Abstract
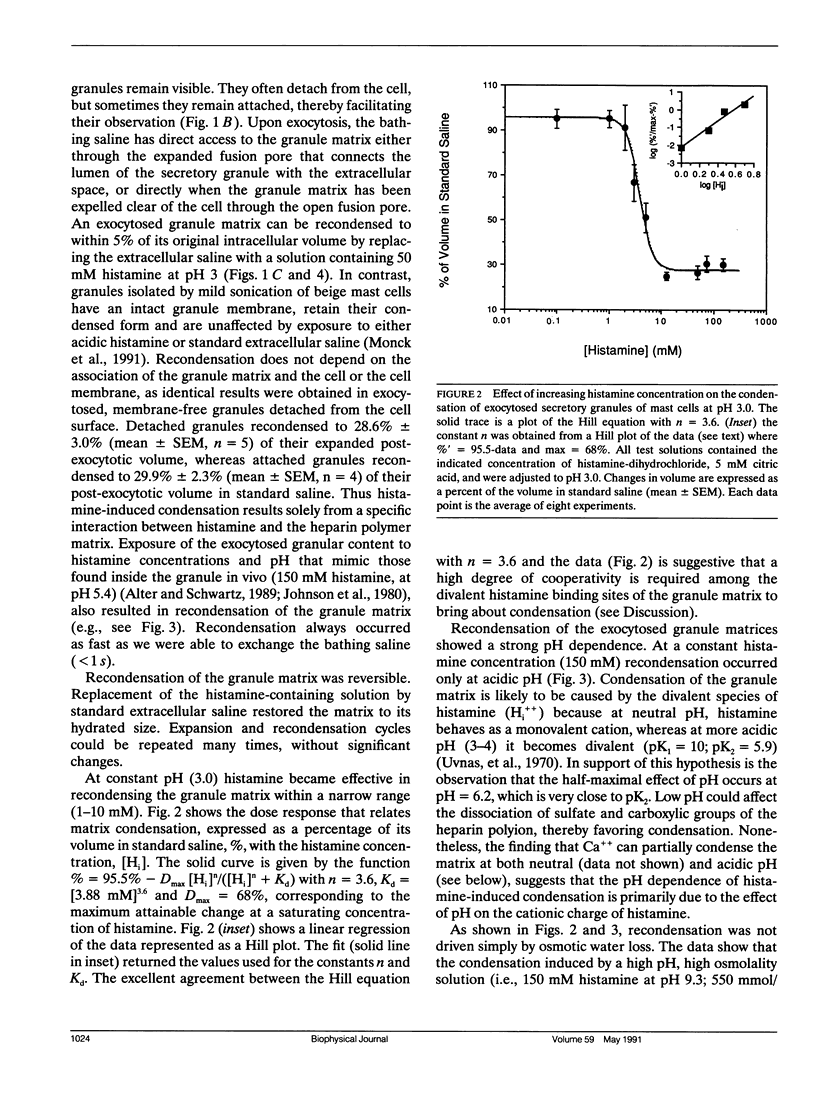
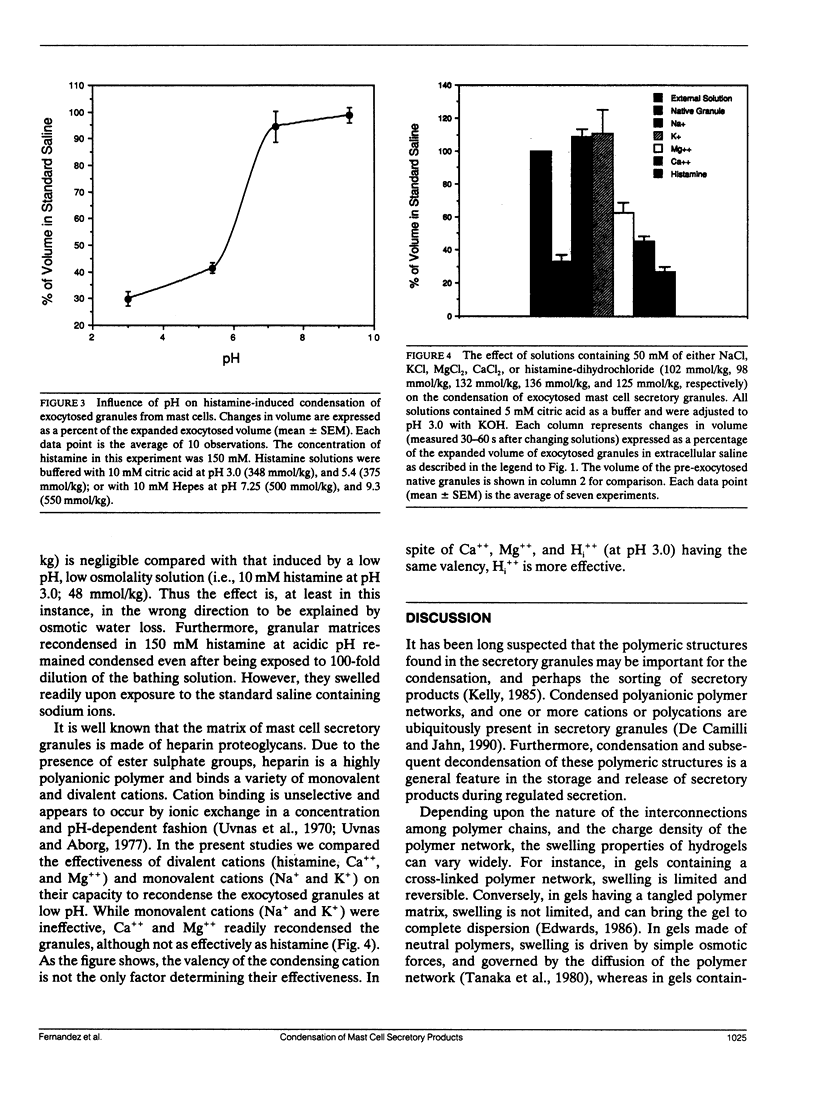
We have investigated the mechanisms responsible for the condensation and decondensation of secretory products that occur in mast cell secretion. We show here that the hydrated matrix of an exocytosed secretory granule can be recondensed to its original volume by exposure to acidic solutions containing histamine at concentrations that mimic those found in vivo. Recondensation by acidic histamine began in the range of 1-10 mM with a dose response curve that was accurately predicted by a Hill type equation with four highly cooperative binding sites and a half maximum concentration of [Hi++] = 3.9 mM. Recondensation by histamine showed a sigmoidal dependency on pH (critical range pH 5.5-6.5) and was fully reversible. These experiments suggest that histamine, possibly by binding to anionic sites in the protein-heparin complex of the granule matrix, triggers a change in the polymeric structures of the granule matrix from an extended coil to a collapsed globular state. This may be a useful model for understanding the condensation of secretory products into dense core granules and their subsequent decondensation upon exocytosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter S. C., Schwartz L. B. Effect of histamine and divalent cations on the activity and stability of tryptase from human mast cells. Biochim Biophys Acta. 1989 Jun 27;991(3):426–430. doi: 10.1016/0304-4165(89)90068-8. [DOI] [PubMed] [Google Scholar]
- Breckenridge L. J., Almers W. Final steps in exocytosis observed in a cell with giant secretory granules. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1945–1949. doi: 10.1073/pnas.84.7.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Jahn R. Pathways to regulated exocytosis in neurons. Annu Rev Physiol. 1990;52:625–645. doi: 10.1146/annurev.ph.52.030190.003205. [DOI] [PubMed] [Google Scholar]
- Edwards S. F. The theory of macromolecular networks. Biorheology. 1986;23(6):589–603. doi: 10.3233/bir-1986-23610. [DOI] [PubMed] [Google Scholar]
