Abstract
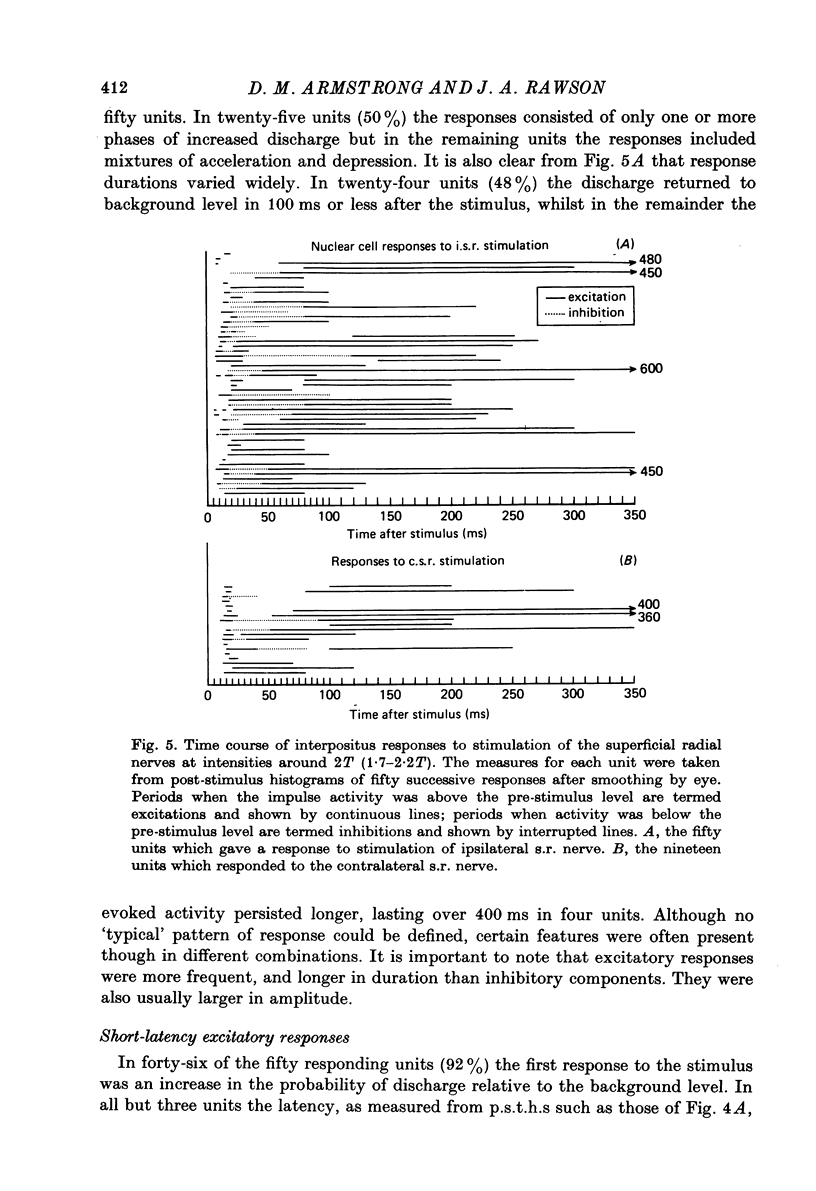
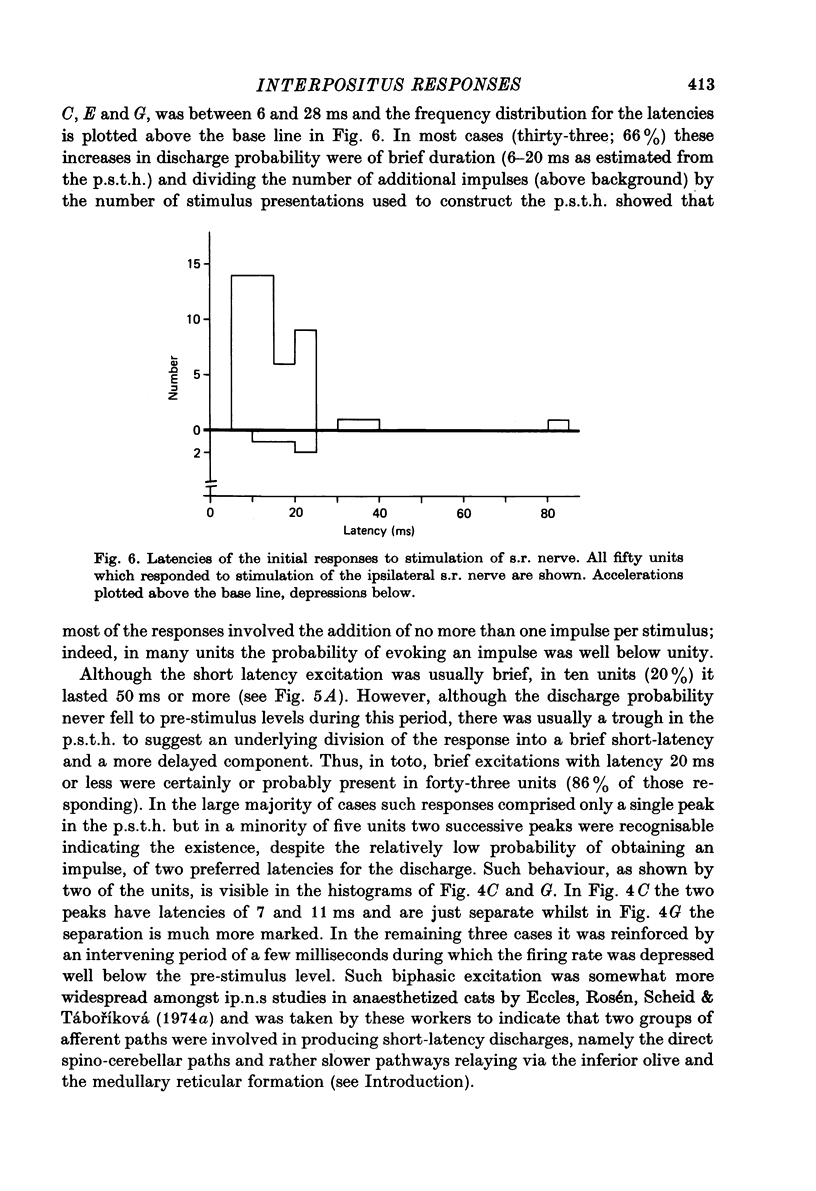
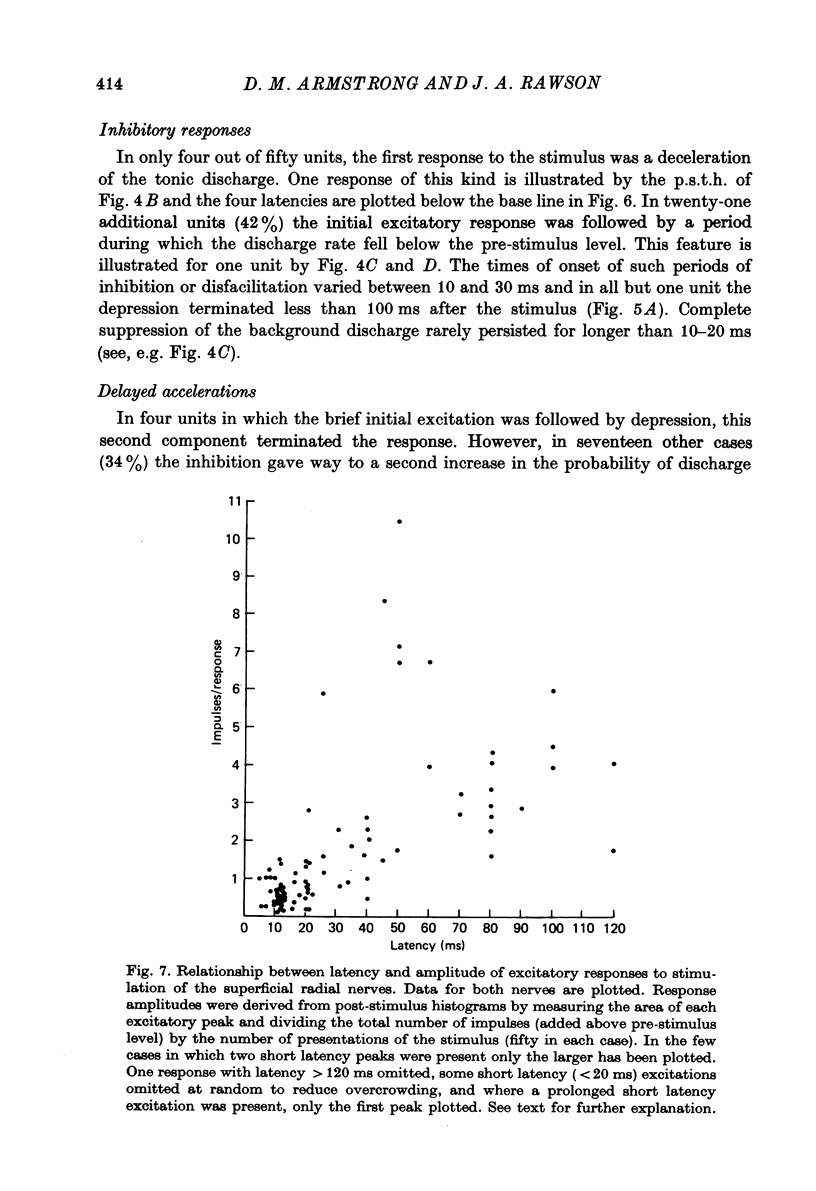
1. A method is described which permitted stable extracellular recordings to be made from 115 neurones in nucleus interpositus of the cerebellum in unanaesthetized free-to-move cats. At least 95% of the neurones were cerebellar efferent cells since they were antidromically invaded following electrical stimulation of the brachium conjunctivum in the region of the contralateral red nucleus. 2. In cats in a state of quiet wakefulness the majority of interpositus neurones were tonically active at rates ranging from 12 to 77 impulses/sec (over-all mean 34/sec). The remaining neurones were silent or discharged only a few impulses throughout observation periods of a few minutes. 3. Cutaneous afferent volleys elicited by single shocks to the superficial radial nerves in the forearm at intensities too weak to evoke a flexion reflex or behavioural arousal produced changes in firing frequency in 62% of eighty-one cells tested. Response patterns varied widely but in 86% of the responding cells the earliest change was a short latency (6--20 ms) increase in discharge probability which from post-stimulus time histograms was found usually to average around one impulse per stimulus. In only four cells (8%) the earliest response was a depression of the tonic firing. However, in many cells the initial acceleration was followed by a reduction in firing frequency which lasted between 10 and 85 ms. 4. In 56% of the responding cells a longer latency (25--80 ms) acceleration was present. Such accelerations varied widely in duration (from 55 to 550 ms) but most commonly lasted 100--200 ms. These responses were usually the most prominent feature in the response pattern: in the majority of neurones between two and five impulses were added per stimulus. 5. Considering the whole time course of the responses, the net effect of nerve volleys was to produce an increase in nuclear cell output. 6. These neurones which were influenced by nerve stimulation also discharged in response to taps to the forepaws. 