Abstract
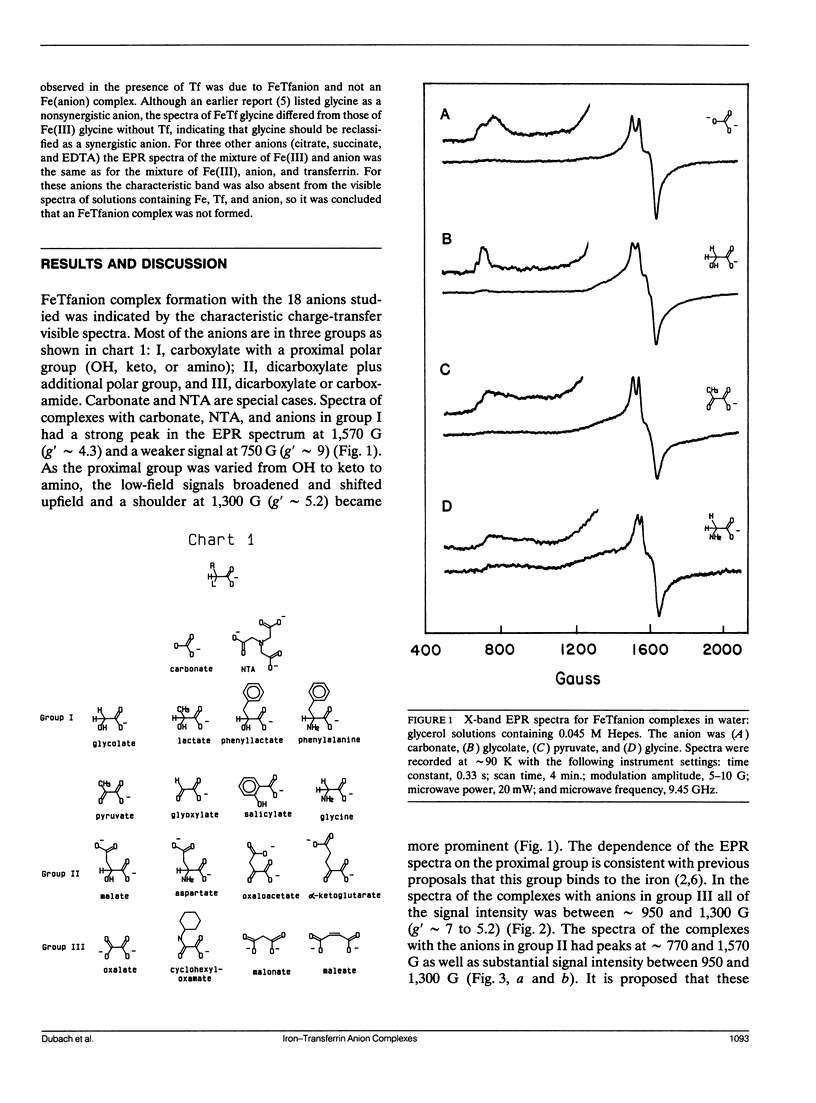
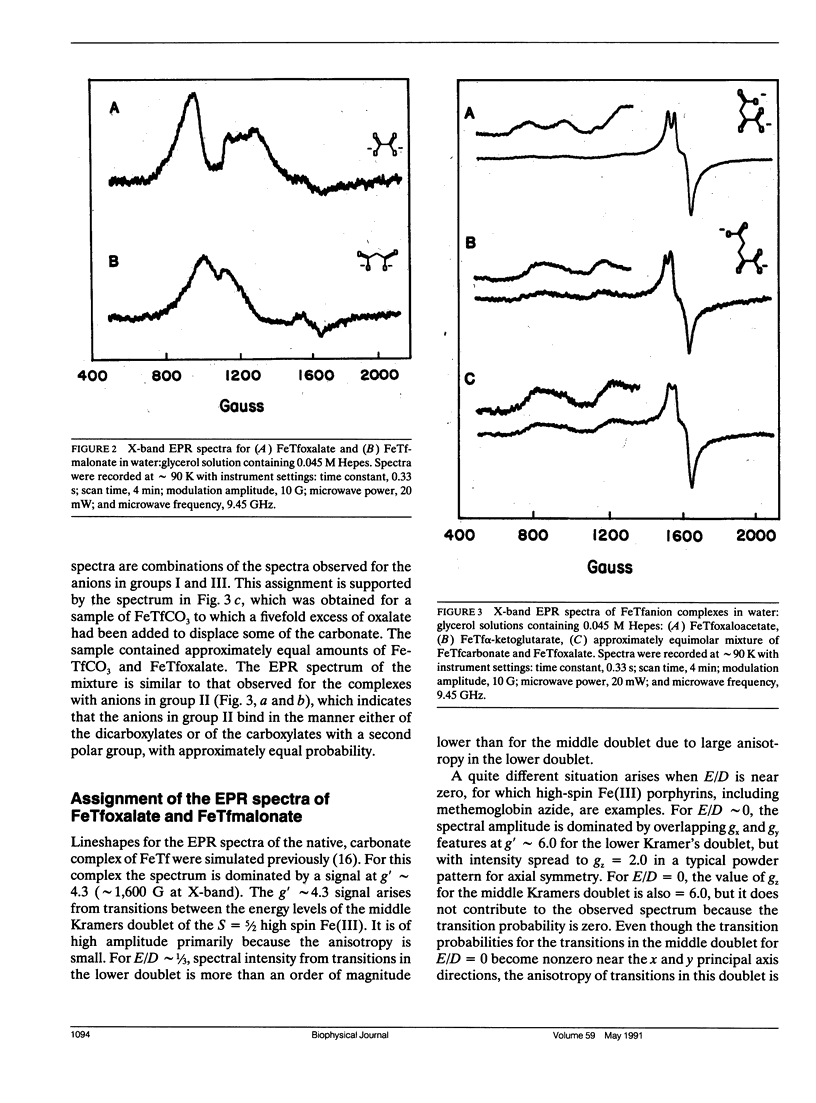
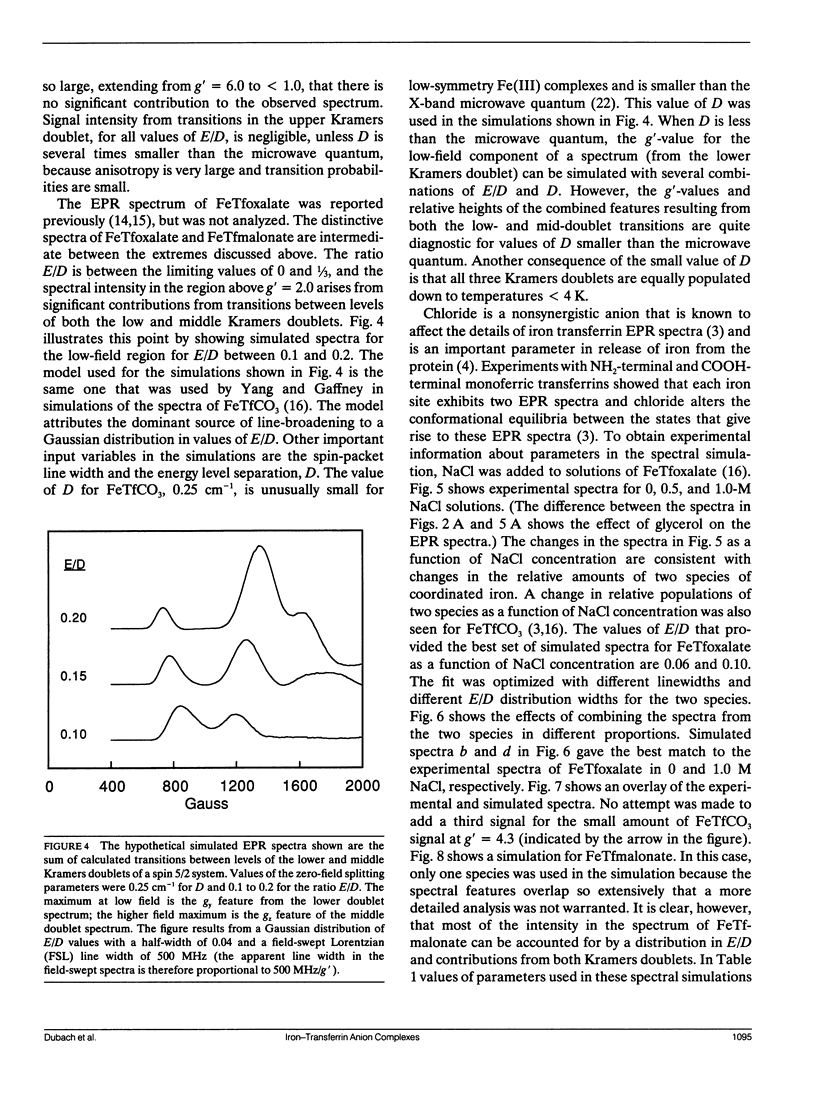
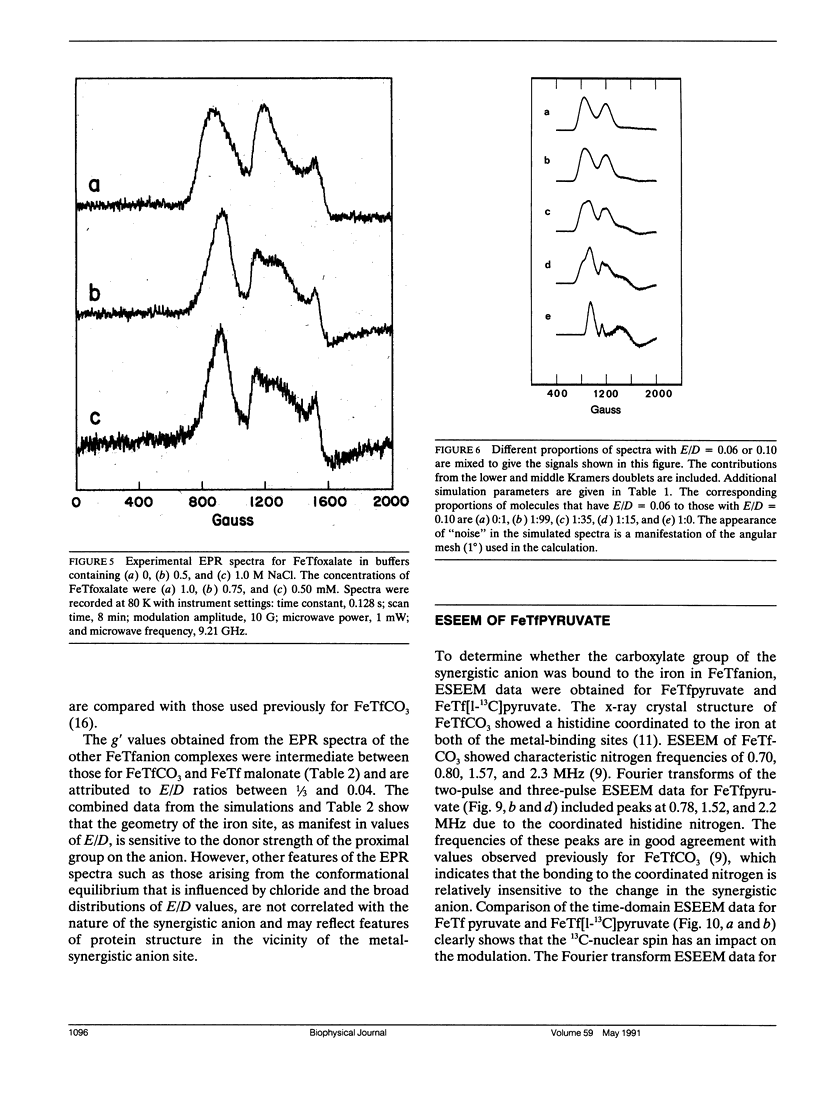
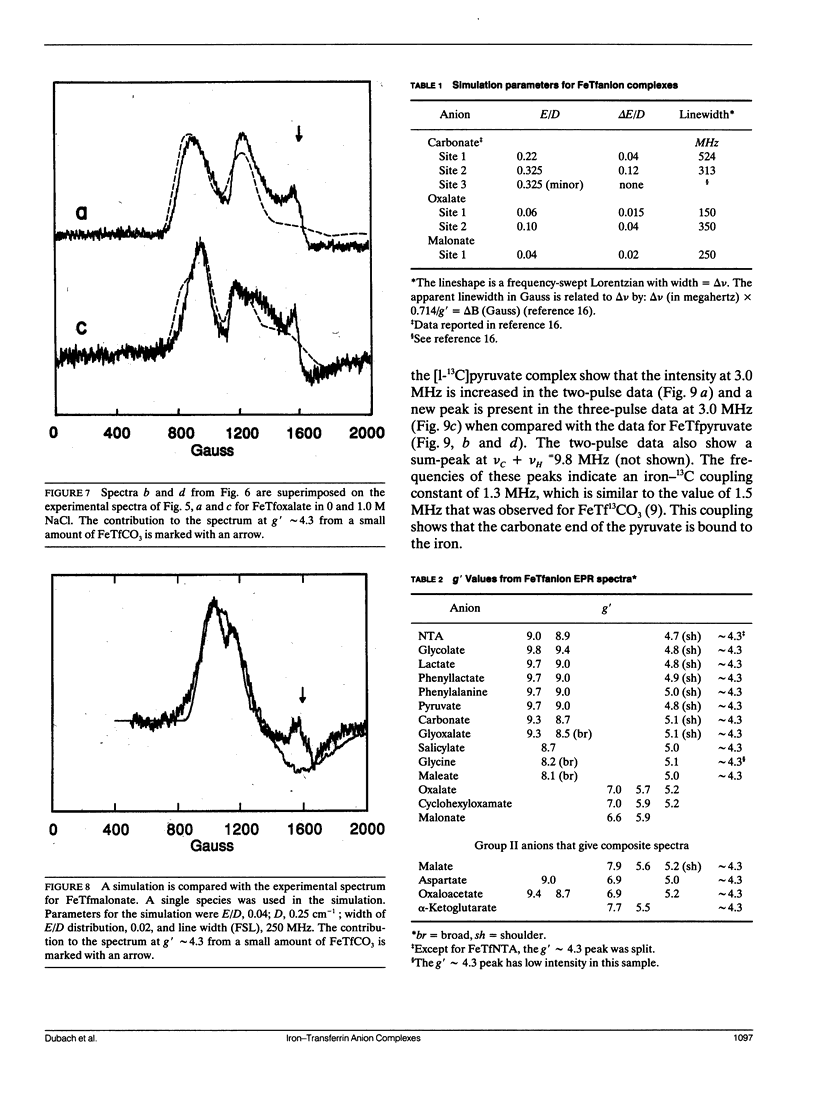
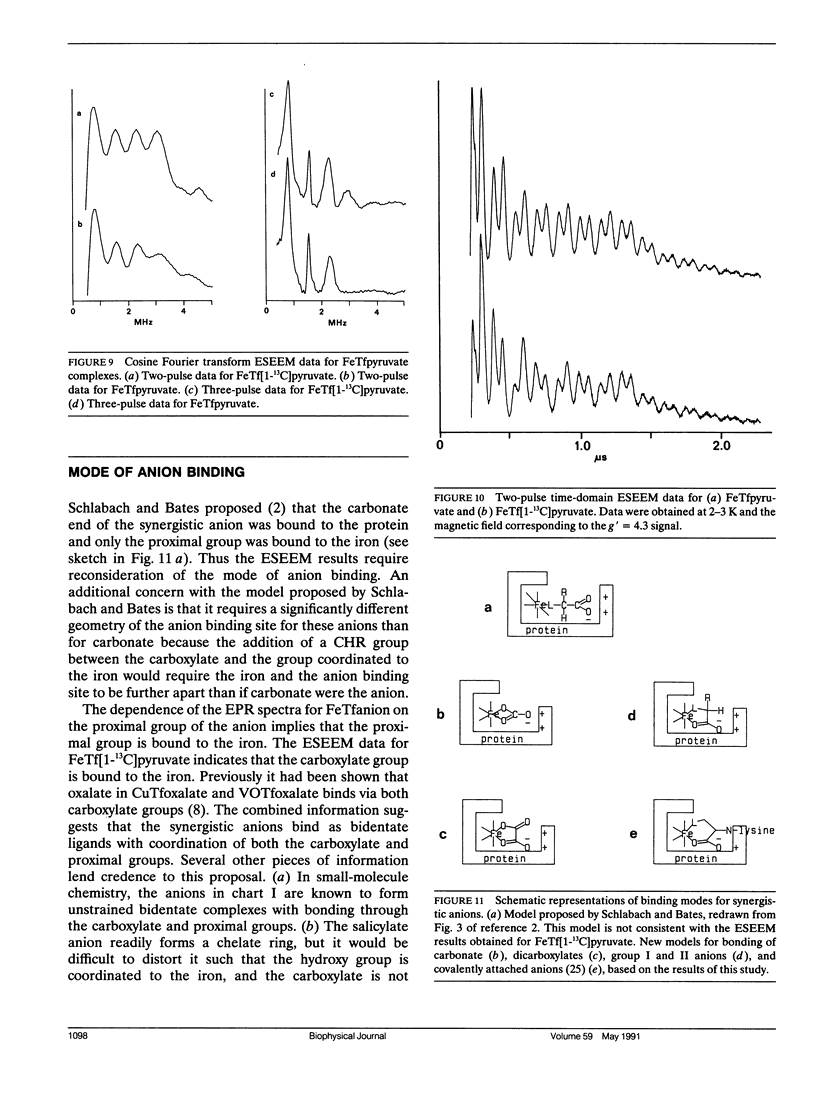
Continuous wave (cw) X-band EPR spectra at approximately 90 K were obtained for iron-transferrin-anion complexes with 18 anions. Each anion had a carboxylate group and at least one other polar moiety. As the second polar group was varied from hydroxyl to carbonyl to amine to carboxylate, the EPR spectra changed from a dominant signal at g' approximately 4.3 with a second smaller peak at g' approximately 9 to a broad signal with intensity between g' approximately 5 and 7. Computer simulation indicated that the changes in the EPR spectra were due to changes in the zero