Mechanisms of toxicity of individual PCB congeners depend on the planarity of a molecule (Safe 1994), as well as on molecular weight and biotransformation rate (Rose et al. 2002). Similarly to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the coplanar non-ortho-substituted PCBs activate aryl hydrocarbon receptor (AhR) and AhR-dependent signal transduction pathways (van den Berg et al. 1998). A majority of the adverse effects of these compounds is thought to be mediated through AhR activation. Therefore, the toxic potencies of dioxin-like PCBs can be expressed in terms of toxic equivalency factors (TEFs) relative to TCDD as the reference toxicant. The TEF values of individual PCBs multiplied by their respective concentrations can be used to yield TCDD toxic equivalents (TEQs) (van den Berg et al. 1998). In contrast, a distinct set of AhR-independent effects, including neurotoxicity, (anti)estrogenicity, and tumor promotion, has been found after exposure to noncoplanar ortho-substituted PCBs (Brouwer et al. 1999; Hansen 1998; Machala et al. 2003; Robertson and Hansen 2001); however, the modes of action of nondioxin-like PCBs are often not clear.
The biological activities of PCBs have been reported to include both estrogenic and anti-estrogenic effects in various in vitro and in vivo models (Cooke et al. 2001; Hansen 1998). TCDD and other AhR agonists, including dioxin-like PCBs, have been frequently reported to have antiestrogenic activity (Buchanan et al. 2000, 2002; Oenga et al. 2004; Safe and Wörmke 2003). Several modes of antiestrogenic action of AhR agonists might include repression of 17β-estradiol (E2)-dependent gene expression by interactions of activated AhR with DNA regions of E2 responsive gene promoters (see Oenga et al. 2004, Safe and Wörmke 2003), inhibition of E2-induced cell cycle proteins and uterine epithelial mitogenesis (Buchanan et al. 2002; Wang et al. 1998), or effects of PCBs on E2 metabolism (Pang et al. 1999; van Duursen et al. 2003). In contrast, the exact mechanisms of estrogenic or antiestrogenic activities of nondioxin-like PCBs are still not fully characterized. The reported results are often contradictory, derived from data obtained in different in vitro or in vivo models (Hansen 1998). The majority of studies found that low-molecular-weight PCBs elicit estrogenic activity both in vitro and in vivo (Arcaro et al. 1999; Nesaretnam and Darbre 1997; Rogers and Denison 2000; Rose et al. 2002). In contrast, the three most prevalent nondioxin-like PCBs, 2,2′,3,4,4′,5′-hexachlorobiphenyl (PCB 138), 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB 153), and 2,2′,3,4,4′,5,5′-heptachloro-biphenyl (PCB 180), have been reported to be antiestrogenic in MCF-7 cells (Bonenfeld-Jorgensen et al. 2001). However, estrogenic/ antiestrogenic potencies of a large set of PCB congeners have not yet been determined in a single in vitro bioassay. Taken together, there is only limited information on effects of prevalent nondioxin-like PCBs and complex PCB mixtures in mammalian blood and tissues.
One essential question is whether the chronic exposure to low doses of environmental persistent organic pollutants (POPs), including PCBs, has endocrine-disrupting effects on exposed human populations (Brouwer et al. 1999; Daston et al. 2003). There are only limited data on estrogenic and dioxin-like activities of complex samples of organic compounds collected from human blood. Sonnenschein et al. (1995) and Soto et al. (1997) reported the development of a serum extraction method for separation of POPs and endogenous steroids. Recently, this extraction and fractionation technique has been adapted for combined chemical and in vitro assay analysis in human blood, allowing for discrimination of effects of endogenous hormones and xenoestrogens (Fernandez et al. 2004). However, results of direct measurements of estrogen receptor (ER)-mediated activity of serum extracts or total POPs fractions in a comprehensive set of human subjects have not yet been published. More information is available concerning in vitro bioassays of dioxin-like activity in human blood contaminated with PCBs. The total TEQ values determined in human female serum and follicular fluid by the DR-CALUX (dioxin receptor–chemically activated luciferase expression) assay have been reported to correlate well with the sum of four major PCB congeners: 153, 138, 180, and 118 (Pauwels et al. 2000). The possible impact of environmental endocrine disruptors on breast cancer, male reproductive tract problems, or prostate cancer is questionable (Chen et al. 2003; Safe 2004). Nevertheless, estrogens play a significant role in, for example, testicular function (O’Donnell et al. 2001). Because the levels of endogenous estrogens in males are considerably lower than in females, possible estrogenic/ antiestrogenic impact of high levels of contamination could be more pronounced in males. Therefore, determination of in vitro estrogenic/antiestrogenic activities of extracts of human male blood samples collected from a PCB-contaminated area could yield more information about the impact of PCBs and/or other POPs on estrogen-dependent signaling.
Since 1959, several thousand tons of residues from the Chemko Strážske chemical plant in the Michalovce district, Slovakia, have been deposited in the nearby river and water reservoir sediments. This has resulted in widespread contamination of the environment, leading to high human exposure. Serum PCB concentrations in subjects from six different districts of Slovakia suggest that levels are three to six times higher in subjects from the Michalovce district (Kočan et al. 2001). When serum levels of 15 PCBs were compared in residents of two districts in eastern Slovakia, one with extensive environmental contamination from a former PCB production site (Michalovce) and the other matched on geography but with background PCB levels, the age-adjusted geometric means for the sum of 15 measured PCB congeners were statistically significantly higher in subjects from the Michalovce district for both sexes: 3327.6 versus 1331.4 ng/g lipid in males, 2751.8 versus 992.2 ng/g lipid in females (Pavúk et al. 2004).
As a part of a large epidemiologic study, the PCBRisk project (Trnovec et al. 2000), we investigated effects of extensive contamination with PCBs on human serum dioxin-like, estrogenic, and antiestrogenic activities of serum extracts from subjects living in the contaminated area. In this study, the ER-mediated activities of individual PCB congeners, which were identified as principal contaminants present in serum of human population in the studied area, were investigated using the T47D breast cancer cell line stably transfected with the luciferase reporter gene under control of estrogen-responsive elements, detecting the direct activation of ER (the ER-CALUX assay) (Legler et al. 1999). In the second step of the study, effects of chronic PCB exposure on antiestrogenic/estrogenic and dioxin-like activities exerted by extracts of human male sera (150 human male serum samples) were assessed and compared to concentrations of major POPs and levels of E2 in serum.
Materials and Methods
Chemicals.
