Abstract
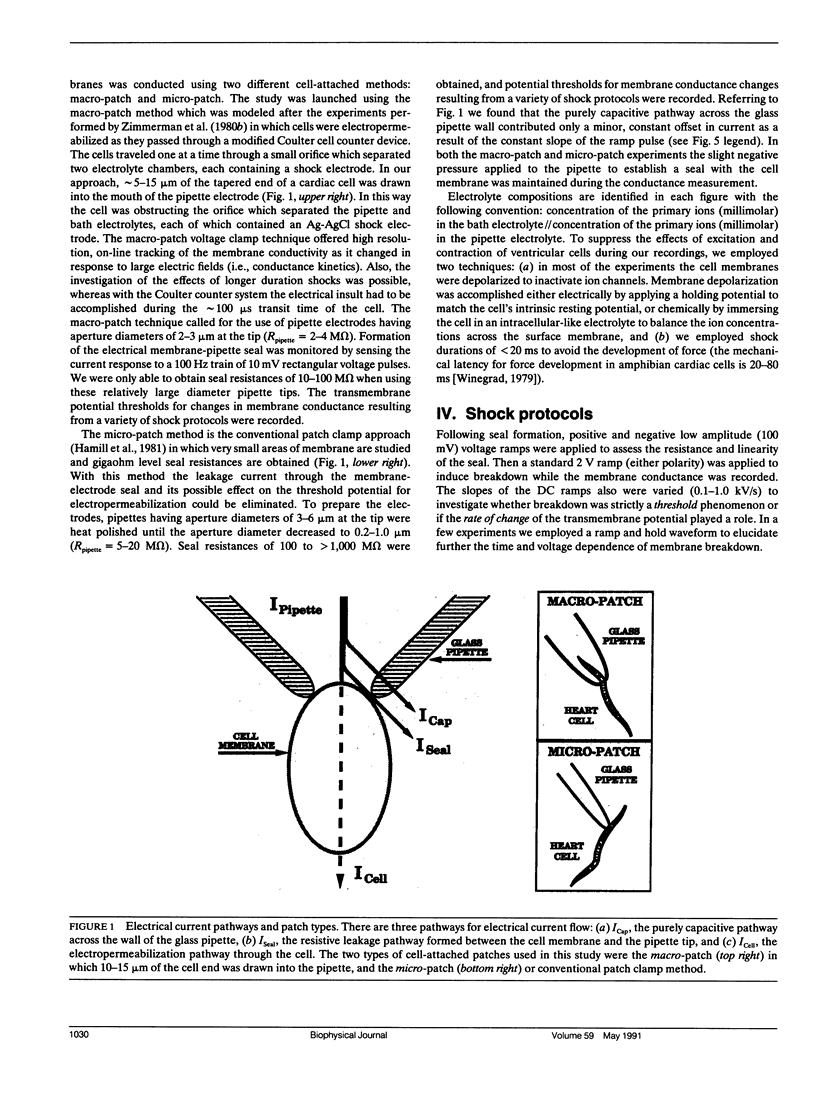
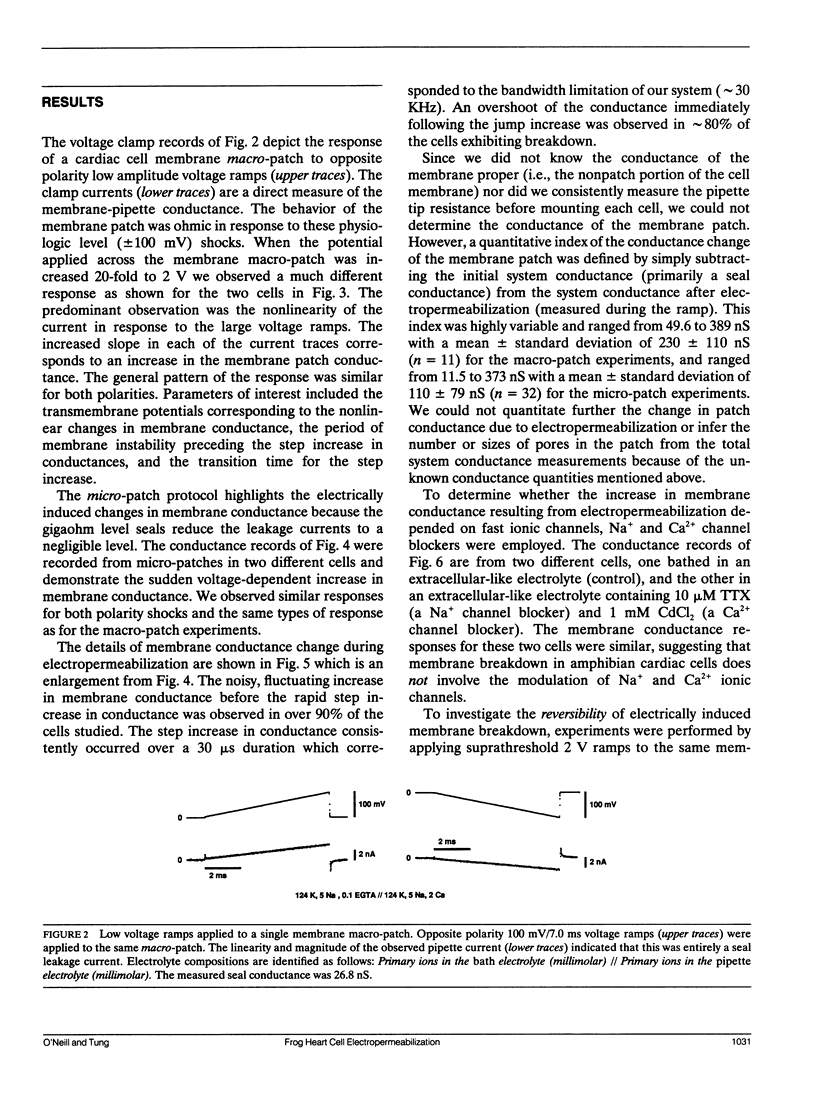
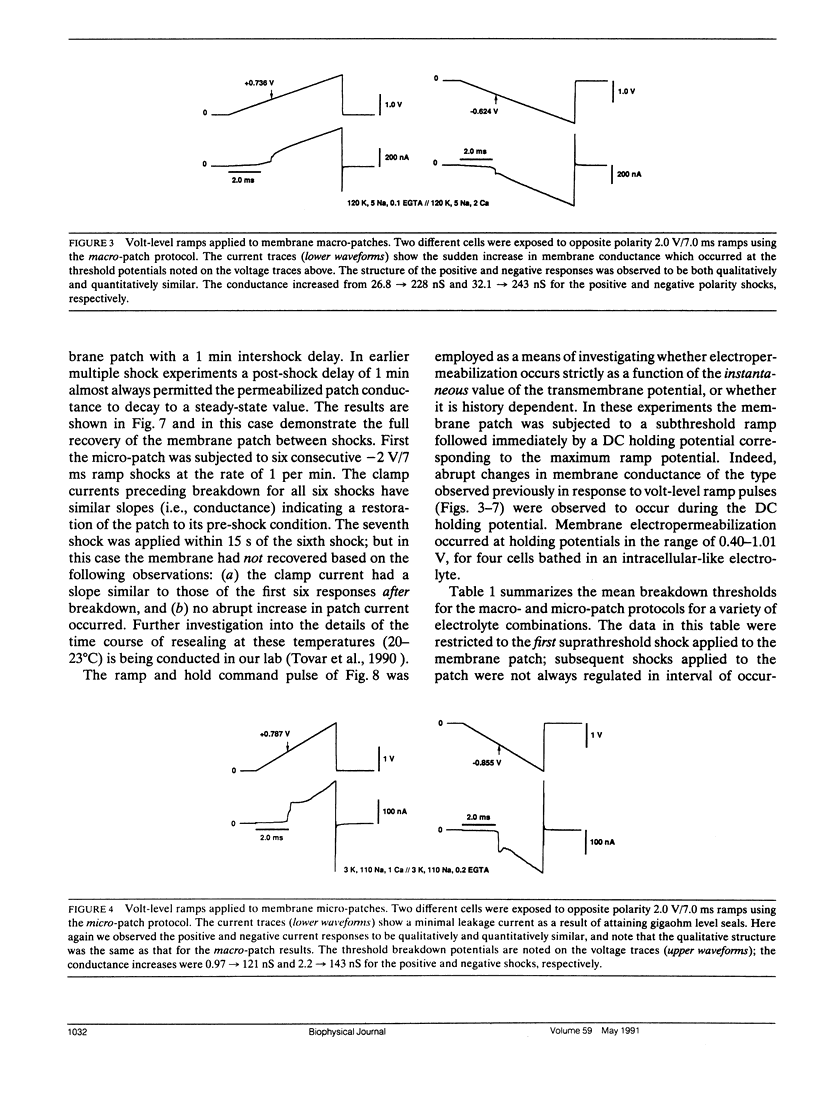
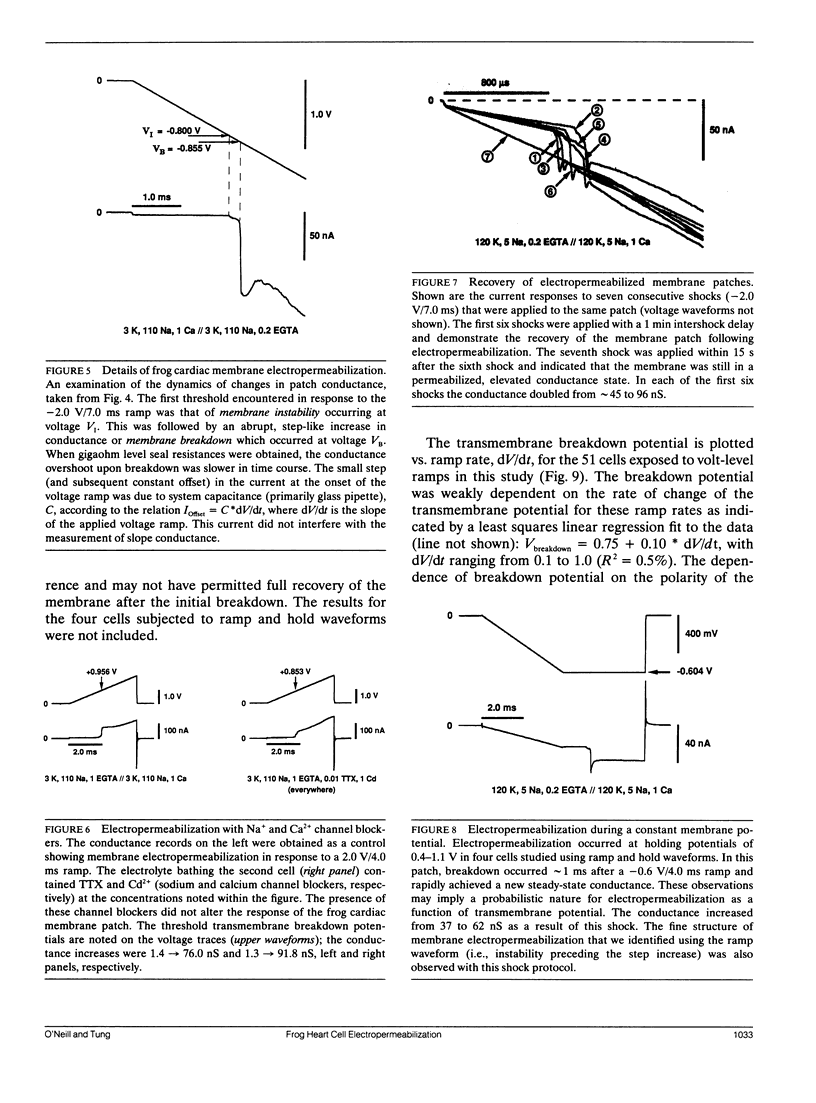
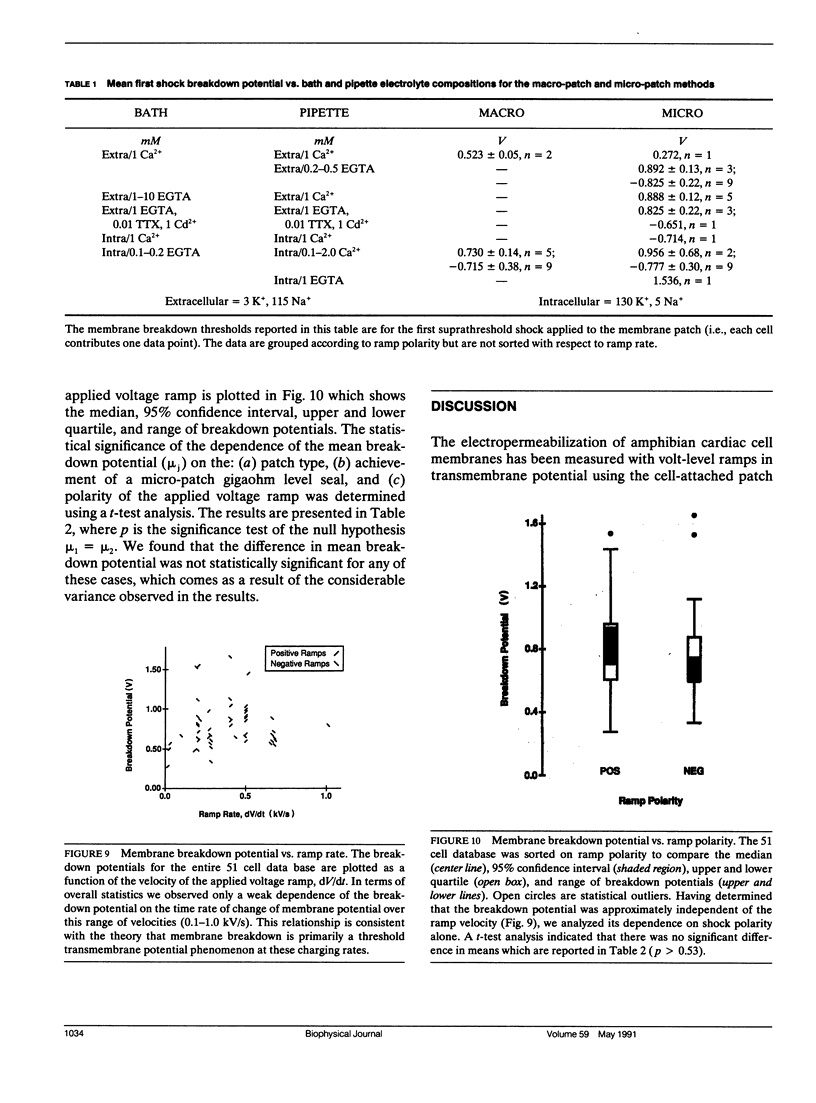
Potential gradients imposed across cell or lipid membranes break down the insulating properties of these barriers if an intensity and time-dependent threshold is exceeded. Potential gradients of this magnitude may occur throughout the body, and in particular in cardiac tissue, during clinical defibrillation, ablation, and electrocution trauma. To study the dynamics of membrane electropermeabilization a cell-attached patch clamp technique was used to directly control the potential across membrane patches of single ventricular cells enzymatically isolated from frog (Rana pipiens) hearts. Ramp waveshapes were used to reveal rapid membrane conductance changes that may have otherwise been obscured using rectangular waveshapes. We observed a step increase (delta t less than 30 microseconds) or breakdown in membrane conductance at transmembrane potential thresholds of 0.6-1.1 V in response to 0.1-1.0 kV/s voltage ramps. Conductance kinetics on a sub-millisecond time scale indicate that breakdown is preceded by a period of instability during which the noise and amplitude of the membrane conductance begin to increase. In some cells membrane breakdown was observed to be fully reversible when using an intershock interval of 1 min (20-23 degrees C). These findings support energetic models of membrane electropermeabilization which describe the formation of membrane pores (or growth of existing pores) to a conducting state (instability), followed by a rapid expansion of these pores when the energy barrier for the formation of hydrophilic pores is overcome (breakdown).
