Abstract
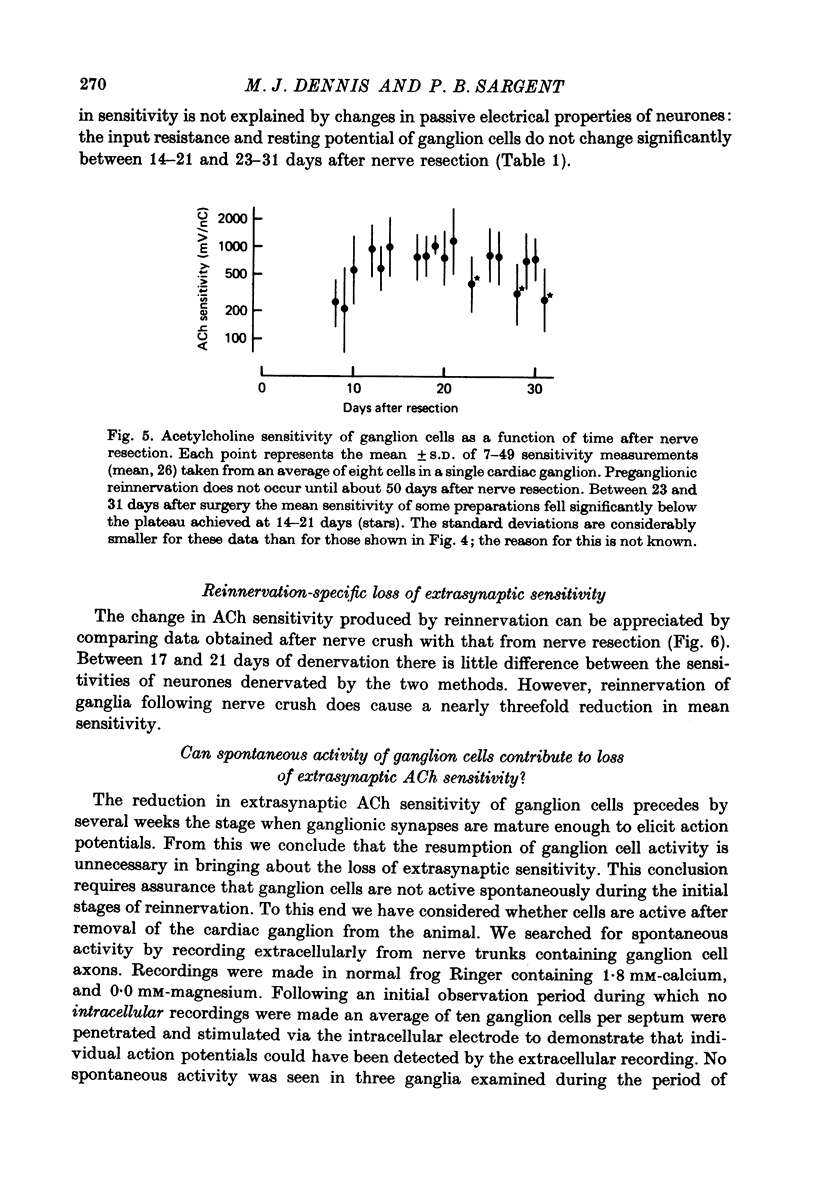
1. The extrasynaptic acetylcholine sensitivity of frog cardiac ganglion cells was measured both after denervation and during the early stages of reinnervation by preganglionic axons. Sensitivity was measured by ionophoretic application of acetylcholine (ACh) to randomly chosen sites on ganglion cell bodies. 2. Extrasynaptic sensitivity rose gradually following denervation and after 3 weeks reached a mean value of approximately 1000 mV/nC. 3. Reinnervation of the cardiac ganglion began about 3 weeks after nerve crush. The ACh sensitivity of ganglion cells fell markedly during the 23--31 day period, to a mean of 184 mV/nC. None of forty-three neurones studied during that period received synaptic inputs sufficient to generate action potentials. 4. Twenty-nine of the forty-three neurones examined 23--31 days after nerve crush had not yet received detectable synaptic inputs, yet even these cells had markedly reduced ACh sensitivity. 5. When reinnervation of cardiac ganglia was delayed by resecting the preganglionic nerves, ACh sensitivity was reduced slightly (43%) between 14--21 and 23--31 days after surgery. Thus, most of the sixfold reduction in sensitivity that occurs during this time after nerve crush is a specific effect of reinnervation. 6. We conclude that loss of extrasynaptic receptors coincides with, or may even precede, the earliest physiological signs of synapse formation. Restoration of action potential activity in the ganglion cells is not essential to initiate this loss.