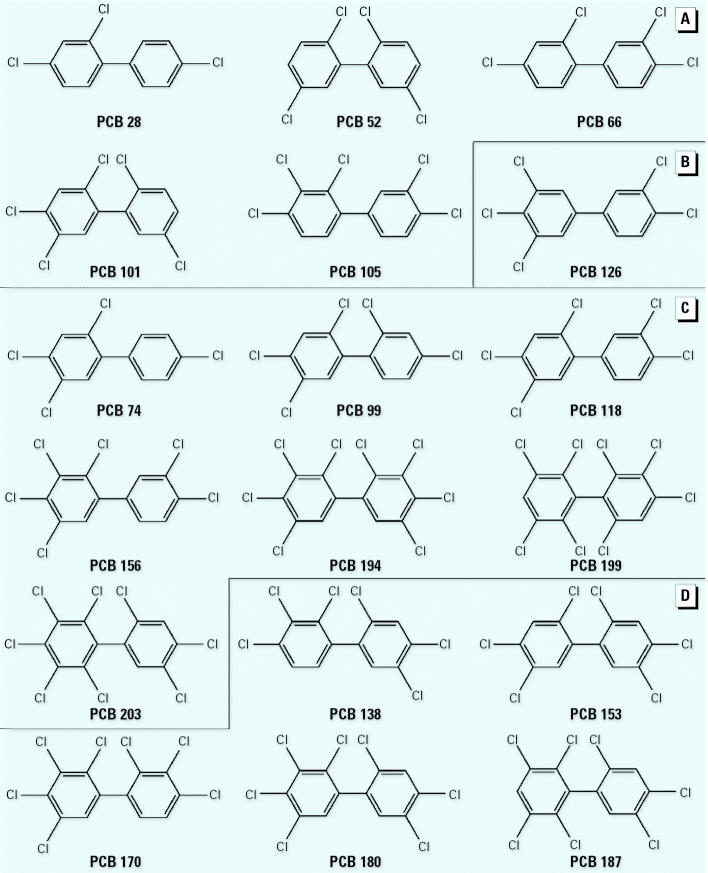
The PCB nomenclature used here is from the International Union of Pure and Applied Chemistry (IUPAC). PCBs 74, 156, 170, 187, 199, and 203 were purchased from Ehrenstorfer (Augsburg, Germany); PCBs 28, 52, 66, 99, 101, 105, 118, 126, 138, 153, 180, and 194 were supplied by Promochem (Wesel, Germany). Purity of all compounds was > 99%. The chemical structure and nomenclature of the PCB congeners we studied is presented in Figure 1. TCDD was supplied by Cambridge Isotope Laboratories, (Andover, MA, USA); E2, cell culture media, and solvents were obtained from Sigma-Aldrich (Prague, Czech Republic). Stock solutions were prepared with dimethyl sulfoxide (DMSO) and stored in the dark. The final concentrations of solvent in the medium did not exceed 0.2% (vol/vol).
Figure 1.
Chemical structures of selected PCB congeners examined for the antiestrogenic/estrogenic activities in human breast carcinoma T47D.Luc cells (ER-CALUX assay). (A) Lower molecular-weight PCBs present in low concentrations in male blood samples. (B) Non-ortho-chlorinated PCB. (C) PCB congeners occurring in relatively higher concentrations in male blood. (D) Prevalent high-molecular-weight PCB congeners.
Blood sampling, extraction, and clean up.
We collected 150 individual male blood samples from residents of two areas of eastern Slovakia, which are differently contaminated with PCBs: the Michalovce district, where commercial PCB mixtures were produced for a number of years (Kočan et al. 2001), and the Stropkov district, which represented the background area. The samples of human male serum (5 mL) were treated with 2 mL methanol and extracted three times with n-hexane:diethyl ether (1:1); the extracts were evaporated and dissolved in 1 mL dichloro-methane (Horander et al. 2004). For determination of overall ER-mediated activity, we replaced the solvent with DMSO in one-half of the crude extract; the second half of the sample was placed on a sulfuric acid-activated silica column and eluted with n-hexane:diethyl ether mixture, evaporated, and redissolved in DMSO (Murk et al. 1997). Using these experimental settings, only persistent compounds were eluted, including PCBs and polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs).
Chemical analysis of POPs.
We determined concentrations of prevalent (non-coplanar) PCB congeners, hexachlorobenzene, and p,p′-DDE by gas chromatography/mass spectrometry (GC/MS) (Kočan et al. 2001, 2004). We calculated TEQs from high performance GC/MS data on blood concentrations of PCDD/PCDFs and non-ortho- and mono-ortho-chlorinated PCBs. The sum of PCBs (∑PCBs) used in the correlation and multivariate statistical analysis was based on the data on concentrations of 17 indicator coplanar and mono-ortho-chlorinated PCB congeners, including PCBs 28, 52, 66, 74, 77, 99, 101, 105, 118, 126, 138, 153, 156, 169, 170, 180, and 189.
Determination of effects associated with AhR activation.
We determined the levels of cytochrome P450 (CYP) 1A1 and CYP1B1 mRNA in human peripheral lymphocytes by RNA extraction and a quantitative reverse-transcriptase-polymerase chain reaction (RT-PCR) method using TaqMan technology (Canton et al. 2003; van Duursen et al. 2005). The in vitro potencies of POPs present in serum to activate AhR were measured in sulfuric acid/silica-treated extracts by a luciferase reporter gene assay (DR-CALUX; BioDetection Systems, Amsterdam, the Netherlands) as described previously (Murk et al. 1996).
ER-mediated activity and determination of E2 in male blood samples.
We determined estrogenic activities of 17 prevalent PCB congeners using the ER-CALUX bioassay (BioDetection Systems) using the human breast carcinoma T47D.Luc cell line, stably transfected with pEREtataLuc construct (Legler et al. 1999; Machala et al. 2004). ER-mediated activity was also determined in the cells treated with either total serum hexane/diethyl ether extracts or with a fraction of POPs obtained by a consequent sulfuric acid/silica fractionation. We determined anti-estrogenicity as a decrease in response to E2 in the cells co-treated with the individual PCB or POPs fraction. Concentrations of E2 were determined by ELISA (ADVIA Centaur Estradiol-6 III assay; Bayer HealthCare, Tarrytown, NY, USA) in 60 samples selected according to stratified PCB levels. We determined cytotoxicity of extracts or individual PCBs by a neutral red uptake assay after a 24-hr exposure.
Data analyses.
All calculations were performed with Microsoft Excel, SlideWritePlus 3.0 for Windows, or Statistica 6.1 for Windows (Microsoft Corporation, Redmond, WA, USA). Nonparametric statistical analyses (Kruskal-Wallis analysis of variance and the Mann-Whitney U test) were used for data analysis. We determined the relationships among biological and chemical data by correlation analysis and multivariate principal component analysis (PCA). We assessed the correlations among the compared parameters using nonparametric Spearman’s rank coefficient (Rs). For the PCA analysis, all the data were normalized using the transformation log (X + 1).
Results
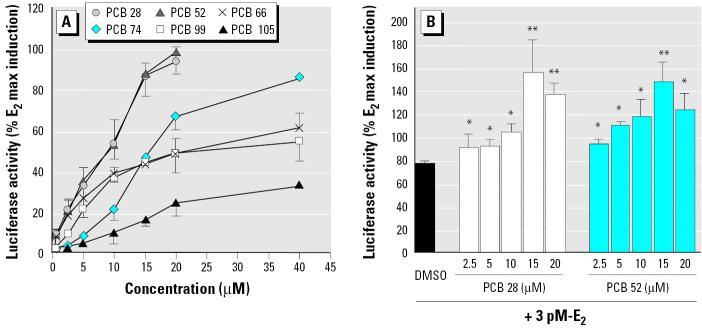
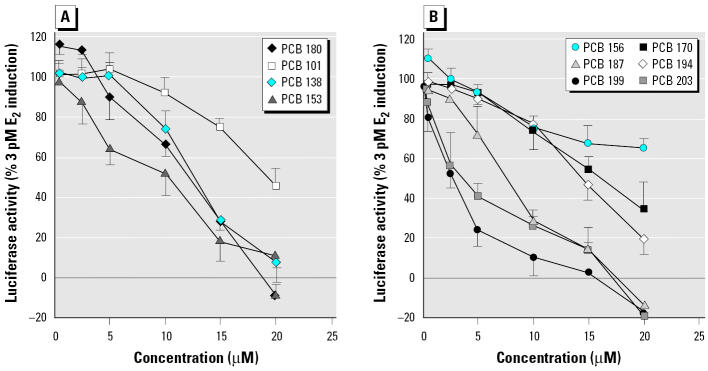
Estrogenic and antiestrogenic potencies of a series of individual PCB congeners, found to be prevalent in the human male blood samples, were determined in the ER-CALUX assay. Lower-molecular-weight PCBs 28, 52, 66, and 74 elicited a significant ER-mediated activity at micromolar concentrations. Pentachlorobiphenyls (PCBs 99 and 105) were only partial ER agonists (Figure 2A). The ER activation by its natural ligand E2 was potentiated when cells were co-treated with trichlorobiphenyls (PCB 28 or 52) (Figure 2B). The most prevalent PCB congeners, 138, 153, 170, 180, and 187, as well as octachlorobiphenyls (PCBs 194, 199, and 203) did not induce the ER-dependent luciferase activity (data not shown), but they all significantly decreased the E2-induced luciferase activity (Figure 3). The most potent inhibitors of ER activation were PCBs 199, 203, and 153; however, the IC50 (concentration that inhibits 50% of maximal E2 response) values of all tested congeners were within a narrow concentration range, 2.9–16.0 μM. A partially reconstituted mixture of the most prevalent PCBs, reflecting a typical ratio of concentrations of individual congeners, showed a significantly higher antiestrogenic activity when compared with inhibition potency of PCB 153 (Figure 4). Potent AhR agonists (dioxin-like PCBs 126, 118, 105, and 156) did not significantly affect the ER activation in the ER-CALUX assay. The estrogenic and antiestrogenic effects of PCB congeners, including the data on their cytotoxicity and calculated median effective concentration (EC50) values, are summarized in Table 1.
