Abstract
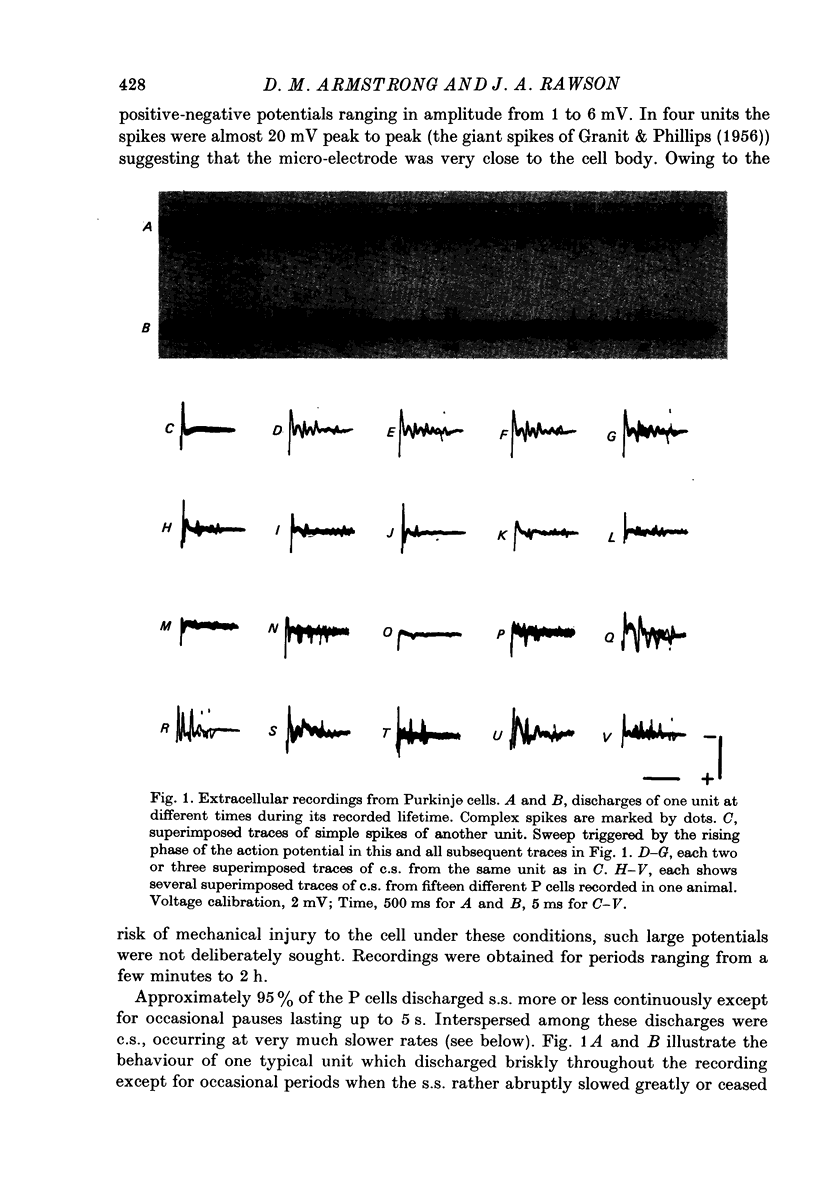
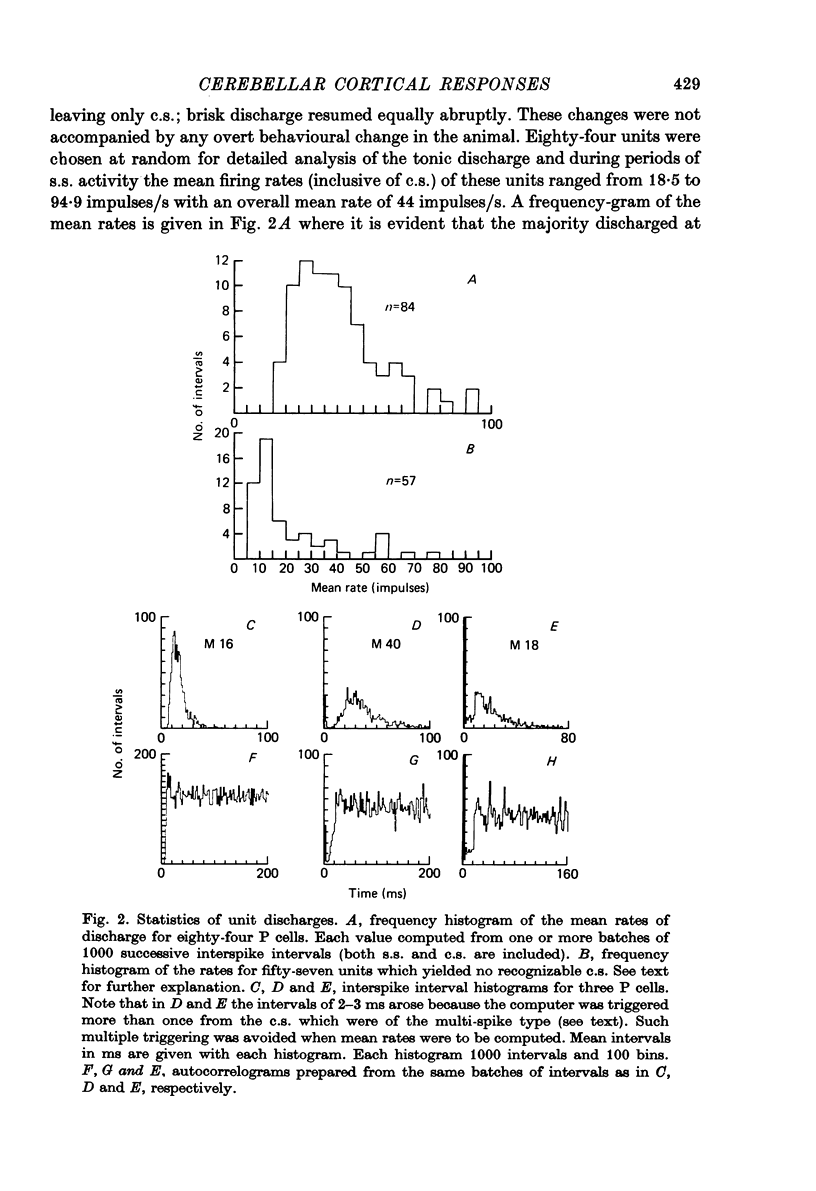
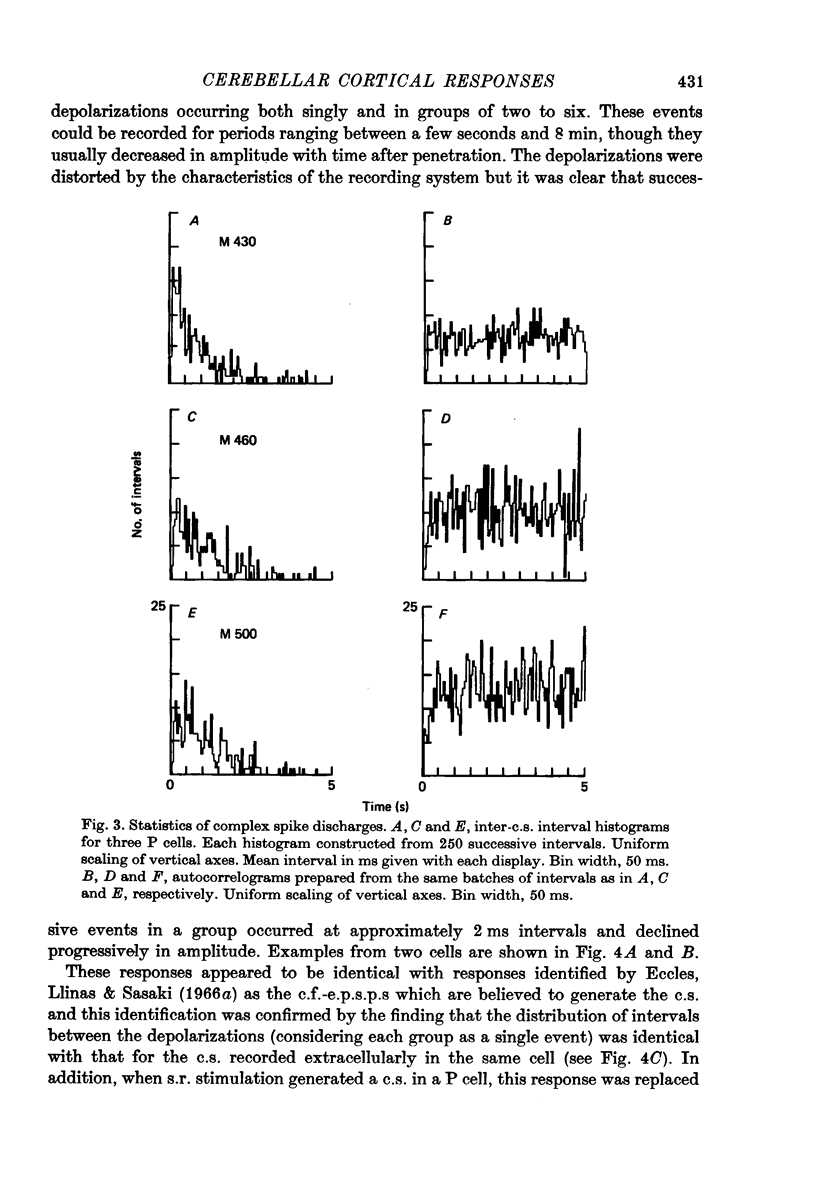
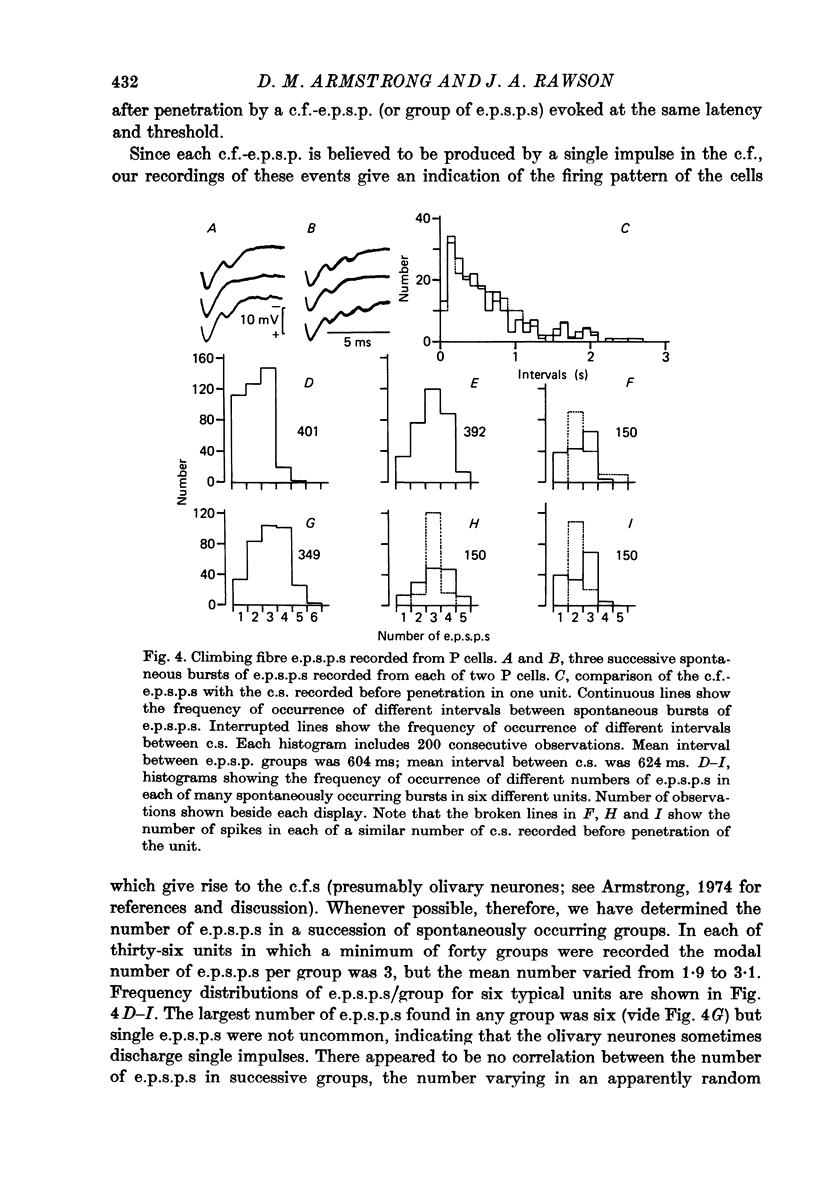
1. Glass-insulated tungsten micro-electrodes were used to record from single neurones in the intermediate zone of the cerebellar cortex of cats in a state of quiet wakefulness. 2. Two hundred and seventy Purkinje (P) cells were recorded extracellularly, 95% of which displayed an irregular tonic discharge at rates between 19 and 95/s (over-all mean 44/s), including complex spikes (c.s.) which occurred at 1.0--2.5/s )over-all mean 1.5/s). The remaining cells discharged c.s. at the usual rate but only one or two simple spikes (s.s.) per minute. C.s. of spike plus wavelet and of multi-spiked type were present in approximately equal numbers of cells. 