Abstract
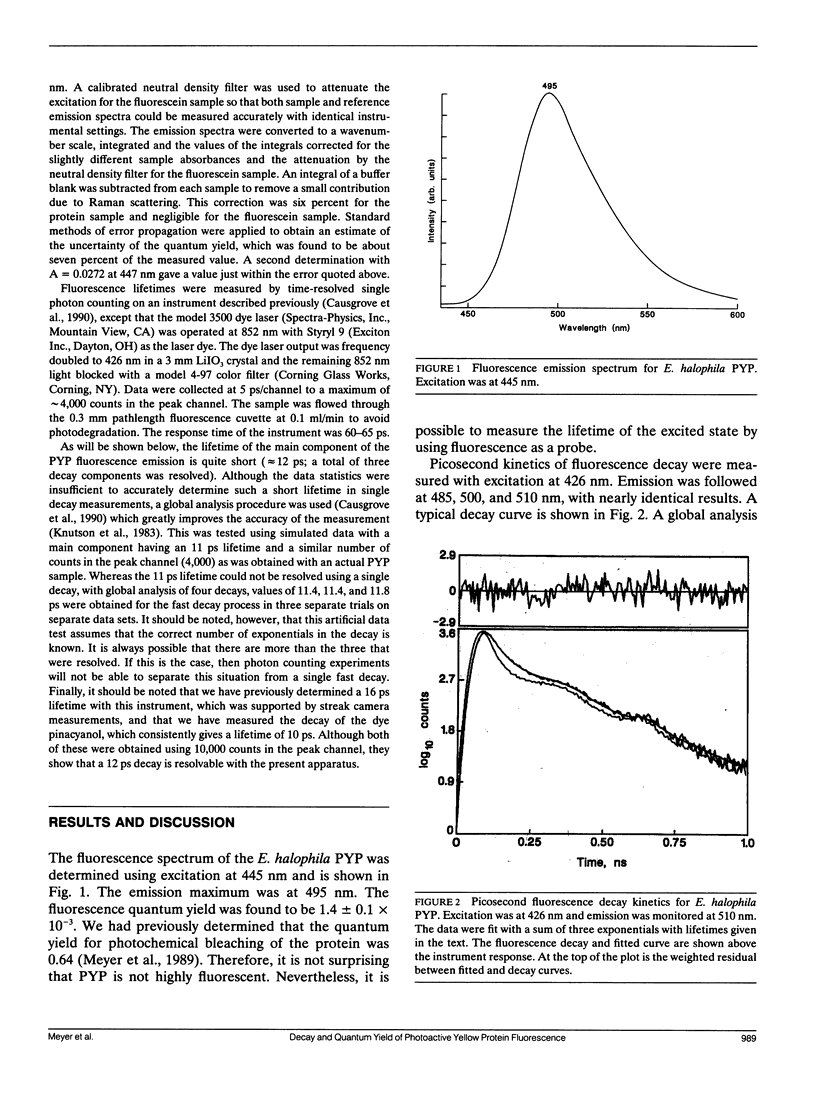
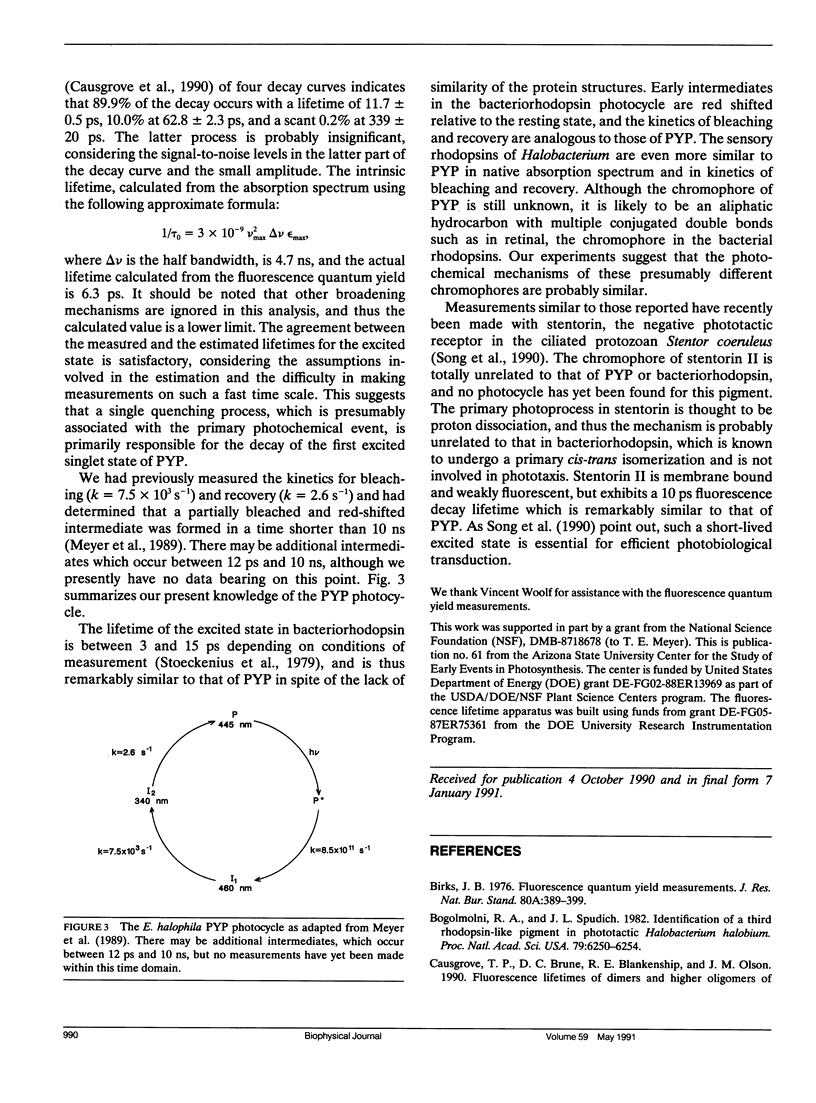
The photoactive yellow protein (PYP) has been previously shown to be partially bleached and red shifted (in less than 10 ns) by a pulse of laser excitation at the wavelength maximum (445 nm), to further bleach (k = 7.5 × 103 s-1), and then to slowly recover in the dark (k = 2.6 s-1) (Meyer, T. E., G. Tollin, J. H. Hazzard, and M. A. Cusanovich. 1989. Biophys. J. 56:559-564). The quantum yield for the formation of the fully bleached form was found to be 0.64. We have now shown that the yellow protein is weakly fluorescent with an emission maximum at 495 nm (which mirrors excitation at 445 nm) and a fluorescence quantum yield of 1.4 × 10-3. Measurement of the picosecond kinetics of the fluorescence decay shows that ∼90% of the emission occurs with a lifetime of 12 ps. This is in good agreement with the quantum yield determination, which suggests that a single quenching process (presumably the photochemical event) is primarily responsible for the excited state decay. The lifetime of the excited state of PYP is remarkably similar to that for the rise of the first photochemical intermediate of bacteriorhodopsin, and underscores the fundamental similarity in their photocycles despite a lack of structural relationship.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogomolni R. A., Spudich J. L. Identification of a third rhodopsin-like pigment in phototactic Halobacterium halobium. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6250–6254. doi: 10.1073/pnas.79.20.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Holden H. M., Rypniewski W. R., Law J. H., Rayment I. The molecular structure of insecticyanin from the tobacco hornworm Manduca sexta L. at 2.6 A resolution. EMBO J. 1987 Jun;6(6):1565–1570. doi: 10.1002/j.1460-2075.1987.tb02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. A., Bergfors T., Sedzik J., Unge T. The three-dimensional structure of P2 myelin protein. EMBO J. 1988 Jun;7(6):1597–1604. doi: 10.1002/j.1460-2075.1988.tb02985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRee D. E., Tainer J. A., Meyer T. E., Van Beeumen J., Cusanovich M. A., Getzoff E. D. Crystallographic structure of a photoreceptor protein at 2.4 A resolution. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6533–6537. doi: 10.1073/pnas.86.17.6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. E., Fitch J. C., Bartsch R. G., Tollin G., Cusanovich M. A. Soluble cytochromes and a photoactive yellow protein isolated from the moderately halophilic purple phototrophic bacterium, Rhodospirillum salexigens. Biochim Biophys Acta. 1990 Apr 26;1016(3):364–370. doi: 10.1016/0005-2728(90)90170-9. [DOI] [PubMed] [Google Scholar]