Figure 2.
Dose-dependent estrogenic effect of the individual PCB congeners (A) and the combined effect of two estrogenic PCB congeners (PCBs 28 and 52) plus 3 pM E2 (B) on induction of luciferase activity in the ER-CALUX assay. Results are expressed as mean ± SD.
*p < 0.05, and **p < 0.01 compared with control.
Figure 3.
Dose-dependent effect of four indicator PCB congeners (A) and six higher chlorinated PCBs (B) on 3 pM E2-induced luciferase activity in T47D.Luc cells. Results are expressed as mean ± SD.
Figure 4.
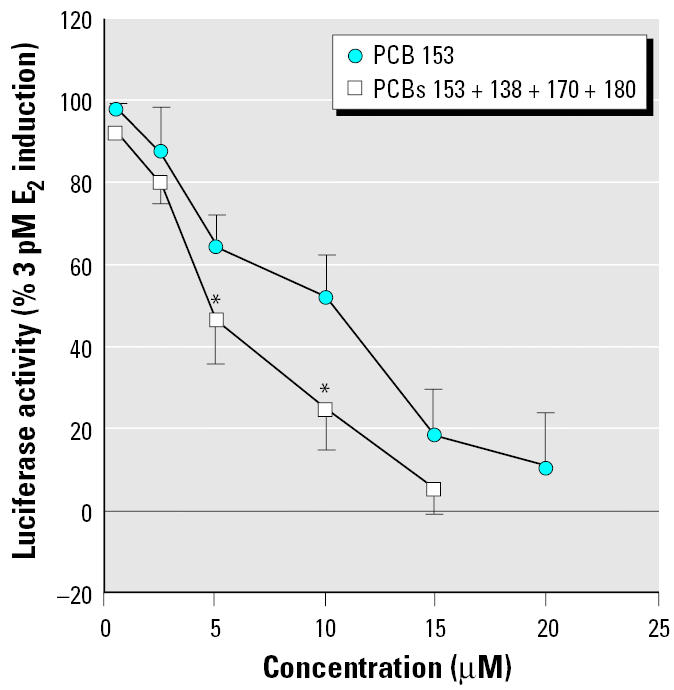
Antiestrogenic potencies of PCB 153 and an artificial mixture of the most prevalent PCBs (ratio of 6:3:5:2 of PCBs 153, 138, 170, and 180). Results are expressed as mean ± SD.
*p < 0.05 compared with an equimolar concentration of PCB 153.
Table 1.
PCB congeners under study, including molecular weights and estrogenic/antiestrogenic and cytotoxic effects determined in ER-CALUX assay using human breast carcinoma T47D.Luc cells.
| ER-activated activity
|
Antiestrogenicity
|
|||||
|---|---|---|---|---|---|---|
| PCB (IUPAC) | Structure | IECa (μM) | IEFb | IC50c (μM) | IhEFd | Cytotoxicity LOECe (μM) |
| 28 | 2,4,4′-Trichlorobiphenyl | 8.23 | 1.65 × 10−7 | NI | NI | > 20 |
| 52 | 2,2′,5,5′-Tetrachlorobiphenyl | 9.52 | 1.42 × 10−7 | NI | NI | > 20 |
| 66 | 2,3′,4,4′-Tetrachlorobiphenyl | 24.31 | 8.56 × 10−8 | NI | NI | > 40 |
| 74 | 2,4,4′,5-Tetrachlorobiphenyl | 17.00 | 1.24 × 10−7 | NI | NI | > 40 |
| 99 | 2,2′,4,4′,5-Pentachlorobiphenyl | WI | WI | NI | NI | > 40 |
| 105 | 2,3,3′,4,4′-Pentachlorobiphenyl | WI | WI | NI | NI | > 40 |
| 118 | 2,3′,4,4′,5-Pentachlorobiphenyl | NI | NI | NI | NI | > 20 |
| 126 | 3,3′,4,4′,5-Pentachlorobiphenyl | NI | NI | NI | NI | > 40 |
| 138 | 2,2′,3,4,4′,5′-Hexachlorobiphenyl | NI | NI | 10.12 | 4.94 × 10−6 | 20 |
| 153 | 2,2′,4,4′,5,5′-Hexachlorobiphenyl | NI | NI | 5.89 | 8.50 × 10−6 | 20 |
| 156 | 2,3,3′,4,4′,5-Hexachlorobiphenyl | NI | NI | WI | WI | 40 |
| 170 | 2,2′,3,3′,4,4′,5-Heptachlorobiphenyl | NI | NI | 16.03 | 3.12 × 10−6 | 25 |
| 180 | 2,2′,3,4,4′,5,5′-Heptachlorobiphenyl | NI | NI | 9.32 | 5.36 × 10−6 | 20 |
| 187 | 2,2′,3,4′,5,5′,6-Heptachlorobiphenyl | NI | NI | 7.48 | 6.68 × 10−6 | 20 |
| 194 | 2,2′,3,3′,4,4′,5,5′-Octachlorobiphenyl | NI | NI | 14.14 | 3.54 × 10−6 | 25 |
| 199 | 2,2′,3,3′,4′,5,6,6′-Octachlorobiphenyl | NI | NI | 2.85 | 1.75 × 10−5 | 20 |
| 203 | 2,2′,3,4,4′,5,5′,6-Octachlorobiphenyl | NI | NI | 3.20 | 1.56 × 10−5 | 20 |
Abbreviations: IEC, induction equivalency concentration; IEF, induction equivalency factor; IhEF, inhibitory equivalency factor; NI, no significant induction/inhibition; WI, weak induction/inhibition (< 50% of estradiol maximum induction; < 50% decrease in induction of 3 pM E2).
Concentration of PCB congener inducing the same level of luciferase activity as the EC50 of the reference inducer E2 (2.08 pM).
Calculated as the ratio between the EC50 of E2 and the concentration of the selected PCB congener inducing the same level of luciferase activity.
Concentration of PCB congener causing 50% decrease in luciferase activity induced by 3 pM E2.
Calculated as the ratio between the IC50 of the synthetic antiestrogen ICI 182,780 (IC50 = 50 pM) and the concentration of the selected PCB congener causing the same level of decrease in luciferase activity induced by 3 pM E2.
Lowest (experimental) concentration of PCB congener causing a significant decrease of cell viability (24-hr exposure Neutral Red uptake assay).