7. The responses to nerve stimulation are compared with those encountered in previous studies using cats anaesthetized with chloralose or barbiturates and with the responses of Purkinje (P) cells and it is suggested that the longer latency excitatory responses result in large part from a reduction in the tonic inhibitory action exerted on the interpositus neurones by Purkinje cells. 8. The possibility is discussed that interpositus responses to cutaneous input from the limbs might contribute (via the rubrospinal system) to the regulation of spinal flexor mechanisms during locomotion and/or contact placing reactions.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. I., Gilbert P. F., Marini R., Schultz W., Yin T. C. Integration of cerebral and peripheral inputs by interpositus neurons in monkey. Exp Brain Res. 1977 Jan 18;27(1):81–99. doi: 10.1007/BF00234827. [DOI] [PubMed] [Google Scholar]
- Allen G. I., Tsukahara N. Cerebrocerebellar communication systems. Physiol Rev. 1974 Oct;54(4):957–1006. doi: 10.1152/physrev.1974.54.4.957. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Cogdell B., Harvey R. Effects of afferent volleys from the limbs on the discharge patterns of interpositus neurones in cats anaesthetized with alpha-chloralose. J Physiol. 1975 Jun;248(2):489–517. doi: 10.1113/jphysiol.1975.sp010985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M. Functional significance of connections of the inferior olive. Physiol Rev. 1974 Apr;54(2):358–417. doi: 10.1152/physrev.1974.54.2.358. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Leonard P., Rawson J. A. A simple micromanipulator for investigation of cerebellar neurones in unrestrained cats. J Physiol. 1975 Feb;245(2):23P–25P. [PubMed] [Google Scholar]
- Armstrong D. M., Rawson J. A. Activity patterns of cerebellar cortical neurones and climbing fibre afferents in the awake cat. J Physiol. 1979 Apr;289:425–448. doi: 10.1113/jphysiol.1979.sp012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Schild R. F. An investigation of the cerebellar corticonuclear projections in the rat using an autoradiographic tracing method. II. Projections from the hemisphere. Brain Res. 1978 Feb 10;141(2):235–249. doi: 10.1016/0006-8993(78)90195-6. [DOI] [PubMed] [Google Scholar]