- Meyer T. E. Isolation and characterization of soluble cytochromes, ferredoxins and other chromophoric proteins from the halophilic phototrophic bacterium Ectothiorhodospira halophila. Biochim Biophys Acta. 1985 Jan 23;806(1):175–183. doi: 10.1016/0005-2728(85)90094-5. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Tollin G., Hazzard J. H., Cusanovich M. A. Photoactive yellow protein from the purple phototrophic bacterium, Ectothiorhodospira halophila. Quantum yield of photobleaching and effects of temperature, alcohols, glycerol, and sucrose on kinetics of photobleaching and recovery. Biophys J. 1989 Sep;56(3):559–564. doi: 10.1016/S0006-3495(89)82703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. E., Yakali E., Cusanovich M. A., Tollin G. Properties of a water-soluble, yellow protein isolated from a halophilic phototrophic bacterium that has photochemical activity analogous to sensory rhodopsin. Biochemistry. 1987 Jan 27;26(2):418–423. doi: 10.1021/bi00376a012. [DOI] [PubMed] [Google Scholar]
- Monaco H. L., Zanotti G., Spadon P., Bolognesi M., Sawyer L., Eliopoulos E. E. Crystal structure of the trigonal form of bovine beta-lactoglobulin and of its complex with retinol at 2.5 A resolution. J Mol Biol. 1987 Oct 20;197(4):695–706. doi: 10.1016/0022-2836(87)90476-1. [DOI] [PubMed] [Google Scholar]
- Newcomer M. E., Jones T. A., Aqvist J., Sundelin J., Eriksson U., Rask L., Peterson P. A. The three-dimensional structure of retinol-binding protein. EMBO J. 1984 Jul;3(7):1451–1454. doi: 10.1002/j.1460-2075.1984.tb01995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchettini J. C., Gordon J. I., Banaszak L. J. The structure of crystalline Escherichia coli-derived rat intestinal fatty acid-binding protein at 2.5-A resolution. J Biol Chem. 1988 Apr 25;263(12):5815–5819. [PubMed] [Google Scholar]
- Song P. S., Kim I. H., Florell S., Tamai N., Yamazaki T., Yamazaki I. Structure and function of the photoreceptor stentorins in Stentor coeruleus. II. Primary photoprocess and picosecond time-resolved fluorescence. Biochim Biophys Acta. 1990 Aug 1;1040(1):58–65. doi: 10.1016/0167-4838(90)90145-6. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]