In the second step of this study, we determined the estrogenic activities of 150 human male serum samples, collected in Michalovce and Stropkov districts, Slovakia, using the ER-CALUX bioassay. The total hexane/ diethyl ether extracts of human male serum samples, containing both endogenous steroids and POPs, showed significant estrogenic responses in the ER-CALUX assay, ranging from 12.5 to 59.2 pg E2 equivalents (EEQ) per milliliter (Table 2). Dioxin-like activities, measured in the POPs fractions by the DR-CALUX bioassay, ranged from 0.2 to 2.9 pg TEQs/mL (Table 2). Weak estrogenic or antiestrogenic activities were found in the fractions of POPs, but only in part of the samples. The POPs fractions from the less polluted background area elicited ER-mediated activity with a higher incidence (18 of 75 samples vs. 8 of 75 samples), whereas anti-estrogenic activity was detected more frequently in the samples from the PCB-polluted region (5 and 17 samples, respectively). However, the ER-mediated activities did not overcome 2 pg EEQs/mL, and only partial estrogenic or antiestrogenic responses (< 40%) were found in positive samples (data not shown). Conversion of concentration units showed that only submicromolar concentrations of prevalent PCBs were present in cultivation medium when serum extracts were applied to cells (data not shown); therefore, only partial antiestrogenic effects of PCBs could be expected in the sample mixtures.
Table 2.
Summary of data from human male serum samples used in multivariate statistical analysis.
| Concentration/mL serum
|
Concentration/g lipid
|
||||||
|---|---|---|---|---|---|---|---|
| No. | Range | Median | Mean | Range | Median | Mean | |
| Estrogenic activity (pg EEQs) | 150 | 12.5–59.2 | 28.2 | 29.2 | 1.3–11.6 | 4.1 | 4.3 |
| E2 (pg) | 60 | < 1.0–43.5 | 15.5 | 15.8 | 0.1–5.4 | 2.0 | 2.1 |
| Dioxin-like activity (pg TEQs) | 144 | 0.2–2.9 | 0.6 | 0.7 | 11.9–434.0 | 83.6 | 92.0 |
| ∑ PCBs/PCDD/PCDFs (pg TEQs) | 100 | 0.05–0.5 | 0.1 | 0.2 | 7.5–57.9 | 18.2 | 20.8 |
| ∑ PCBs (μg) | 150 | 0.0020–0.1755 | 0.0078 | 0.0147 | 0.3458–32.51 | 1.124 | 2.040 |
| p,p′-DDE (μg) | 150 | 0.0017–0.1165 | 0.0119 | 0.0171 | 0.2689–11.16 | 1.800 | 2.219 |
Sum of PCDD/PCDFs and dioxin-like PCBs was calculated as TEQs according to World Health Organization TEF values (van den Berg et al. 1998).
The in vitro bioassay data were compared with data on concentrations of major POPs in the samples obtained in the PCBRisk project. In this large epidemiological study, > 2,000 human blood samples were evaluated for concentrations of PCBs, PCDD/PCDFs, and p,p′-DDE (Kočan et al. 2004). The analytical data for the subset of 150 male samples, which were used here for statistical analysis of in vitro bioassay data only, are summarized in Table 2. Additionally, data on induction of AhR-dependent expression of CYP1A1 and CYP1B1 mRNA in blood lymphocytes, as determined by real-time PCR (Canton et al. 2003; van Duursen et al. 2005), and E2 concentration determined in a substantial part of blood sample extracts were included in the statistical analysis. PCDDs did not contribute significantly to higher levels of TEQs, and concentrations of PCDFs, which might also contribute to the dioxin-like activities, were only marginally increased in highly exposed male subjects (Kočan et al. 2004). High concentrations of p,p′-DDE were found in a majority of samples (Table 2); however, only weak estrogenic and no dioxin-like activity was found for this compound (data not shown). Therefore, modulations of biological effects might be attributed mainly to differences in the concentrations of PCBs.
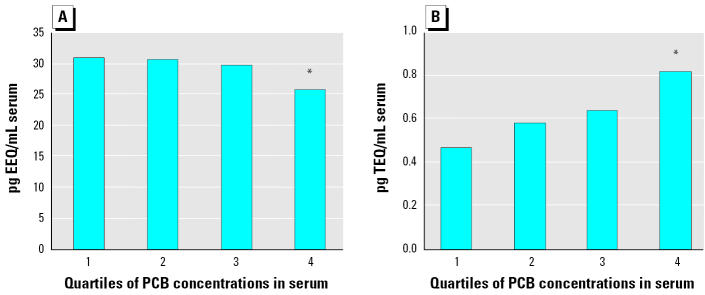
Total estrogenic activity was moderately decreased, while the dioxin-like activity was increased, in samples with high PCB levels ranging from 13.9 to 175.5 ng/mL serum (i.e., 1865.7–32509.4 ng/g lipid; Figure 5). Quartiles presented in Figure 5 are based on concentrations expressed per milliliter of serum. The alternative data set (expressed on a gravimetric basis) showed a very similar pattern of effects. The levels of E2 decreased in the fourth quartile of PCB concentrations, but the decrease was not significant.
Figure 5.
Estrogenic activities (A) and dioxin-like activities (B) of extracts of human male serum samples. Median values of quartiles were stratified according to PCB concentrations.
*Significantly different from groups with lower PCB levels (p = 0.02; Mann-Whitney U test); concentrations of PCBs (μg/mL serum) are as follows: first quartile, 0.0020–0.0055; second quartile, 0.0055–0.0078; third quartile, 0.0079–0.0138; fourth quartile, 0.0139–0.1755.
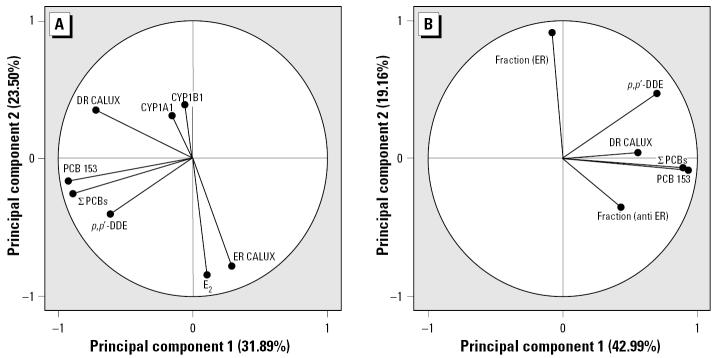
Based on concentration data summarized in Table 2, we performed multivariate PCA to more precisely characterize statistical associations between in vitro bioassay data and levels of major organic contaminants in the blood. The PCA is one of a set of ordination techniques used in data reduction and summarization. As shown in Figure 6A, the first two components explained 55% of the variability of the original data. The axes were aligned with the directions of greatest variation in the data set. The other components were neglected because they did not contribute significantly to the meaningful interpretation of the relationships among biological and chemical parameters. The first principal component axis represented the chemical variables of the male serum extracts, such as ∑PCBs, PCB 153 concentration, p,p′-DDE content, and the biological variable AhR-mediated activity (DR-CALUX assay). The second principal component was associated with the ER-mediated activity of serum extracts (ER-CALUX assay), E2 concentrations, and CYP1A1 and CYP1B1 mRNA expression level. The length and direction of the lines represent the significance of the associated variables. The PCA confirmed a positive relation between overall estrogenicity (12.5–59.2 pg EEQ/ml) and E2 levels (1.0–43.5 pg/ml; Table 2). Further, weak negative relations between ER-mediated activity and expression of CYP1A1 and CYP1B1 mRNAs and between estrogenic and dioxin-like activities were observed (Figure 6A). The associations of the variables were confirmed by bivariate rank correlations computed on original untransformed data. The estrogenic activity of serum extracts correlated with E2 concentrations (Rs = 0.510, p < 0.001). Weak but statistically significant negative correlation between ER-mediated activity and levels of CYP1A1 mRNA (Rs = −0.241, p < 0.05), as well as between E2 concentrations and dioxin-like activity (Rs = −0.227, p < 0.1), were revealed. No correlation was found between E2 concentrations and total PCB levels (Rs = 0.078).
