Abstract
1. These experiments have investigated the contribution made by GABA-mediated inhibitory processes to the orientation tuning of complex cells in the cat's striate cortex. The GABA antagonist bicuculline has been ionophoretically applied to individual complex cells and the modifications produced in their orientation tuning documented. 2. In terms of the type of change produced in orientation tuning by the application of bicuculline, it seems that there are two categories of complex cells. 3. In one of these categories the orientation selectivity was eliminated during bicuculline application. The excitatory input to these cells would therefore appear to be non-orientation specific. Their orientation selectivity is presumably generated by a GABA-mediated inhibitory input. 4. In the other category of complex cells, although the orientation selectivity was decreased during bicuculline application, the cells retained a preference for a range of orientations that was generally centred around the original optimal orientation. It is suggested that for these cells the inhibitory input enhances the orientation tuning of an excitatory input that is already broadly orientation tuned. 5. Comparison of normal orientation tuning curves with those observed during the application of bicuculline provides a basis for estimating the orientation tuning of the GABA-mediated inhibitory input. In all cases, it is clear that at normal resting discharge levels, orientations either side of the optimal, and not those centred on the optimal, generate the most powerful inhibitory input. 6. These results would seem to be best explained by inhibitory interconnexions between cortical columns sensitive to different orientations. This type of lateral interaction between columns may serve to enhance the contrast in the orientation domain for the cortical representation of a specific stimulus orientation. 