- Bloedel J. R. Cerebellar afferent systems: a review. Prog Neurobiol. 1973;2(1):3–68. doi: 10.1016/0301-0082(73)90006-3. [DOI] [PubMed] [Google Scholar]
- Bloedel J. R., Roberts W. J. Functional relationship among neurons of the cerebellar cortex in the absence of anesthesia. J Neurophysiol. 1969 Jan;32(1):75–84. doi: 10.1152/jn.1969.32.1.75. [DOI] [PubMed] [Google Scholar]
- Burton J. E., Onoda N. Interpositus neuron discharge in relation to a voluntary movement. Brain Res. 1977 Jan 31;121(1):167–172. doi: 10.1016/0006-8993(77)90447-4. [DOI] [PubMed] [Google Scholar]
- CHAMBERS W. W., SPRAGUE J. M. Functional localization in the cerebellum. I. Organization in longitudinal cortico-nuclear zones and their contribution to the control of posture, both extrapyramidal and pyramidal. J Comp Neurol. 1955 Aug;103(1):105–129. doi: 10.1002/cne.901030107. [DOI] [PubMed] [Google Scholar]
- Cody F. W., Richardson H. C. Responses of cerebellar interpositus nuclear neurones to trigeminal inputs in the cat [proceedings]. J Physiol. 1978 Apr;277:62P–63P. [PubMed] [Google Scholar]
- Courville J., Brodal A. Rubro-cerebellar connections in the cat: an experimental study with silver impregnation methods. J Comp Neurol. 1966 Mar;126(3):471–485. doi: 10.1002/cne.901260309. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Rantucci T., Rosén I., Scheid P., Táboríková H. Somatotopic studies on cerebellar interpositus neurons. J Neurophysiol. 1974 Nov;37(6):1449–1459. doi: 10.1152/jn.1974.37.6.1449. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Rosén I., Scheid P., Táboríková H. Patterns of convergence onto interpositus neurons from peripheral afferents. J Neurophysiol. 1974 Nov;37(6):1438–1448. doi: 10.1152/jn.1974.37.6.1438. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Rosén I., Scheid P., Táboríková H. Temporal patterns of responses of interpositus neurons to peripheral afferent stimulation. J Neurophysiol. 1974 Nov;37(6):1424–1437. doi: 10.1152/jn.1974.37.6.1424. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Sabah N. H., Táboríková H. Excitatory and inhibitory responses of neurones of the cerebellar fastigial nucleus. Exp Brain Res. 1974 Jan 22;19(1):61–77. doi: 10.1007/BF00233395. [DOI] [PubMed] [Google Scholar]