Figure 6.
Principal component analysis of the measured parameters of the serum samples. Abbreviations: Fraction (ER), number of estrogenic samples of the fraction of POPs; fraction (anti-ER), number of anti-estrogenic samples of the fraction of POPs; ∑PCBs, serum of 17 PCB congeners.
The PCA for the fractions of persistent compounds explained 60% of the total variability in the data set (Figure 6B). The first principal component was associated with a number of fractions with antiestrogenic activity (anti-ER), ∑PCBs, PCB 153 content, and AhR-mediated activity (DR-CALUX assay). The second principal component axis represented only the estrogenic activity of fractions (ER). PCA analysis showed that anti-estrogenic activity of fractions depended on the concentrations of PCB congeners and TCDD equivalents obtained in the DR-CALUX assay (Rs = 0.246–0.275, p < 0.01).
Discussion
PCBs have been reported to be both estrogenic and antiestrogenic, based on various in vitro and in vivo models. Lower-molecular-weight PCBs are reportedly estrogenic, with the exception of dioxin-like 3,3′,4,4′-tetrachlorobiphenyl (PCB 77), which elicited anti-estrogenicity in vivo and also in some in vitro models (Ramamoorthy et al. 1999). 2,2′,6,6′-Tetrachlorobiphenyl (PCB 54), a fully ortho-substituted compound not occurring in the environment at significant levels (Hansen 1998), was estrogenic both in the MCF-7 cell focus assay and in the rat uterotrophic assay (Arcaro et al. 1999). 3,3′,5,5′-Tetrachlorobiphenyl (PCB 80), another model congener, was a weak ER agonist both in vivo and in vitro, while, surprisingly, PCB 52 was inactive in the same models (Nesaretnam and Darbre 1997). PCB 66 and PCB 95 (2,2′,3,5′,6-pentachlorobiphenyl) have been reported to be estrogenic in BG1LucE2 cells at 10 μM concentrations, whereas coplanar PCB 77 elicited no ER-mediated activity in this cellular model (Rogers and Denison 2000). PCB 52 and PCB 77 caused a modest transient uterotrophic effect in weaning rats (Rose et al. 2002); however, PCB 77 attenuated the increase in uterine weight and cell proliferation in another study (Jansen et al. 1993). The uterotrophic effects after exposure to less persistent PCB congeners showed nonlinear dose responses, and they decreased rapidly (Rose et al. 2002). However, all the above data have been obtained from various models and assays, and estrogenic/ antiestrogenic effects of both lower chlorinated and higher chlorinated PCBs present in the environment have not yet been examined systematically in one assay.
In our study, PCBs 28, 52, 66, 74, 99 and 105, all found at significant levels in male serum samples, induced the ER-mediated activity at micromolar concentrations (Table 1), suggesting that ER activation could be one of the potential modes of action of low-molecular-weight PCBs. However, the decrease of total estrogenic activity and E2 levels observed in human serum samples of males exposed to high PCB levels (Figure 5A) indicated that PCB mixtures elicited an overall antiestrogenic effect. Therefore, the ER-mediated activity of lower-chlorinated PCBs appears to have only a limited toxicologic significance, perhaps with the exception of acute transient exposure to PCBs (Rose et al. 2002).
Unlike low-molecular-weight PCBs, the dioxin-like and prevalent high-molecular-weight PCB congeners are considered to be antiestrogenic. TCDD, a model toxicant for dioxin-like PCBs, exhibits potent anti-estrogenic activity (Buchanan et al. 2000, 2002; Cooke et al. 2001; Safe and Wörmke 2003). TCDD has little effect on total ER levels (Gierthy et al. 1996), and no direct binding to ER has been reported (see Safe and Wörmke 2003). Recently, inhibition of ER-mediated cell proliferation by coplanar PCBs has been reported in breast cancer cell lines (Oenga et al. 2004). TCDD or coplanar PCBs did not inhibit E2-induced activity of a reporter construct containing the promoter insert from creatine kinase B in T47D cells, while dioxin-like compounds, including PCB 77 and PCB 126, prevented activation of other reporter constructs in both MCF-7 and T47D cells, although only at levels as high as 10 μM (Ramamoorthy et al. 1999). This suggests that a type of reporter construct can affect detection of antiestrogenic activity. One possible mechanism of antiestrogenic activity of AhR ligands is the direct inhibition of E2-responsive genes through binding to inhibitory dioxin responsive elements (iDRE) in their promoter regions. Functional iDREs have been identified in promoter regions of pS2, c-fos, Hsp27 and cathepsin D genes (reviewed by Safe and Wörmke 2003). In the present study, anti-estrogenicity was not elicited by coplanar PCB 126 (Table 1) in the T47D.Luc cells used in the ER-CALUX assay. The lack of anti-estrogenic activity of coplanar PCBs observed in the T47D.Luc cell line might be explained by the missing iDREs in the reporter construct, which contains three tandem repeats of the consensus estrogen-responsive element (ERE) oligonucleotide (Legler et al. 1999).
On the other hand, this cellular model allowed us to investigate a direct activation of ER and/or perturbation of E2-induced ER activation. While the low-molecular-weight PCBs elicited ER activation and ER-dependent gene expression, prevalent and more persistent high-molecular-weight PCB congeners were antiestrogenic (Table 1, Figures 3 and 4). Pulses of exposure to more labile mixtures of lower chlorinated PCBs may contribute to transient endocrine disruption, including an increase in estrogenic activity (Hansen 1998; Rose et al. 2002). PCB 153 was estrogenic in the acute 2-day immature rat uterine weight assay, albeit at very high concentrations (Li et al. 1994). Antiestrogenic effects of three prevalent congeners (PCBs 138, 153, and 180) have been found both in a reporter gene and cell proliferation MCF-7 assays (Bonenfeld-Jorgensen et al. 2001). This is in accordance with our data on antiestrogenicity of high-molecular-weight PCBs in the ER-CALUX assay (Table 1). Inhibition of ER activation by hexa-, hepta- and octachlorinated biphenyls and suppression of estrogenic signaling found in serum of males chronically exposed to PCBs (Figures 3–5) suggest that PCB 153 and other prevalent congeners could contribute to overall antiestrogenic response. Contribution of hydroxy- and methylsulfonyl-PCB metabolites, p,p′-DDE and methylsulfonyl-p,p′-DDE metabolites to antiestrogenic activities of POPs might also be of importance. Because both low- and high-molecular-weight PCBs and POPs metabolites elicited their effects on estrogenic activity at similar micromolar concentrations (Bonenfeld-Jorgensen et al. 2001; Letcher et al. 2002; Machala et al. 2004; this study), it might be expected that the anti-estrogenic effects of prevalent higher chlorinated PCBs would prevail in human male blood. Nevertheless, antiestrogenic effects of PCBs detected in human male serum appeared to be less important, when compared with the levels of E2, the major contributor of the overall estrogenic activity.
In vitro bioassays are a suitable tool for exposure assessment of dioxin-like and (anti)estrogenic compounds (van den Berg et al. 1998; Zacharewski 1997). However, currently only limited data are available on dioxin-like activities found in human female serum and follicular fluid; the TEQs determined by the DR-CALUX assay have been reported to correlate well with the sum of four major PCB congeners 153, 138, 180, and 118 (Pauwels et al. 2000). The data on the ER-mediated activity in human blood samples are still limited. Rasmussen et al. (2003) reported estrogenic activity in female serum by E-Screen assay, however, they did not observe any correlation between estrogenicity and concentrations of individual endocrine disruptors. In the present study, a decrease of total estrogenic activity and increased dioxin-like activity were found in serum samples from human males chronically exposed to PCBs. However, as shown in Figure 5, correlations with PCB concentrations were significant only in subjects with high exposure levels.