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardy G. H., Coltorti F., Stewart R. B., Greene H. L., Ivey T. D. Catheter-mediated electrical ablation: the relation between current and pulse width on voltage breakdown and shock-wave generation. Circ Res. 1988 Aug;63(2):409–414. doi: 10.1161/01.res.63.2.409. [DOI] [PubMed] [Google Scholar]
- Bardy G. H., Sawyer P. L. Biophysical and anatomical considerations for safe and efficacious catheter ablation of arrhythmias. Clin Cardiol. 1990 Jun;13(6):425–433. doi: 10.1002/clc.4960130611. [DOI] [PubMed] [Google Scholar]
- Benz R., Beckers F., Zimmermann U. Reversible electrical breakdown of lipid bilayer membranes: a charge-pulse relaxation study. J Membr Biol. 1979 Jul 16;48(2):181–204. doi: 10.1007/BF01872858. [DOI] [PubMed] [Google Scholar]
- Benz R., Conti F. Reversible electrical breakdown of squid giant axon membrane. Biochim Biophys Acta. 1981 Jul 6;645(1):115–123. doi: 10.1016/0005-2736(81)90518-6. [DOI] [PubMed] [Google Scholar]
- Benz R., Conti F. Structure of the squid axon membrane as derived from charge-pulse relaxation studies in the presence of absorbed lipophilic ions. J Membr Biol. 1981 Apr 15;59(2):91–104. doi: 10.1007/BF01875707. [DOI] [PubMed] [Google Scholar]
- Benz R., Zimmermann U. The resealing process of lipid bilayers after reversible electrical breakdown. Biochim Biophys Acta. 1981 Jan 8;640(1):169–178. doi: 10.1016/0005-2736(81)90542-3. [DOI] [PubMed] [Google Scholar]
- Bhatt D. L., Gaylor D. C., Lee R. C. Rhabdomyolysis due to pulsed electric fields. Plast Reconstr Surg. 1990 Jul;86(1):1–11. doi: 10.1097/00006534-199007000-00001. [DOI] [PubMed] [Google Scholar]