- Eccles J. C. The cerebellum as a computer: patterns in space and time. J Physiol. 1973 Feb;229(1):1–32. doi: 10.1113/jphysiol.1973.sp010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I., Lundberg A. An electromyographic analysis of muscular activity in the hindlimb of the cat during unrestrained locomotion. Acta Physiol Scand. 1969 Apr;75(4):614–630. doi: 10.1111/j.1748-1716.1969.tb04415.x. [DOI] [PubMed] [Google Scholar]
- Freeman J. A. Responses of cat cerebellar Purkinje cells to convergent inputs from cerebral cortex and peripheral sensory systems. J Neurophysiol. 1970 Nov;33(6):697–712. doi: 10.1152/jn.1970.33.6.697. [DOI] [PubMed] [Google Scholar]
- Gordon M., Rubia F. J., Strata P. The effect of pentothal on the activity evoked in the cerebellar cortex. Exp Brain Res. 1973 Mar 29;17(1):50–62. doi: 10.1007/BF00234563. [DOI] [PubMed] [Google Scholar]
- Ito M., Yoshida M., Obata K., Kawai N., Udo M. Inhibitory control of intracerebellar nuclei by the purkinje cell axons. Exp Brain Res. 1970;10(1):64–80. doi: 10.1007/BF00340519. [DOI] [PubMed] [Google Scholar]
- Loeb G. E., Bak M. J., Duysens J. Long-term unit recording from somatosensory neurons in the spinal ganglia of the freely walking cat. Science. 1977 Sep 16;197(4309):1192–1194. doi: 10.1126/science.897663. [DOI] [PubMed] [Google Scholar]
- MASSION J., ALBE-FESSARD D. DUALIT'E DES VOIES SENSORIELLES AFF'ERENTES CONTR OLANT L'ACTIVIT'E DU NOYAU ROUGE. Electroencephalogr Clin Neurophysiol. 1963 Jun;15:435–454. doi: 10.1016/0013-4694(63)90065-8. [DOI] [PubMed] [Google Scholar]
- Massion J. The mammalian red nucleus. Physiol Rev. 1967 Jul;47(3):383–436. doi: 10.1152/physrev.1967.47.3.383. [DOI] [PubMed] [Google Scholar]
- Matsushita M., Iwahori N. Structural organization of the interpositus and the dentate nuclei. Brain Res. 1971 Dec 10;35(1):17–36. doi: 10.1016/0006-8993(71)90592-0. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng. 1972 Sep;10(5):662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- Mortimer J. A. Cerebellar responses to teleceptive stimuli in alert monkeys. Brain Res. 1975 Jan 17;83(3):369–390. doi: 10.1016/0006-8993(75)90831-8. [DOI] [PubMed] [Google Scholar]