Our data suggest that exposure to high levels of PCBs might affect E2 blood levels, although this was not significant. Currently, there is little information available about a possible modulation of steroid hormone levels after exposure to PCBs. In a recently published study, a weak but significant negative correlation was found between serum levels of the prevalent PCB 153 congener and testosterone in young men, and E2 concentrations (within a concentration range of 43–144 pM) were also slightly decreased in the more exposed subjects (Richthoff et al. 2003). The concentrations of PCB 153 (23–250 ng/g lipid) found in these subjects were significantly lower that those observed both in the present study and in previous studies in eastern Slovakia (Kočan et al. 2001, 2004). The concentrations of PCB 153 ranged from 115 to 8,631 ng/g lipid in 150 Slovak male serum samples included in the present study. Another experimental study in rats exposed to PCB mixtures also reported lower testosterone and E2 serum levels and suppression of brain aromatase activity (Hany et al. 1999). Decreased E2 concentrations could be associated with AhR activation by dioxin-like PCBs, leading to enhanced CYP1A/CYP1B1-catalyzed metabolism of E2 (Gierthy et al. 1996; Spink et al. 1990). Induction of CYP1A1 and CYP1B1 mRNAs in lymphocytes is considered to reflect increased exposure to dioxin-like compounds (Canton et al. 2003; Hanaoka et al. 2002). Within the epidemiologic study, Canton et al. (2004) found increased levels of CYP1A1 and CYP1B1 mRNA only in lymphocytes of males exposed to very high levels of PCBs (fourth quartile). This finding suggests that a physiologically significant AhR-dependent induction of E2-metabolizing CYP enzymes might occur in liver and other target tissues.
Besides CYP1A1, 1A2, and 1B1 iso-enzymes, CYP3A4 has been suggested to play a major role in hydroxylation of E2 (Badawi et al. 2000; Hayes et al. 1996; Pang et al. 1999; Spink et al. 1990; Takemoto et al. 2004; Yamazaki et al. 1998). Induction of CYP3A4 is a consequence of exposure to prevalent nondioxin-like PCBs (Gillette et al. 2002; Parkinson et al. 1981). Therefore, both coplanar and noncoplanar PCBs could increase E2 metabolism and reduce blood E2 concentrations.
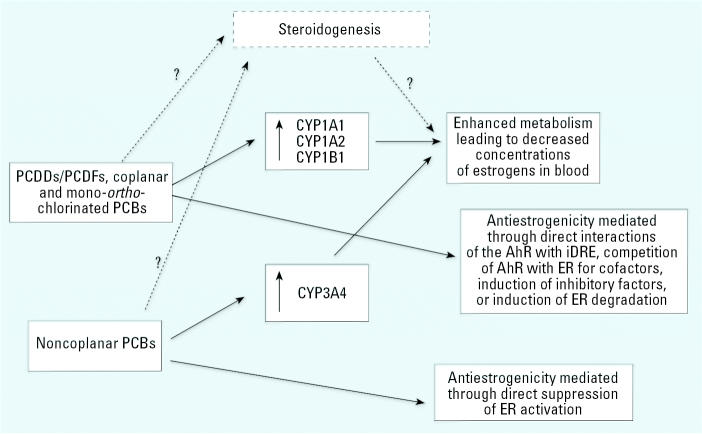
Both dioxin-like and nondioxin-like PCBs might affect estrogen signaling by multiple mechanisms, as summarized in Figure 7. Obviously, this list of modes of action is not complete; PCBs might also potentially disrupt the pathways associated with the perturbation of hypothalamus–pituitary–gonadal axis hormone signaling and steroidogenesis, as another potential mechanism of E2 modulations by PCBs.
Figure 7.
Potential mechanisms of antiestrogenic effects of coplanar and noncoplanar PCBs.
As outlined in the recent review by Safe (2004), it is currently not possible to directly attribute increased incidence of breast cancer or disorders of the male reproductive tract, for example, to endocrine disruption associated with organochlorine exposure. A number of adverse impacts of high PCB contamination have been identified, including perturbations of thyroid function, immunity, or neuro-developmental processes (Robertson and Hansen 2001). However, it was not possible to associate any of the adverse effects observed within the frames of the PCBRisk project with antiestrogenicity of PCBs.
In summary, significant associations between exposure to PCBs and overall (anti)estrogenic and dioxin-like activities in the present study were found only at high exposure levels. Although the prevalent noncoplanar PCBs elicited antiestrogenicity in the ER-CALUX assay, when tested as individual compounds or as a partially reconstituted mixture, a significant estrogenic activity was determined in whole-serum extracts. Moreover, only weak or negligible anti/estrogenic activities were found in serum extract fractions containing exclusively POPs including PCBs. Due to the presence of E2 in human male blood and its dominant role in total estrogenic activity of serum samples, reduction of E2 levels might be a more significant antiestrogenic effect of high PCB exposure. This mode of action, associated with induction of CYP1A1, CYP1A2, CYP1B1 and/or CYP3A4 enzymes or perturbation of steroidogenesis and endocrine signaling, preceding the biosynthesis of estrogens, deserves further attention.
Footnotes
This work was presented in part at the PCB Workshop, 13–15 June 2004, Urbana-Champaign, Illinois, USA, and at the Dioxin2004 Symposium, 6–10 September 2004, Berlin, Germany.
We thank all collaborators involved in the PCBRisk project for their enormous effort in collection of samples and for fruitful discussion and support, especially Å. Bergman and L. Hovander (Stockholm University, Sweden), M.B.M. van Duursen (IRAS, University of Utrecht, the Netherlands), and S. Jursa (Slovak Medical University, Bratislava, Slovakia). We thank M. Gájová for her assistance with extraction and fractionation of male blood samples.
This work was supported by the European Union (project no. QLK4-CT-2000-00488) and by the Czech Ministry of Agriculture (MZE 0002716201).
References
- Arcaro KF, Yi L, Seegal RF, Vakharia DD, Yang Y, Spink DC, et al. 2,2′,6,6′-Tetrachlorobiphenyl is estrogenic in vitro and in vivo. J Cell Biochem. 1999;72:94–102. [PubMed] [Google Scholar]
- Badawi AF, Cavalieri EL, Rogan EG. Effect of chlorinated hydrocarbons on expression of cytochrome P450 1A1, 1A2 and 1B1 and 2- and 4-hydroxylation of 17β-estradiol in female Sprague-Dawley rats. Carcinogenesis. 2000;21:1593–1599. [PubMed] [Google Scholar]
- Bonenfeld-Jorgensen EC, Andersen HR, Rasmussen TH, Vinggaard AM. Effect of highly bioaccumulated poly-chlorinated biphenyl congeners on estrogen and androgen receptor activity. Toxicology. 2001;158:141–153. doi: 10.1016/s0300-483x(00)00368-1. [DOI] [PubMed] [Google Scholar]
- Brouwer A, Longnecker MP, Birnbaum LS, Cogliano J, Kostyniak P, Moore J, et al. Characterization of potential endocrine-related health effects at low-dose levels of exposure to PCBs. Environ Health Perspect. 1999;107(suppl 4):639–649. doi: 10.1289/ehp.99107s4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan DL, Ohsako S, Tohyama C, Cooke PS, Iguchi T. Dioxin inhibition of estrogen-induced mouse uterine epithelial mitogenesis involves changes in cyclin and transforming growth factor-beta expression. Toxicol Sci. 2002;66:62–68. doi: 10.1093/toxsci/66.1.62. [DOI] [PubMed] [Google Scholar]
- Buchanan DL, Sato T, Peterson RE, Cooke PS. Antiestrogenic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in mouse uterus: critical role of the aryl hydrocarbon receptor in stromal tissue. Toxicol Sci. 2000;57:302–311. doi: 10.1093/toxsci/57.2.302. [DOI] [PubMed] [Google Scholar]
- Canton RF, Besselink HT, Sanderson JT, Botschuyver S, Brouwer B, van den Berg M. Expression of CYP1A and 1B1 mRNA in blood lymphocytes from two district populations in Slovakia compared to total TEQs in blood as measured by the DRE-CALUX® assay. Organohalogen Compounds. 2003;64:215–218. [Google Scholar]
- Chen C, Weiss NS, Stanczyk FZ, Lewis SK, DiTommaso D, Etzioni R, et al. Endogenous sex hormones and prostate cancer risk: a case-control study nested within the carotene and retinol efficacy trial. Cancer Epidemiol Biomarkers Prev. 2003;12:1410–1416. [PubMed] [Google Scholar]
- Cooke PS, Sato T, Buchanan DL. 2001. Disruption of steroid hormone signaling by PCBs. In: PCBs. Recent Advances in Environmental Toxicology and Health Effects (Robertson LW, Hansen LG, eds). Lexington, KY:University Press of Kentucky, 257–263.