- Chang D. C., Reese T. S. Changes in membrane structure induced by electroporation as revealed by rapid-freezing electron microscopy. Biophys J. 1990 Jul;58(1):1–12. doi: 10.1016/S0006-3495(90)82348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L. V., Sukharev S. I., Popov S. V., Pastushenko V. F., Sokirko A. V., Abidor I. G., Chizmadzhev Y. A. The electrical breakdown of cell and lipid membranes: the similarity of phenomenologies. Biochim Biophys Acta. 1987 Sep 3;902(3):360–373. doi: 10.1016/0005-2736(87)90204-5. [DOI] [PubMed] [Google Scholar]
- Crowley J. M. Electrical breakdown of bimolecular lipid membranes as an electromechanical instability. Biophys J. 1973 Jul;13(7):711–724. doi: 10.1016/S0006-3495(73)86017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov D. S. Electric field-induced breakdown of lipid bilayers and cell membranes: a thin viscoelastic film model. J Membr Biol. 1984;78(1):53–60. doi: 10.1007/BF01872532. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S., Jain R. K. Membrane stability. Biochim Biophys Acta. 1984 Dec 4;779(4):437–468. doi: 10.1016/0304-4157(84)90020-0. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escande-Géraud M. L., Rols M. P., Dupont M. A., Gas N., Teissié J. Reversible plasma membrane ultrastructural changes correlated with electropermeabilization in Chinese hamster ovary cells. Biochim Biophys Acta. 1988 Apr 7;939(2):247–259. doi: 10.1016/0005-2736(88)90068-5. [DOI] [PubMed] [Google Scholar]
- Frazier D. W., Krassowska W., Chen P. S., Wolf P. D., Dixon E. G., Smith W. M., Ideker R. E. Extracellular field required for excitation in three-dimensional anisotropic canine myocardium. Circ Res. 1988 Jul;63(1):147–164. doi: 10.1161/01.res.63.1.147. [DOI] [PubMed] [Google Scholar]
- Gass G. V., Chernomordik L. V. Reversible large-scale deformations in the membranes of electrically-treated cells: electroinduced bleb formation. Biochim Biophys Acta. 1990 Mar 30;1023(1):1–11. doi: 10.1016/0005-2736(90)90002-6. [DOI] [PubMed] [Google Scholar]