Full text
PDF


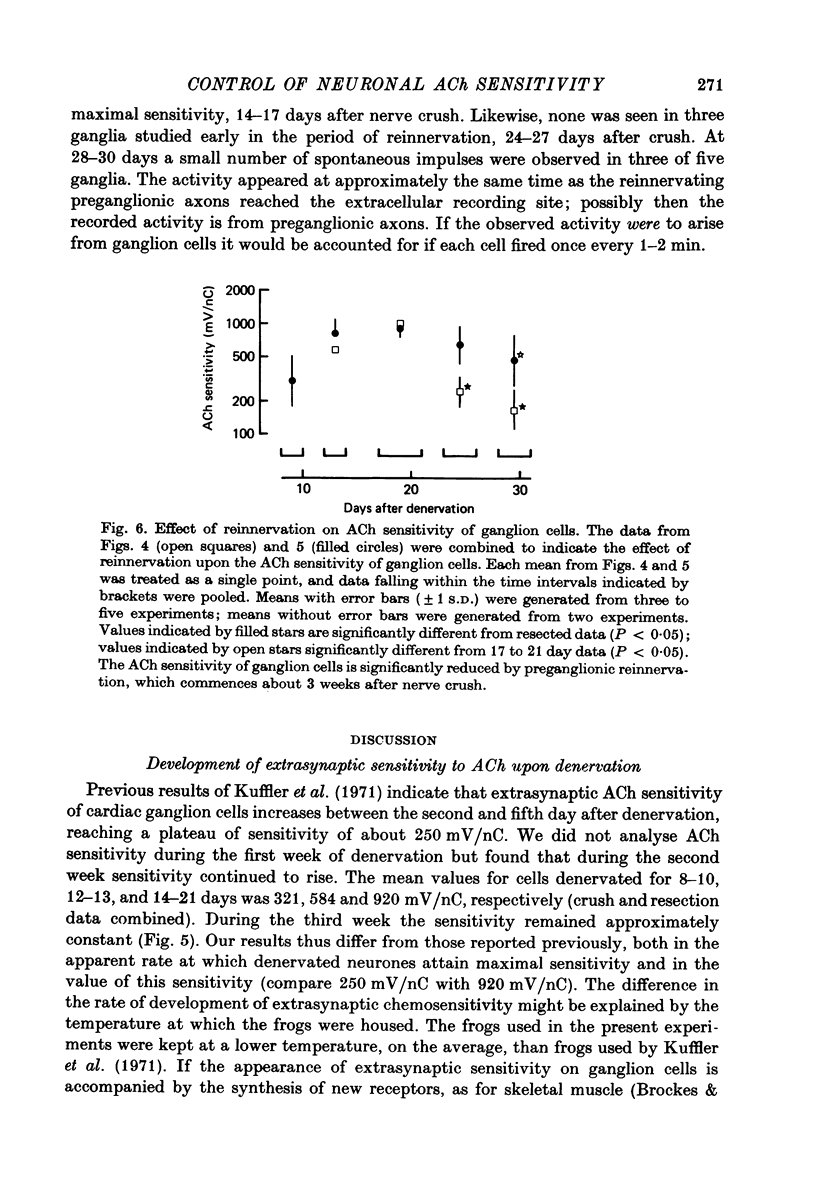




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., McIsaac R. J. Fast and slow mammalian muscles after denervation. Exp Neurol. 1970 Jan;26(1):183–202. doi: 10.1016/0014-4886(70)90099-3. [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Hall Z. W. Synthesis of acetylcholine receptor by denervated rat diaphragm muscle. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1368–1372. doi: 10.1073/pnas.72.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Harris A. J., Kuffler S. W. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Non-transmitting neuromuscular junctions during an early stage of end-plate reinnervation. J Physiol. 1974 Jun;239(3):553–570. doi: 10.1113/jphysiol.1974.sp010582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Sargent P. B. Multiple innervation of normal and re-innervated parasympathetic neurones in the frog cardiac ganglion. J Physiol. 1978 Aug;281:63–75. doi: 10.1113/jphysiol.1978.sp012409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes P. N., Fambrough D. M. Synthesis of acetylcholine receptors by cultured chick myotubes and denervated mouse extensor digitorum longus muscles. Proc Natl Acad Sci U S A. 1976 Jan;73(1):161–164. doi: 10.1073/pnas.73.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman D. B., Witzke F. Trophic regulation of acetylcholine sensitivity of muscle: effect of electrical stimulation. Science. 1972 May 5;176(4034):514–516. doi: 10.1126/science.176.4034.514. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrough D. M. Acetylcholine receptors. Revised estimates of extrajunctional receptor density in denervated rat diaphragm. J Gen Physiol. 1974 Oct;64(4):468–472. doi: 10.1085/jgp.64.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. J., Kuffler S. W., Dennis M. J. Differential chemosensitivity of synaptic and extrasynaptic areas on the neuronal surface membrane in parasympathetic neurons of the frog, tested by microapplication of acetylcholine. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):541–553. doi: 10.1098/rspb.1971.0046. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Fambrough D. M. Acetylcholine receptors. Distribution and extrajunctional density in rat diaphragm after denervation correlated with acetylcholine sensitivity. J Gen Physiol. 1972 Sep;60(3):248–262. doi: 10.1085/jgp.60.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R., Vrbová G. Effect of muscle activity on denervation hypersensitivity. J Physiol. 1970 Sep;210(2):144P–145P. [PubMed] [Google Scholar]