- Daston GP, Cook JC, Kavlock RJ. Uncertainties for endocrine disrupters: our view on progress. Toxicol Sci. 2003;74:245–252. doi: 10.1093/toxsci/kfg105. [DOI] [PubMed] [Google Scholar]
- Fernandez MF, Rivas A, Olea-Serrano F, Cerrillo I, Molina-Molina JM, Araque P, et al. Assessment of total effective xenoestrogen burden in adipose tissue and identification of chemicals responsible for the combined estrogenic effect. Anal Bioanal Chem. 2004;379:163–170. doi: 10.1007/s00216-004-2558-5. [DOI] [PubMed] [Google Scholar]
- Gierthy JF, Spink BC, Figge HL, Pentecost BT, Spink DC. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin, 12-O-tetra-decanoylphorbol-13-acetate and 17β-estradiol on estrogen receptor regulation in MCF-7 human breast cancer cells. J Cell Biochem. 1996;60:173–184. doi: 10.1002/(sici)1097-4644(19960201)60:2<173::aid-jcb2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Gillette JS, Hansen LG, Rose RL. Metabolic effects of episodic polychlorinated biphenyl (PCB) congeners. Rev Toxicol Environ Toxicol. 2002;4:129–159. [Google Scholar]
- Hanaoka T, Yamano Y, Pan G, Hara K, Ichiba M, Zhang J, et al. Cytochrome P450 1B1 mRNA levels in peripheral blood cells and exposure to polycyclic aromatic hydrocarbons in Chinese coke oven workers. Sci Total Environ. 2002;296:27–33. doi: 10.1016/s0048-9697(02)00070-0. [DOI] [PubMed] [Google Scholar]
- Hansen LG. Stepping backward to improve assessment of PCB congener toxicities. Environ Health Perspect. 1998;106(suppl 1):171–189. doi: 10.1289/ehp.98106s1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hany J, Lilienthal H, Sarasin A, Roth-Härer A, Fastabend A, Dunemann L, et al. Developmental exposure of rats to a reconstituted PCB mixture or Aroclor 1254: effects on organ weights, aromatase activity, sex hormone levels, and sweet preference behavior. Toxicol Appl Pharmacol. 1999;158:231–243. doi: 10.1006/taap.1999.8710. [DOI] [PubMed] [Google Scholar]
- Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 17β-Estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci USA. 1996;93:9776–9781. doi: 10.1073/pnas.93.18.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovander L, Linderholm L, Athanasiadou M, Athanassiadis I, Trnovec T, Kočan A. Analysis of PCB and PCB metabo-lites in humans from eastern Slovakia. Organohalogen Compounds. 2004;66:3525–3531. [Google Scholar]
- Jansen HT, Cooke PS, Porcelli J, Liu T-C, Hansen LG. Estrogenic and antiestrogenic actions of PCBs in the female rat: in vitro and in vivo studies. Reprod Toxicol. 1993;7:237–248. doi: 10.1016/0890-6238(93)90230-5. [DOI] [PubMed] [Google Scholar]
- Kočan A, Drobná B, Petrík J, Jursa S, Chovancová J, Čonka K, et al. Human exposure to PCBs and some other persistent organochlorines in eastern Slovakia as a consequence of former PCB production. Organohalogen Compounds. 2004;66:3539–3546. [Google Scholar]
- Kočan A, Petrík J, Jursa S, Chovancová J, Drobná B. Environmental contamination with polychlorinated biphenyls in the area of their former manufacture in Slovakia. Chemosphere. 2001;43:595–600. doi: 10.1016/s0045-6535(00)00411-2. [DOI] [PubMed] [Google Scholar]
- Legler J, van den Brink CE, Brouwer A, Murk AJ, van der Saag PT, Vethaak AD, et al. Development of a stably transfected estrogen receptor-mediated luciferase reporter gene assay in the human T47D breast cancer cell line. Toxicol Sci. 1999;48:55–66. doi: 10.1093/toxsci/48.1.55. [DOI] [PubMed] [Google Scholar]
- Letcher RJ, Lemmen JG, van der Burg B, Brouwer A, Bergman A, Giesy JP, et al. In vitro antiestrogenic effects of aryl methyl sulfone metabolites of polychlorinated biphenyls and 2,2-bis(4-chlorophenyl)-1,1-dichloroethene on 17 beta-estradiol-induced gene expression in several bioassay systems. Toxicol Sci. 2002;69:362–372. doi: 10.1093/toxsci/69.2.362. [DOI] [PubMed] [Google Scholar]
- Li M-H, Hansen LG. Responses of prepubertal female rats to environmental PCBs with high and low dioxin equivalencies. Fundam Appl Toxicol. 1996;33:282–293. doi: 10.1006/faat.1996.0166. [DOI] [PubMed] [Google Scholar]
- Li M-H, Zhao Y-D, Hansen LG. Multiple dose toxicokinetic influence on the estrogenicity of 2,2′,4,4′,5,5′-hexachloro-biphenyl. Bull Environ Contam Toxicol. 1994;53:583–590. doi: 10.1007/BF00199030. [DOI] [PubMed] [Google Scholar]
- Machala M, Bláha L, Lehmler H-J, Plíšková M, Májková Z, Kapplová P, et al. Toxicity of hydroxylated and quinoid PCB metabolites: inhibition of gap junctional inter-cellular communication and activation of aryl hydrocarbon and estrogen receptors in hepatic and mammary cells. Chem Res Toxicol. 2004;17:340–347. doi: 10.1021/tx030034v. [DOI] [PubMed] [Google Scholar]
- Machala M, Bláha L, Vondráček J, Trosko JE, Scott J, Upham BL. Inhibition of gap junctional intercellular communication by noncoplanar polychlorinated biphenyls: inhibitory potencies and screening for potential mode(s) of action. Toxicol Sci. 2003;76:102–111. doi: 10.1093/toxsci/kfg209. [DOI] [PubMed] [Google Scholar]
- Moore M, Mustain M, Daniel K, Chen I, Safe S, Zacharewski T, et al. Antiestrogenic activity of hydroxylated poly-chlorinated biphenyl congeners identified in human serum. Toxicol Appl Pharmacol. 1997;142:160–168. doi: 10.1006/taap.1996.8022. [DOI] [PubMed] [Google Scholar]
- Murk AJ, Legler J, Denison MS, Giesy JP, van der Guchte C, Brouwer A. Chemical-activated luciferase gene expression (CALUX): a novel in vitro bioassay for Ah receptor active compounds in sediments and pore water. Fundam Appl Toxicol. 1996;33:149–160. doi: 10.1006/faat.1996.0152. [DOI] [PubMed] [Google Scholar]
- Murk AJ, Leonards PEG, Bulder AS, Jonas AS, Rozemeijer MJC, Denison MS, et al. The CALUX (chemical-activated luciferase expression) assay, adapted and validated for measuring TCDD-equivalents in blood plasma. Environ Toxicol Chem. 1997;16:1583–1589. [Google Scholar]
- Nesaretnam K, Darbre P. 3,5,3′,5′-tetrachlorobiphenyl is a weak oestrogen agonist in vitro and in vivo. J Steroid Biochem Mol Biol. 1997;62:409–418. doi: 10.1016/s0960-0760(97)00062-9. [DOI] [PubMed] [Google Scholar]
- O’Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocr Rev. 2001;22:289–318. doi: 10.1210/edrv.22.3.0431. [DOI] [PubMed] [Google Scholar]