- Glaser R. W., Leikin S. L., Chernomordik L. V., Pastushenko V. F., Sokirko A. I. Reversible electrical breakdown of lipid bilayers: formation and evolution of pores. Biochim Biophys Acta. 1988 May 24;940(2):275–287. doi: 10.1016/0005-2736(88)90202-7. [DOI] [PubMed] [Google Scholar]
- Gross D., Loew L. M., Webb W. W. Optical imaging of cell membrane potential changes induced by applied electric fields. Biophys J. 1986 Aug;50(2):339–348. doi: 10.1016/S0006-3495(86)83467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Jones J. L., Jones R. E., Balasky G. Microlesion formation in myocardial cells by high-intensity electric field stimulation. Am J Physiol. 1987 Aug;253(2 Pt 2):H480–H486. doi: 10.1152/ajpheart.1987.253.2.H480. [DOI] [PubMed] [Google Scholar]
- Jones J. L., Lepeschkin E., Jones R. E., Rush S. Response of cultured myocardial cells to countershock-type electric field stimulation. Am J Physiol. 1978 Aug;235(2):H214–H222. doi: 10.1152/ajpheart.1978.235.2.H214. [DOI] [PubMed] [Google Scholar]
- Jones J. L., Proskauer C. C., Paull W. K., Lepeschkin E., Jones R. E. Ultrastructural injury to chick myocardial cells in vitro following "electric countershock". Circ Res. 1980 Mar;46(3):387–394. doi: 10.1161/01.res.46.3.387. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Ashikawa I., Saita N., Yoshimura H., Itoh H., Nagayama K., Ikegami A. Electroporation of cell membrane visualized under a pulsed-laser fluorescence microscope. Biophys J. 1988 Jun;53(6):1015–1019. doi: 10.1016/S0006-3495(88)83181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. Y. Formation and resealing of pores of controlled sizes in human erythrocyte membrane. Nature. 