- Jones R., Vrbová G. Two factors responsible for the development of denervation hypersensitivity. J Physiol. 1974 Feb;236(3):517–538. doi: 10.1113/jphysiol.1974.sp010450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Dennis M. J., Harris A. J. The development of chemosensitivity in extrasynaptic areas of the neuronal surface after denervation of parasympathetic ganglion cells in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):555–563. doi: 10.1098/rspb.1971.0047. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The number of transmitter molecules in a quantum: an estimate from iontophoretic application of acetylcholine at the neuromuscular synapse. J Physiol. 1975 Oct;251(2):465–482. doi: 10.1113/jphysiol.1975.sp011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T., Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol. 1972 Mar;221(2):493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H. Control of ACh sensitivity in rat muscle fibers. Cold Spring Harb Symp Quant Biol. 1976;40:263–274. doi: 10.1101/sqb.1976.040.01.027. [DOI] [PubMed] [Google Scholar]
- MILEDI R. Properties of regenerating neuromuscular synapses in the frog. J Physiol. 1960 Nov;154:190–205. doi: 10.1113/jphysiol.1960.sp006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- McMahan U. J., Kuffler S. W. Visual identification of synaptic boutons on living ganglion cells and of varicosities in postganglionic axons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):485–508. doi: 10.1098/rspb.1971.0044. [DOI] [PubMed] [Google Scholar]
- Roper S. The acetylcholine sensitivity of the surface membrane of multiply-innervated parasympathetic ganglion cells in the mudpuppy before and after partial denervation. J Physiol. 1976 Jan;254(2):455–473. doi: 10.1113/jphysiol.1976.sp011240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent P. B., Dennis M. J. Formation of synapses between parasympathetic neurones deprived of preganglionic innervation. Nature. 1977 Aug 4;268(5619):456–458. doi: 10.1038/268456a0. [DOI] [PubMed] [Google Scholar]
- Schmidt H., Stefani E. Action potentials in slow muscle fibres of the frog during regeneration of motor nerves. J Physiol. 1977 Sep;270(2):507–517. doi: 10.1113/jphysiol.1977.sp011965. [DOI] [PMC free article] [PubMed] [Google Scholar]