- Oenga GN, Spink DC, Carpenter DO. TCDD and PCBs inhibit breast cancer cell proliferation. Toxicol In Vitro. 2004;18:811–819. doi: 10.1016/j.tiv.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Pang S, Cao JQ, Katz BH, Hayes CL, Sutter TR, Spink DC. Inductive and inhibitory effects of non-ortho-substituted polychlorinated biphenyls on estrogen metabolism and human cytochromes P450 1A1 and 1B1. Biochem Pharmacol. 1999;58:29–38. doi: 10.1016/s0006-2952(99)00070-2. [DOI] [PubMed] [Google Scholar]
- Parkinson A, Robertson L, Safe L, Safe S. Polychlorinated biphenyls as inducers of hepatic microsomal enzymes: structure-activity rules. Chem Biol Interact. 1981;30:271–285. doi: 10.1016/0009-2797(80)90050-2. [DOI] [PubMed] [Google Scholar]
- Pauwels AN, Cenijn PH, Schepens PJC, Brouwer A. Comparison of chemical-activated luciferase gene expression bioassay and gas chromatography for PCB determination in human serum and follicular fluid. Environ Health Perspect. 2000;108:553–557. doi: 10.1289/ehp.00108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavúk M, Cerhan JR, Lynch CF, Schecter A, Petrik J, Chovancova J, et al. Environmental exposure to PCBs and cancer incidence in eastern Slovakia. Chemosphere. 2004;54:1509–1520. doi: 10.1016/j.chemosphere.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy K, Gupta MS, Sun G, McDougal A, Safe SH. 3,3′4,4′-Tetrachlorobiphenyl exhibits antiestrogenic and antitumorigenic activity in the rodent uterus and mammary cells and in human breast cancer cells. Carcinogenesis. 1999;20:115–123. doi: 10.1093/carcin/20.1.115. [DOI] [PubMed] [Google Scholar]
- Rasmussen TH, Nielsen F, Andersen HR, Nielsen JB, Weihe P, Grandjean P. Assessment of xenoestrogenic exposure by a biomarker approach: application of the E-Screen bioassay to determine estrogenic response of serum extracts. Environ Health. 2003;2:12. doi: 10.1186/1476-069X-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richthoff J, Rylander L, Jönsson BAG, Åkesson H, Hagmar L, Nilsson-Ehle P, et al. Serum levels of 2,2′,4,4′,5,5′-hexa-chlorobiphenyl (CB-153) in relation to markers of reproductive function in young males from the general Swedish population. Environ Health Perspect. 2003;111:409–413. doi: 10.1289/ehp.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LW, Hansen LG. 2001. PCBs. Recent Advances in Environmental Toxicology and Health Effects. Lexington, KY:The University Press of Kentucky.
- Rogers JM, Denison MS. Recombinant cell bioassays for endocrine disruptors: development of a stably transfected human ovarian cell line for the detection of estrogenic and anti-estrogenic chemicals. In Vitro Mol Toxicol. 2000;13:67–82. [PubMed] [Google Scholar]
- Rose RL, Khan MA, Li M-H, Gillette JS, Hansen LG. Endocrine offects of episodic polychlorinated biphenyl (PCB) congeners. Rev Toxicol Environ Toxicol. 2002;2:1–18. [Google Scholar]
- Safe SH. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol. 1994;24:87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- Safe SH. Endocrine disruptors and human health: is there a problem? Toxicology. 2004;205:3–10. doi: 10.1016/j.tox.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Safe SH, Wörmke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chem Res Toxicol. 2003;16:807–816. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- Sonnenschein C, Soto AM, Fernandez MF, Olea N, Olea-Serrano MF, Ruiz-Lopez MD. Development of a marker of estrogenic exposure in human serum. Clin Chem. 1995;41:1888–1895. [PubMed] [Google Scholar]
- Soto AM, Fernandez MF, Luizzi MF, Oles Karasko AS, Sonnenschein C. Developing a marker of exposure to xenoestrogen mixtures in human serum. Environ Health Perspect. 1997;105(suppl 3):647–654. doi: 10.1289/ehp.97105s3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spink DC, Lincoln DW, II, Dickerman HW, Gierthy JF. 2,3,7,8-Tetrachlorodibenzo-p-dioxin causes an extensive alteration of 17β-estradiol metabolism in MCF-7 breast tumor cells. Proc Natl Acad Sci USA. 1990;87:6917–6921. doi: 10.1073/pnas.87.17.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K, Nakajima M, Fujiki Y, Katoh M, Gonzalez FJ, Yokoi T. Role of the aryl hydrocarbon receptor and Cyp1b1 in the antiestrogenic activity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Arch Toxicol. 2004;78:309–315. doi: 10.1007/s00204-004-0550-7. [DOI] [PubMed] [Google Scholar]
- Trnovec T, Kočan A, Langer P, Šovčíková E, Tajtáková M, Bergman Å, et al. Project proposal. Evaluating human health risk from low-dose and long-term exposure to polychlorinated biphenyls. Endocr Regul. 2000;34:167–168. [PubMed] [Google Scholar]
- van den Berg M, Birnbaum L, Bosveld AT, Brunström B, Cook P, Feeley M, et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect. 1998;106:775–792. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duursen MB, Sanderson JT, van den Berg M. Cytochrome P450 1A1 and 1B1 in human blood lymphocytes are not suitable as biomarkers of exposure to dioxin-like compounds: polymorphisms and interindividual variation in expression and inducibility. Toxicol Sci. 2005;85:703–712. doi: 10.1093/toxsci/kfi089. [DOI] [PubMed] [Google Scholar]
- van Duursen MB, Sanderson JT, van der Bruggen M, van der Linden J, van den Berg M. Effects of several dioxin-like compounds on estrogen metabolism in the malignant MCF-7 and nontumorigenic MCF-10A human mammary epithelial cell lines. Toxicol Appl Pharmacol. 2003;190:241–250. doi: 10.1016/s0041-008x(03)00166-2. [DOI] [PubMed] [Google Scholar]
- Wang WL, Smith R, III, Safe S. Aryl hydrocarbon receptor-mediated antiestrogenicity in MCF-7 cells: modulation of hormone-induced cell cycle enzymes. Arch Biochem Biophys. 1998;356:239–248. doi: 10.1006/abbi.1998.0782. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Takeyoshi M, Yakabe Y, Sawaki M, Imatanaka N, Takatsuki M. Comparison of reporter gene assay and immature rat uterotrophic assay of twenty-three chemicals. Toxicology. 2002;170:21–30. [Google Scholar]
- Yamazaki H, Shaw PM, Guengerich FP, Shimada T. Roles of cytochromes P450 1A2 and 3A4 in the oxidation of estradiol and estrone in human liver microsomes. Chem Res Toxicol. 1998;11:659–665. doi: 10.1021/tx970217f. [DOI] [PubMed] [Google Scholar]
- Zacharewski T. In vitro bioassay for assessing estrogenic substances. Environ Sci Technol. 1997;31:613–623. [Google Scholar]