field splitting parameter ratio, E/D, from approximately 1/3 for carbonate anion to approximately 0.04 for malonate anion. Observation of iron-13C coupling in the electron spin echo envelope modulation (ESEEM) for iron transferrin [1-13C]pyruvate indicated that the carboxylate group was bound to the iron. It is proposed that all of the anions behave as bidentate ligands, with coordination to the iron through both the carboxylate and proximal groups, and the carboxyl group serves as a bridge between the iron and a positively charged group on the protein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Aasa R., Malmström B. G., Vänngård T. Bicarbonate and the binding of iron to transferrin. J Biol Chem. 1967 May 25;242(10):2484–2490. [PubMed] [Google Scholar]
- Aisen P., Pinkowitz R. A., Leibman A. EPR and other studies of the anion-binding sites of transferrin. Ann N Y Acad Sci. 1973 Dec 31;222:337–346. doi: 10.1111/j.1749-6632.1973.tb15272.x. [DOI] [PubMed] [Google Scholar]
- Anderson B. F., Baker H. M., Dodson E. J., Norris G. E., Rumball S. V., Waters J. M., Baker E. N. Structure of human lactoferrin at 3.2-A resolution. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1769–1773. doi: 10.1073/pnas.84.7.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. T., Patch M. G., Carrano C. J. Affinity labels for the anion-binding site in ovotransferrin. Biochemistry. 1988 Aug 23;27(17):6276–6282. doi: 10.1021/bi00417a012. [DOI] [PubMed] [Google Scholar]
- Bailey S., Evans R. W., Garratt R. C., Gorinsky B., Hasnain S., Horsburgh C., Jhoti H., Lindley P. F., Mydin A., Sarra R. Molecular structure of serum transferrin at 3.3-A resolution. Biochemistry. 1988 Jul 26;27(15):5804–5812. doi: 10.1021/bi00415a061. [DOI] [PubMed] [Google Scholar]