- Gerdes H. H., Rosa P., Phillips E., Baeuerle P. A., Frank R., Argos P., Huttner W. B. The primary structure of human secretogranin II, a widespread tyrosine-sulfated secretory granule protein that exhibits low pH- and calcium-induced aggregation. J Biol Chem. 1989 Jul 15;264(20):12009–12015. [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986 Oct 24;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Fumagalli G., Zanini A., Meldolesi J. Sorting of three secretory proteins to distinct secretory granules in acidophilic cells of cow anterior pituitary. J Cell Biol. 1987 Oct;105(4):1579–1586. doi: 10.1083/jcb.105.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. G., Carty S. E., Fingerhood B. J., Scarpa A. The internal pH of mast cell granules. FEBS Lett. 1980 Oct 20;120(1):75–79. doi: 10.1016/0014-5793(80)81050-7. [DOI] [PubMed] [Google Scholar]
- Kelly R. B. Pathways of protein secretion in eukaryotes. Science. 1985 Oct 4;230(4721):25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- Monck J. R., Oberhauser A. F., Alvarez de Toledo G., Fernandez J. M. Is swelling of the secretory granule matrix the force that dilates the exocytotic fusion pore? Biophys J. 1991 Jan;59(1):39–47. doi: 10.1016/S0006-3495(91)82196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Anderson R. G. The condensing vacuole of exocrine cells is more acidic than the mature secretory vesicle. Nature. 1987 Mar 5;326(6108):77–79. doi: 10.1038/326077a0. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Rabenstein D. L., Ludowyke R., Lagunoff D. Proton nuclear magnetic resonance studies of mast cell histamine. Biochemistry. 1987 Nov 3;26(22):6923–6926. doi: 10.1021/bi00396a010. [DOI] [PubMed] [Google Scholar]
- Tam P. Y., Verdugo P. Control of mucus hydration as a Donnan equilibrium process. Nature. 1981 Jul 23;292(5821):340–342. doi: 10.1038/292340a0. [DOI] [PubMed] [Google Scholar]
- Uvnäs B., Aborg C. H., Bergendorff A. Storage of histamine in mast cells. Evidence for an ionic binding of histamine to protein carboxyls in the granule heparin-protein complex. Acta Physiol Scand Suppl. 1970;336:1–26. [PubMed] [Google Scholar]
- Uvnäs B., Aborg C. H. On the cation exchanger properties of rat mast cell granules and their storage of histamine. Acta Physiol Scand. 1977 Jul;100(3):309–314. doi: 10.1111/j.1748-1716.1977.tb05955.x. [DOI] [PubMed] [Google Scholar]
- Verdugo P. Goblet cells secretion and mucogenesis. Annu Rev Physiol. 1990;52:157–176. doi: 10.1146/annurev.ph.52.030190.001105. [DOI] [PubMed] [Google Scholar]
- Verdugo P. Hydration kinetics of exocytosed mucins in cultured secretory cells of the rabbit trachea: a new model. Ciba Found Symp. 1984;109:212–225. doi: 10.1002/9780470720905.ch15. [DOI] [PubMed] [Google Scholar]