7. Increasing the resting discharge level of a complex cell, without blocking the action of GABA appeared to increase the gain of the inhibitory mechanisms acting on the cell. The normal excitatory responses to optimal or near optimal orientations were greatly reduced, or replaced by inhibitory responses, and non-optimal orientations produced only inhibitory responses. These inhibitory effects were blocked on the context of other observations in the literature. It is tentatively suggested that the interneurones providing the inhibitory drive to complex cells receive an input from recurrent collaterals of the recipient complex cells. Their other inputs would derive from neighbouring colums and from the afferent input to the parent column. The inputs from neighbouring columns would mediate the lateral inhibitory interactions in the orientation domain, and the recurrent collateral feed-back the decreased responsiveness at high resting discharge levels.
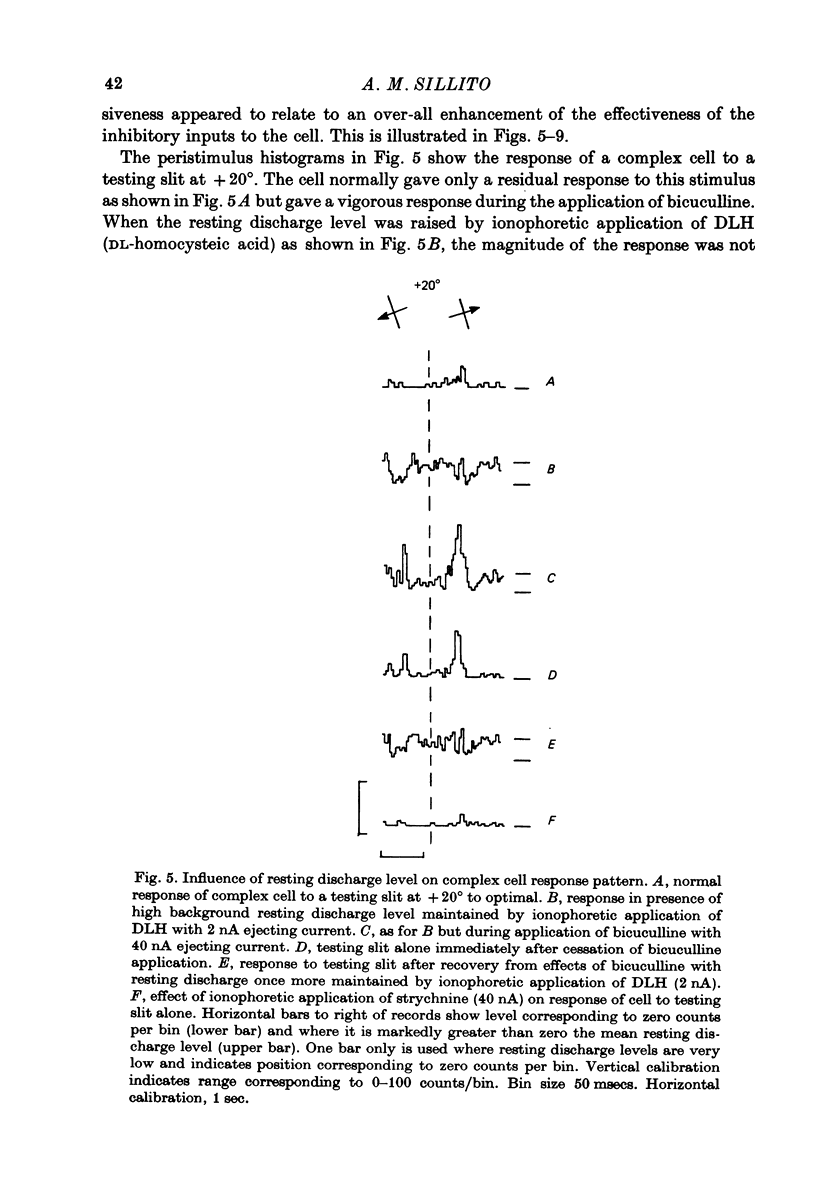
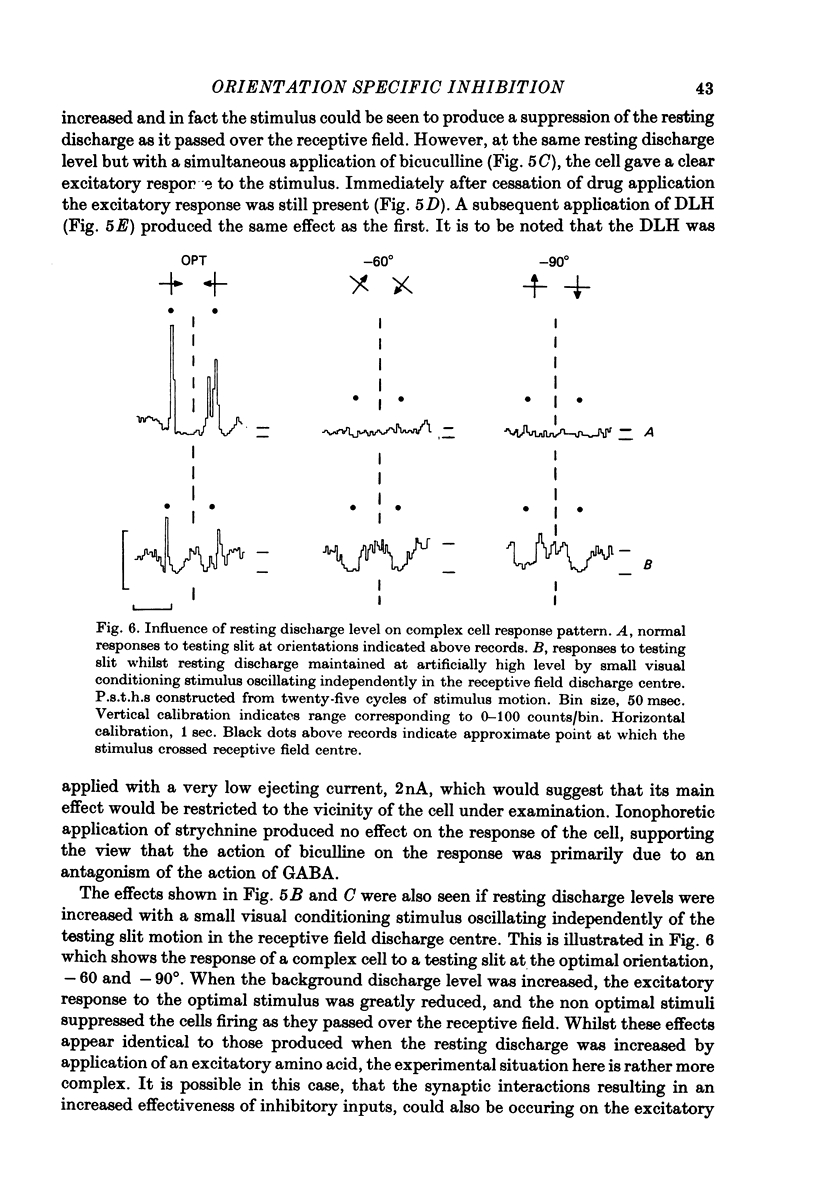