- Bates G. W., Schlabach M. R. The nonspecific binding of Fe3+ to transferrin in the absence of synergistic anions. J Biol Chem. 1975 Mar 25;250(6):2177–2181. [PubMed] [Google Scholar]
- Bates G. W., Wernicke J. The kinetics and mechanism of iron(3) exchange between chelates and transferrin. IV. The reaction of transferrin with iron(3) nitrilotriacetate. J Biol Chem. 1971 Jun 10;246(11):3679–3685. [PubMed] [Google Scholar]
- Campbell R. F., Chasteen N. D. An anion binding study of vanadyl(IV) human serotransferrin. Evidence for direct linkage to the metal. J Biol Chem. 1977 Sep 10;252(17):5996–6001. [PubMed] [Google Scholar]
- Eaton S. S., Dubach J., Eaton G. R., Thurman G., Ambruso D. R. Electron spin echo envelope modulation evidence for carbonate binding to iron(III) and copper(II) transferrin and lactoferrin. J Biol Chem. 1990 May 5;265(13):7138–7141. [PubMed] [Google Scholar]
- Eaton S. S., Dubach J., More K. M., Eaton G. R., Thurman G., Ambruso D. R. Comparison of the electron spin echo envelope modulation (ESEEM) for human lactoferrin and transferrin complexes of copper(II) and vanadyl ion. J Biol Chem. 1989 Mar 25;264(9):4776–4781. [PubMed] [Google Scholar]
- Kretchmar S. A., Teixeira M., Huynh B. H., Raymond K. N. Mössbauer studies of electrophoretically purified monoferric and diferric human transferrin. Biol Met. 1988;1(1):26–32. doi: 10.1007/BF01128014. [DOI] [PubMed] [Google Scholar]
- Najarian R. C., Harris D. C. Oxalate and spin-labeled oxalate as probes of the anion binding site of human transferrin. Metal to anion distance. J Biol Chem. 1978 Jan 10;253(1):38–42. [PubMed] [Google Scholar]
- Schlabach M. R., Bates G. W. The synergistic binding of anions and Fe3+ by transferrin. Implications for the interlocking sites hypothesis. J Biol Chem. 1975 Mar 25;250(6):2182–2188. [PubMed] [Google Scholar]
- Tipton P. A., McCracken J., Cornelius J. B., Peisach J. Electron spin echo envelope modulation studies of pyruvate kinase active-site complexes. Biochemistry. 1989 Jul 11;28(14):5720–5728. doi: 10.1021/bi00440a004. [DOI] [PubMed] [Google Scholar]
- Yang A. S., Gaffney B. J. Determination of relative spin concentration in some high-spin ferric proteins using E/D-distribution in electron paramagnetic resonance simulations. Biophys J. 1987 Jan;51(1):55–67. doi: 10.1016/S0006-3495(87)83311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