- Orlovskii G. N. Rabota kletok Purkin'e pri lokomotsii. Biofizika. 1972 Sep-Oct;17(5):891–896. [PubMed] [Google Scholar]
- Orlovskii G. N. Rabota neironov mozzhechkovykh iader pri lokomotsii. Biofizika. 1972 Nov-Dec;17(6):1119–1126. [PubMed] [Google Scholar]
- Orlovsky G. N. Activity of rubrospinal neurons during locomotion. Brain Res. 1972 Nov 13;46:99–112. doi: 10.1016/0006-8993(72)90008-x. [DOI] [PubMed] [Google Scholar]
- Talbott R. E., Towe A. L., Kennedy T. T. Physiological and histological classification of cerebellar neurons in chloralose-anesthetized cats. Exp Neurol. 1967 Sep;19(1):46–64. doi: 10.1016/0014-4886(67)90006-4. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J Neurophysiol. 1968 Sep;31(5):785–797. doi: 10.1152/jn.1968.31.5.785. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. I. Nuclear cell output. J Neurophysiol. 1970 Jul;33(4):527–536. doi: 10.1152/jn.1970.33.4.527. [DOI] [PubMed] [Google Scholar]
- Toyama K., Tsukahara N., Kosaka K., Matsunami K. Synaptic excitation of red nucleus neurones by fibres from interpositus nucleus. Exp Brain Res. 1970;11(2):187–198. doi: 10.1007/BF00234322. [DOI] [PubMed] [Google Scholar]
- Tsukahara N., Bando T. Red nuclear and interposate nuclear excitation of pontine nuclear cells. Brain Res. 1970 Apr 14;19(2):295–298. doi: 10.1016/0006-8993(70)90442-7. [DOI] [PubMed] [Google Scholar]
- Tsukahara N., Korn H., Stone J. Pontine relay from cerebral cortex to cerebellar cortex and nucleus interpositus. Brain Res. 1968 Sep;10(3):448–453. doi: 10.1016/0006-8993(68)90213-8. [DOI] [PubMed] [Google Scholar]
- Tsukahara N. The properties of the cerebello-pontine reverberating circuit. Brain Res. 1972 May 12;40(1):67–71. doi: 10.1016/0006-8993(72)90108-4. [DOI] [PubMed] [Google Scholar]
- Yu J., Tarnecki R., Chambers W. W., Liu C. N., Konorski J. Mechanisms mediating ipsilateral limb hyperflexion after cerebellar paravermal cortical ablation or cooling. Exp Neurol. 1973 Jan;38(1):144–156. doi: 10.1016/0014-4886(73)90015-0. [DOI] [PubMed] [Google Scholar]