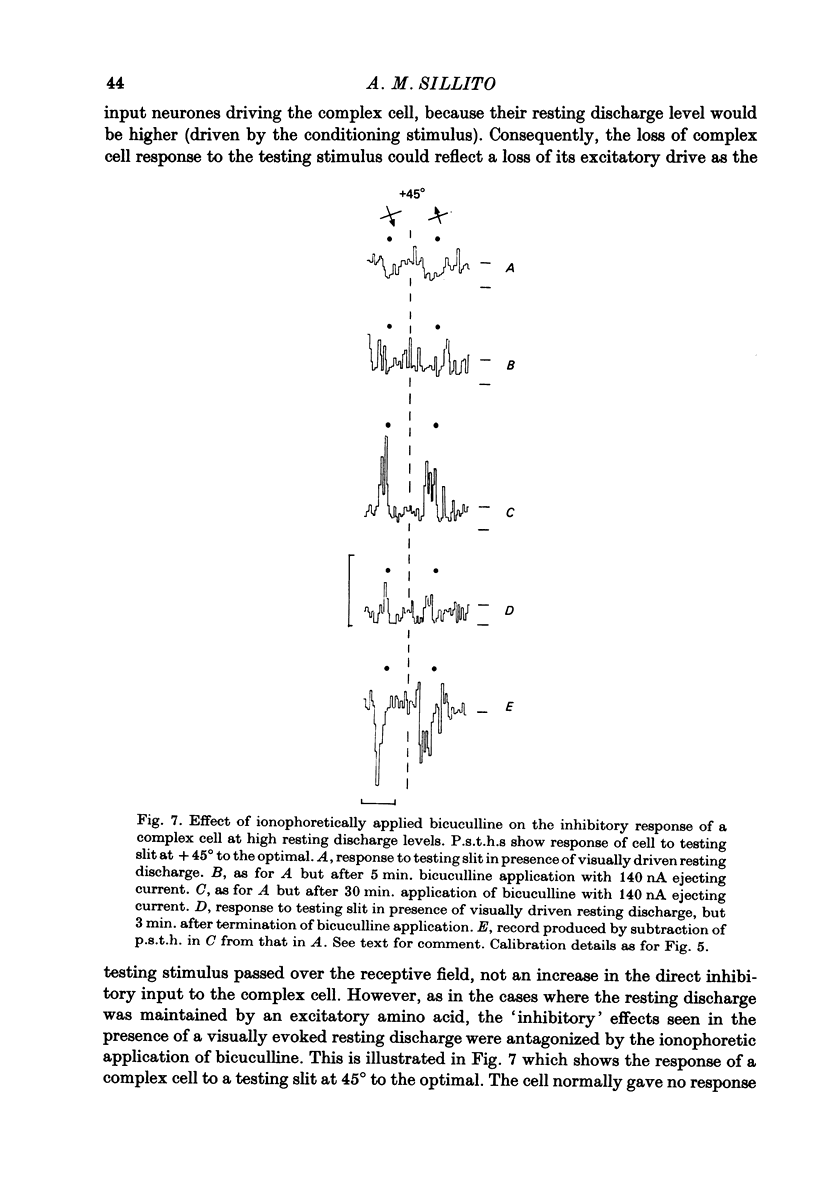
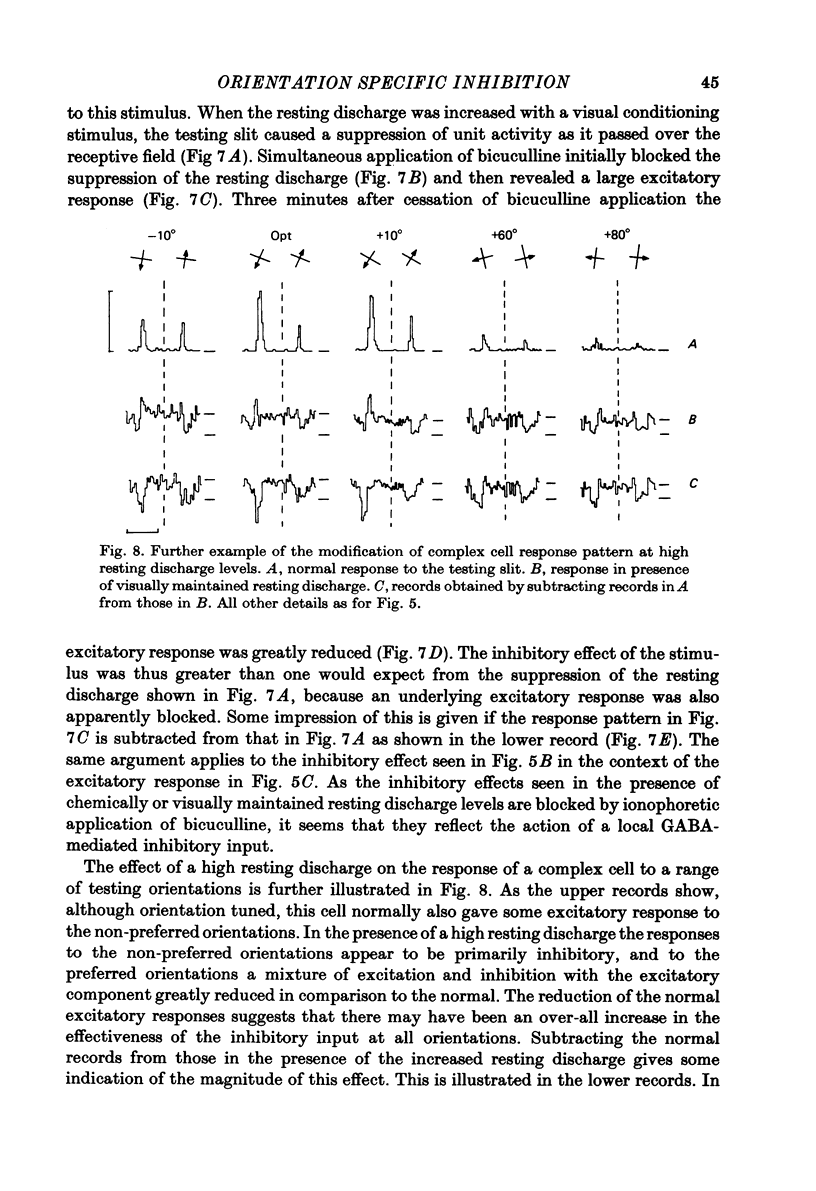
Full text
PDF



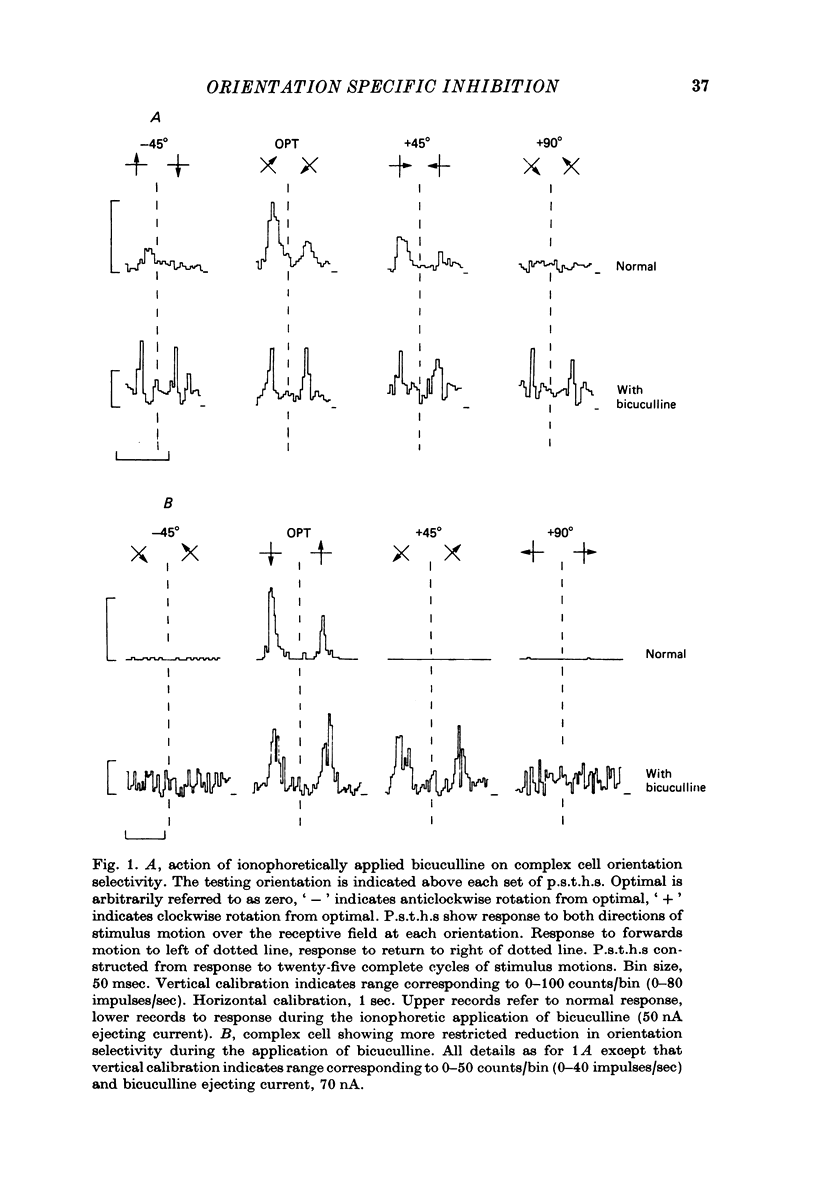
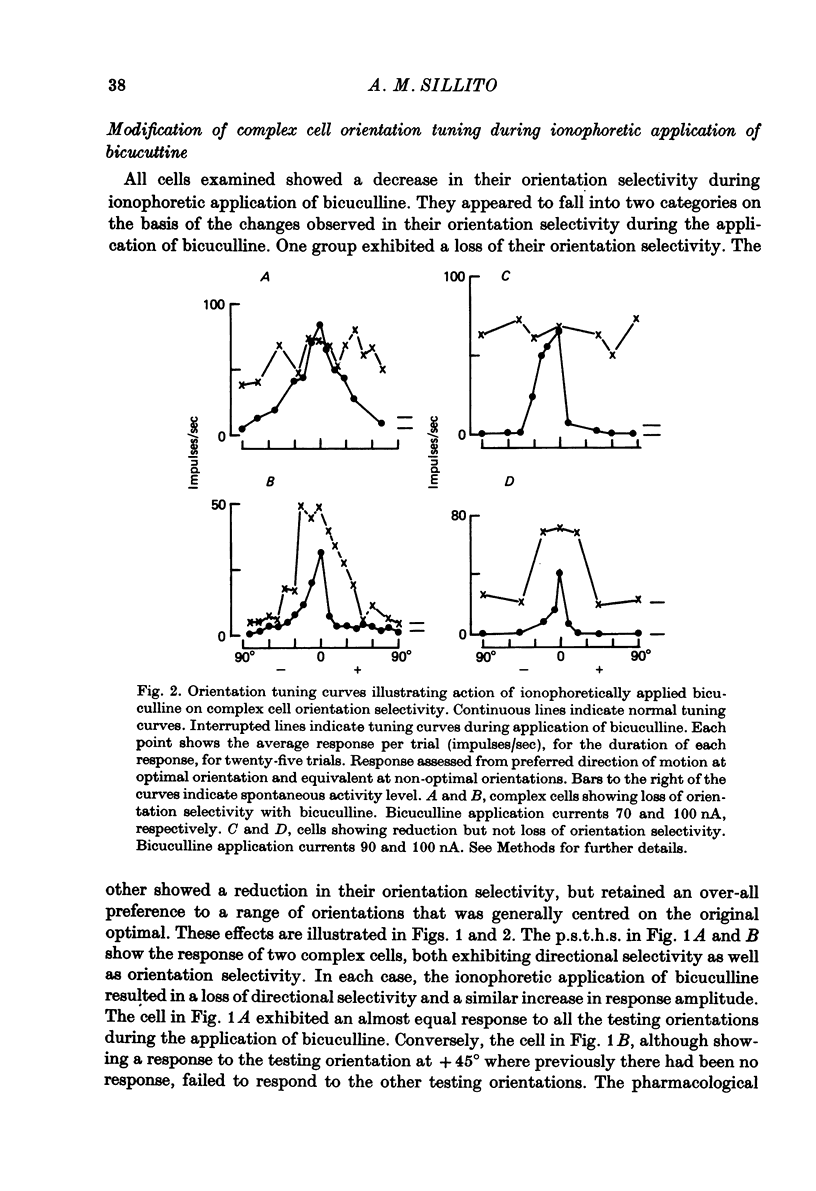

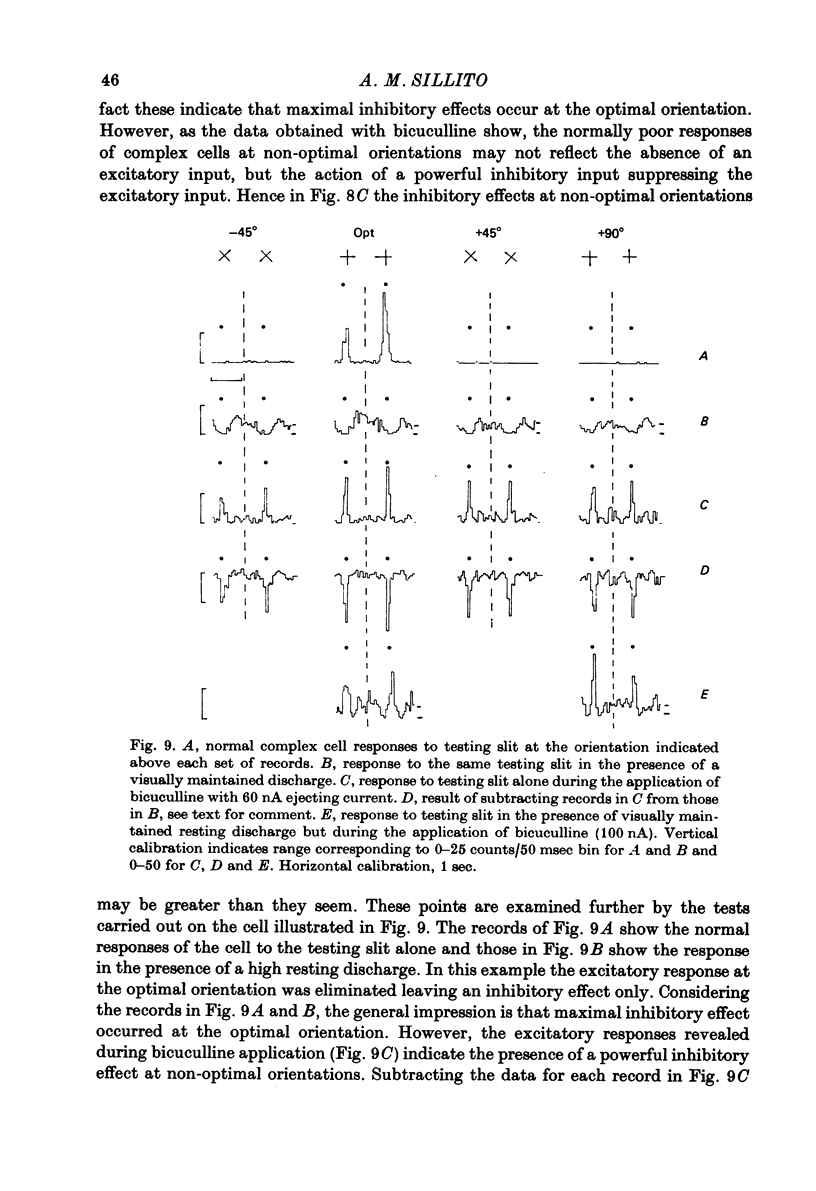



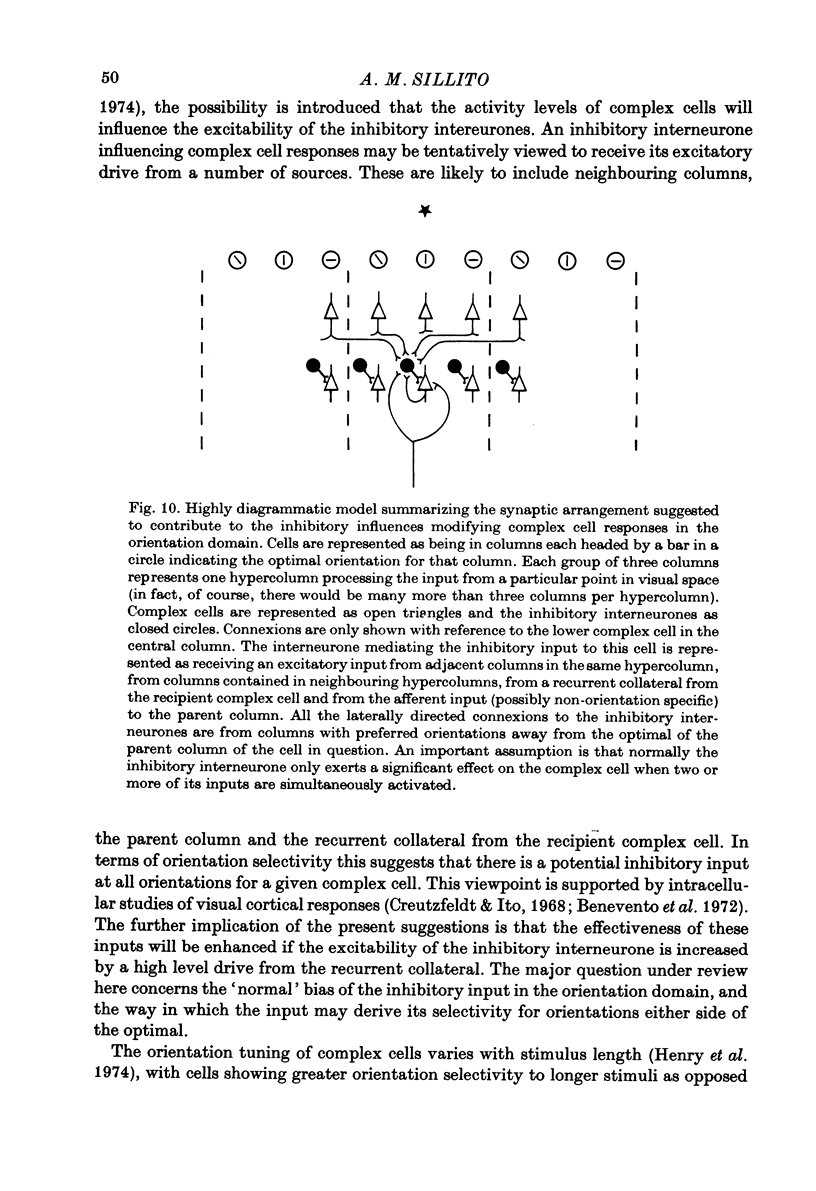



Selected References
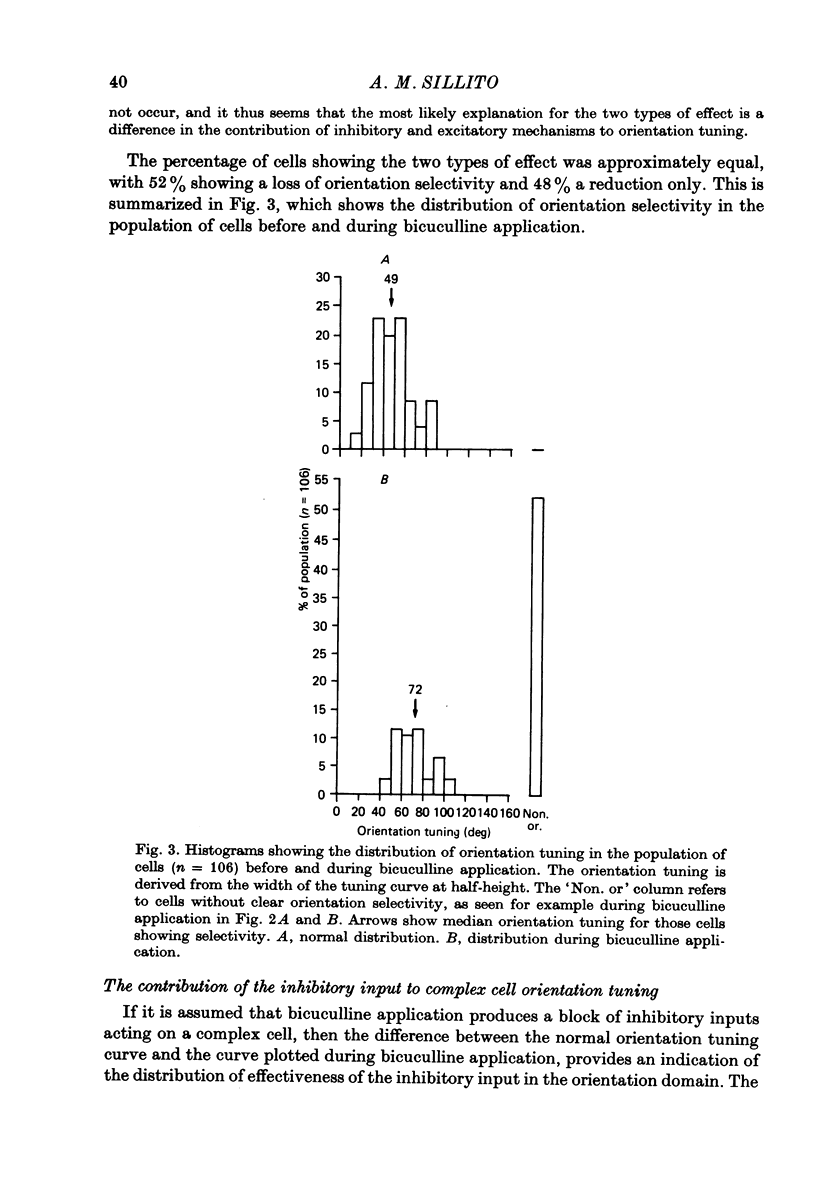
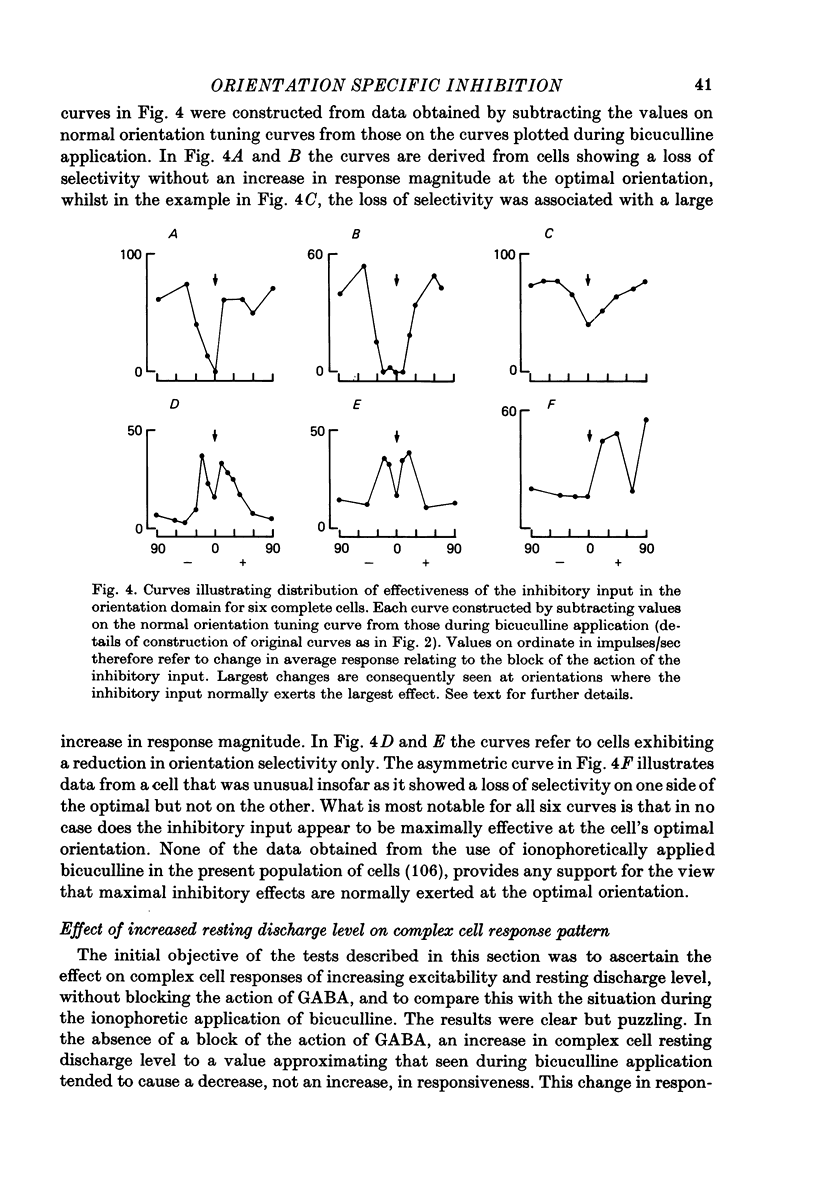
These references are in PubMed. This may not be the complete list of references from this article.