1977 Aug 4;268(5619):438–441. doi: 10.1038/268438a0. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. Y. Voltage-induced conductance in human erythrocyte membranes. Biochim Biophys Acta. 1979 Jul 5;554(2):479–497. doi: 10.1016/0005-2736(79)90386-9. [DOI] [PubMed] [Google Scholar]
- Klee M., Plonsey R. Stimulation of spheroidal cells--the role of cell shape. IEEE Trans Biomed Eng. 1976 Jul;23(4):347–354. doi: 10.1109/tbme.1976.324597. [DOI] [PubMed] [Google Scholar]
- Krassowska W., Frazier D. W., Pilkington T. C., Ideker R. E. Potential distribution in three-dimensional periodic myocardium--Part II: Application to extracellular stimulation. IEEE Trans Biomed Eng. 1990 Mar;37(3):267–284. doi: 10.1109/10.52328. [DOI] [PubMed] [Google Scholar]
- Lee R. C., Gaylor D. C., Bhatt D., Israel D. A. Role of cell membrane rupture in the pathogenesis of electrical trauma. J Surg Res. 1988 Jun;44(6):709–719. doi: 10.1016/0022-4804(88)90105-9. [DOI] [PubMed] [Google Scholar]
- Mehrle W., Hampp R., Zimmermann U. Electric pulse induced membrane permeabilization. Spatial orientation and kinetics of solute efflux in freely suspended and dielectrophoretically aligned plant mesophyll protoplasts. Biochim Biophys Acta. 1989 Jan 30;978(2):267–275. doi: 10.1016/0005-2736(89)90124-7. [DOI] [PubMed] [Google Scholar]
- Mir L. M., Banoun H., Paoletti C. Introduction of definite amounts of nonpermeant molecules into living cells after electropermeabilization: direct access to the cytosol. Exp Cell Res. 1988 Mar;175(1):15–25. doi: 10.1016/0014-4827(88)90251-0. [DOI] [PubMed] [Google Scholar]