3. Presumed climbing fibre-e.p.s.p.s were recorded from fifty-six P cells and occurred both singly and in groups of two to six e.p.s.p.s at an intra-group frequency of about 500/s. The cells giving rise to the c.f.s therefore discharge propagated impulses both singly and in short bursts as previously reported for anesthetized animals. A single e.p.s.p. can give rise to more than one spike in the multi-spiked type of c.s., and probably to a complete c.s. event. 4. Following spontaneous c.s. the interval to the next s.s. varied from 8 to 600 ms. There was an inverse correlation between duration of the post-c.s. interval and the rate at which s.s. were discharged in the preceding 100 ms. The duration exceeded the mean s.s. interval provided s.s. rate was less than 40--50/s, and the post-c.s. interval would then constitute a real interruption of s.s. discharge. 5. When the superficial radial (s.r.) nerves were stimulated with single shocks too weak to produce a behavioural response changes in discharge pattern were detected in eighty-eight of 151 P cells tested. The initial responses were almost always excitatory and consisted in seventy-two cells of a c.s., in eleven of a c.s. preceded by a brief increase in s.s. and in two cases of a s.s. discharge alone. The spino-olivo-cerebellar paths responsible for the c.s. showed transmission characteristics similar to those reported for animals anaesthetized with barbiturates. 6. C.s. were readily evoked by tapping or squeezing the forepaws. 