- Asanuma H., Rosén I. Spread of mono- and polysynaptic connections within cat's motor cortex. Exp Brain Res. 1973 Mar 19;16(5):507–520. doi: 10.1007/BF00234477. [DOI] [PubMed] [Google Scholar]
- Benevento L. A., Creutzfeldt O. D., Kuhnt U. Significance of intracortical inhibition in the visual cortex. Nat New Biol. 1972 Jul 26;238(82):124–126. doi: 10.1038/newbio238124a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Tobin E. A. Lateral inhibition between orientation detectors in the cat's visual cortex. Exp Brain Res. 1972;15(4):439–440. doi: 10.1007/BF00234129. [DOI] [PubMed] [Google Scholar]
- Carpenter R. H., Blakemore C. Interactions between orientations in human vision. Exp Brain Res. 1973 Oct 26;18(3):287–303. doi: 10.1007/BF00234599. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O., Ito M. Functional synaptic organization of primary visual cortex neurones in the cat. Exp Brain Res. 1968;6(4):324–352. doi: 10.1007/BF00233183. [DOI] [PubMed] [Google Scholar]
- Fisken R. A., Garey L. J., Powell T. P. The intrinsic, association and commissural connections of area 17 on the visual cortex. Philos Trans R Soc Lond B Biol Sci. 1975 Nov 20;272(919):487–536. doi: 10.1098/rstb.1975.0099. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Powell T. P. An experimental study of the termination of the lateral geniculo-cortical pathway in the cat and monkey. Proc R Soc Lond B Biol Sci. 1971 Oct 12;179(1054):41–63. doi: 10.1098/rspb.1971.0080. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977 Jun;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F. The marking of electrode tip positions in nervous tissue. J Physiol. 1971;214 (Suppl):12P–12P. [PubMed] [Google Scholar]
- Henry G. H., Dreher B., Bishop P. O. Orientation specificity of cells in cat striate cortex. J Neurophysiol. 1974 Nov;37(6):1394–1409. doi: 10.1152/jn.1974.37.6.1394. [DOI] [PubMed] [Google Scholar]
- Hoffman K. P., Stone J. Conduction velocity of afferents to cat visual cortex: a correlation with cortical receptive field properties. Brain Res. 1971 Sep 24;32(2):460–466. doi: 10.1016/0006-8993(71)90340-4. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Sequence regularity and geometry of orientation columns in the monkey striate cortex. J Comp Neurol. 1974 Dec 1;158(3):267–293. doi: 10.1002/cne.901580304. [DOI] [PubMed] [Google Scholar]
- Kelly J. P., Van Essen D. C. Cell structure and function in the visual cortex of the cat. J Physiol. 1974 May;238(3):515–547. doi: 10.1113/jphysiol.1974.sp010541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVay S. Synaptic patterns in the visual cortex of the cat and monkey. Electron microscopy of Golgi preparations. J Comp Neurol. 1973 Jul 1;150(1):53–85. doi: 10.1002/cne.901500104. [DOI] [PubMed] [Google Scholar]
- Lee B. B., Albus K., Heggelund P., Hulme M. J., Creutzfeldt O. D. The depth distribution of optimal stimulus orientations for neurones in cat area 17. Exp Brain Res. 1977 Mar 30;27(3-4):301–314. doi: 10.1007/BF00235505. [DOI] [PubMed] [Google Scholar]
- Palmer L. A., Rosenquist A. C. Visual receptive fields of single striate corical units projecting to the superior colliculus in the cat. Brain Res. 1974 Feb 15;67(1):27–42. doi: 10.1016/0006-8993(74)90295-9. [DOI] [PubMed] [Google Scholar]
- Rose D., Blakemore C. Effects of bicuculline on functions of inhibition in visual cortex. Nature. 1974 May 24;249(455):375–377. doi: 10.1038/249375a0. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. Inhibitory processes underlying the directional specificity of simple, complex and hypercomplex cells in the cat's visual cortex. J Physiol. 1977 Oct;271(3):699–720. doi: 10.1113/jphysiol.1977.sp012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito A. M. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J Physiol. 1975 Sep;250(2):305–329. doi: 10.1113/jphysiol.1975.sp011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito A. M. The effectiveness of bicuculline as an antagonist of GABA and visually evoked inhibition in the cat's striate cortex. J Physiol. 1975 Sep;250(2):287–304. doi: 10.1113/jphysiol.1975.sp011055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito A. M. The spatial extent of excitatory and inhibitory zones in the receptive field of superficial layer hypercomplex cells. J Physiol. 1977 Dec;273(3):791–803. doi: 10.1113/jphysiol.1977.sp012124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito A. M., Versiani V. The contribution of excitatory and inhibitory inputs to the length preference of hypercomplex cells in layers II and III of the cat's striate cortex. J Physiol. 1977 Dec;273(3):775–790. doi: 10.1113/jphysiol.1977.sp012123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W., Tretter F., Cynader M. Organization of cat striate cortex: a correlation of receptive-field properties with afferent and efferent connections. J Neurophysiol. 1975 Sep;38(5):1080–1098. doi: 10.1152/jn.1975.38.5.1080. [DOI] [PubMed] [Google Scholar]
- Toyama K., Kimura M., Shiida T., Takeda T. Convergence of retinal inputs onto visual cortical cells: II. A study of the cells disynaptically excited from the lateral geniculate body. Brain Res. 1977 Dec 2;137(2):221–231. doi: 10.1016/0006-8993(77)90335-3. [DOI] [PubMed] [Google Scholar]
- Toyama K., Maekawa K., Takeda T. Convergence of retinal inputs into visual cortical cells: I. A study of the cells monosynaptically excited from the lateral geniculate body. Brain Res. 1977 Dec 2;137(2):207–220. doi: 10.1016/0006-8993(77)90334-1. [DOI] [PubMed] [Google Scholar]
- Winfield D. A., Powell T. P. The termination of thalamo-cortical fibres in the visual cortex of the cat. J Neurocytol. 1976 Jun;5(3):269–281. doi: 10.1007/BF01175115. [DOI] [PubMed] [Google Scholar]