- Mitra R., Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985 Nov;249(5 Pt 2):H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Needham D., Hochmuth R. M. Electro-mechanical permeabilization of lipid vesicles. Role of membrane tension and compressibility. Biophys J. 1989 May;55(5):1001–1009. doi: 10.1016/S0006-3495(89)82898-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann F., Zimmermann U., Pilwat G. Release and uptake of haemoglobin and ions in red blood cells induced by dielectric breakdown. Biochim Biophys Acta. 1975 Jul 3;394(3):449–462. doi: 10.1016/0005-2736(75)90296-5. [DOI] [PubMed] [Google Scholar]
- Serpersu E. H., Kinosita K., Jr, Tsong T. Y. Reversible and irreversible modification of erythrocyte membrane permeability by electric field. Biochim Biophys Acta. 1985 Feb 14;812(3):779–785. doi: 10.1016/0005-2736(85)90272-x. [DOI] [PubMed] [Google Scholar]
- Sowers A. E., Lieber M. R. Electropore diameters, lifetimes, numbers, and locations in individual erythrocyte ghosts. FEBS Lett. 1986 Sep 15;205(2):179–184. doi: 10.1016/0014-5793(86)80893-6. [DOI] [PubMed] [Google Scholar]
- Sugar I. P., Förster W., Neumann E. Model of cell electrofusion. Membrane electroporation, pore coalescence and percolation. Biophys Chem. 1987 May 9;26(2-3):321–335. doi: 10.1016/0301-4622(87)80033-9. [DOI] [PubMed] [Google Scholar]
- Yabe S., Smith W. M., Daubert J. P., Wolf P. D., Rollins D. L., Ideker R. E. Conduction disturbances caused by high current density electric fields. Circ Res. 1990 May;66(5):1190–1203. doi: 10.1161/01.res.66.5.1190. [DOI] [PubMed] [Google Scholar]
- Zimmermann U., Beckers F., Coster H. G. The effect of pressure on the electrical breakdown in the membranes of Valonia utricularis. Biochim Biophys Acta. 1977 Jan 21;464(2):399–346. doi: 10.1016/0005-2736(77)90014-1. [DOI] [PubMed] [Google Scholar]
- Zimmermann U. Electrical breakdown, electropermeabilization and electrofusion. Rev Physiol Biochem Pharmacol. 1986;105:176–256. [PubMed] [Google Scholar]