7. Excitatory responses to nerve stimulation were usually followed by a depression of the tonic s.s. discharge. Its duration ranged widely in different cells (from 10 to 500 ms) and it would coincide with equally variable periods of facilitation previously seen in neurones of nucleus interpositus. It is therefore likely that such facilitations of the cerebellar nuclear cells result at least in part from reductions in the tonic inhibitory input from the P cells. 8. Thirty-six units were classes as 'probable cortical interneurones'. They discharged more regularly, at rates between 9 and 28/s. Twenty such units (56%) responded to s.r. stimulation with a brief excitation not usually followed by any pronounced depression.
Full text
PDF



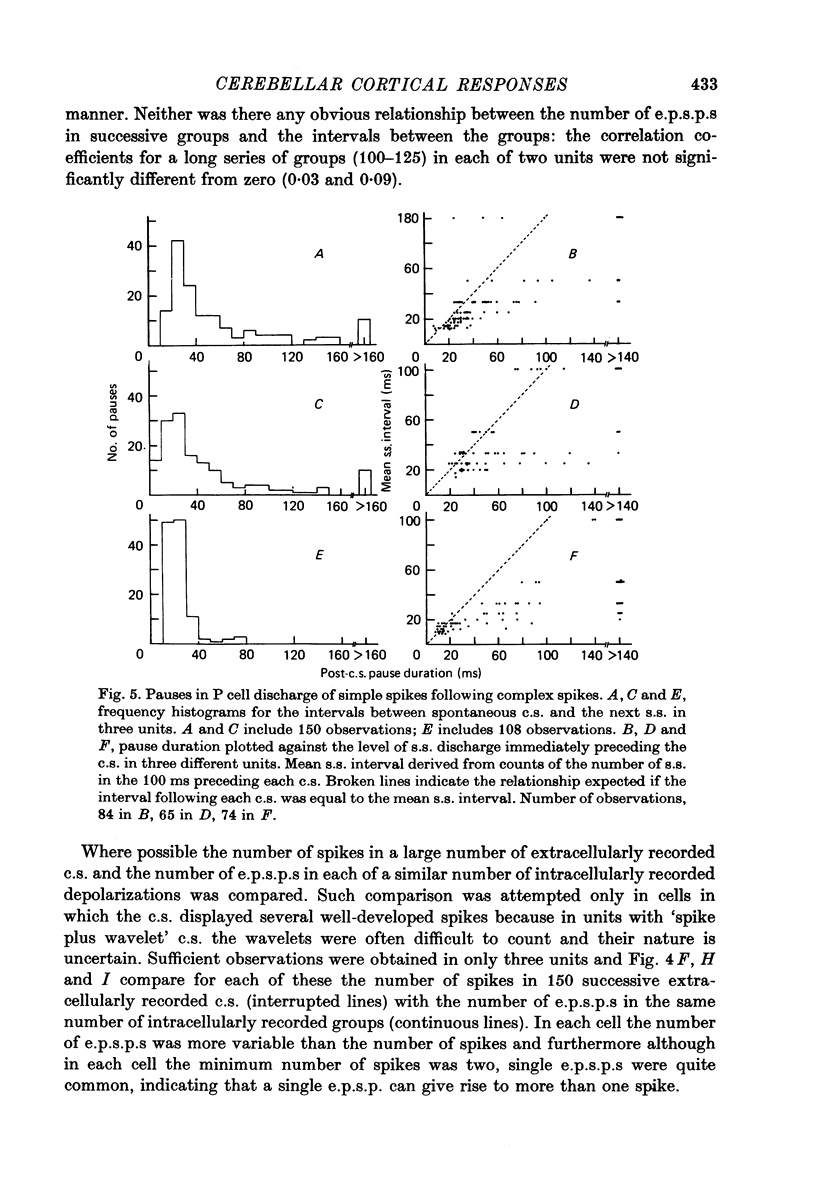

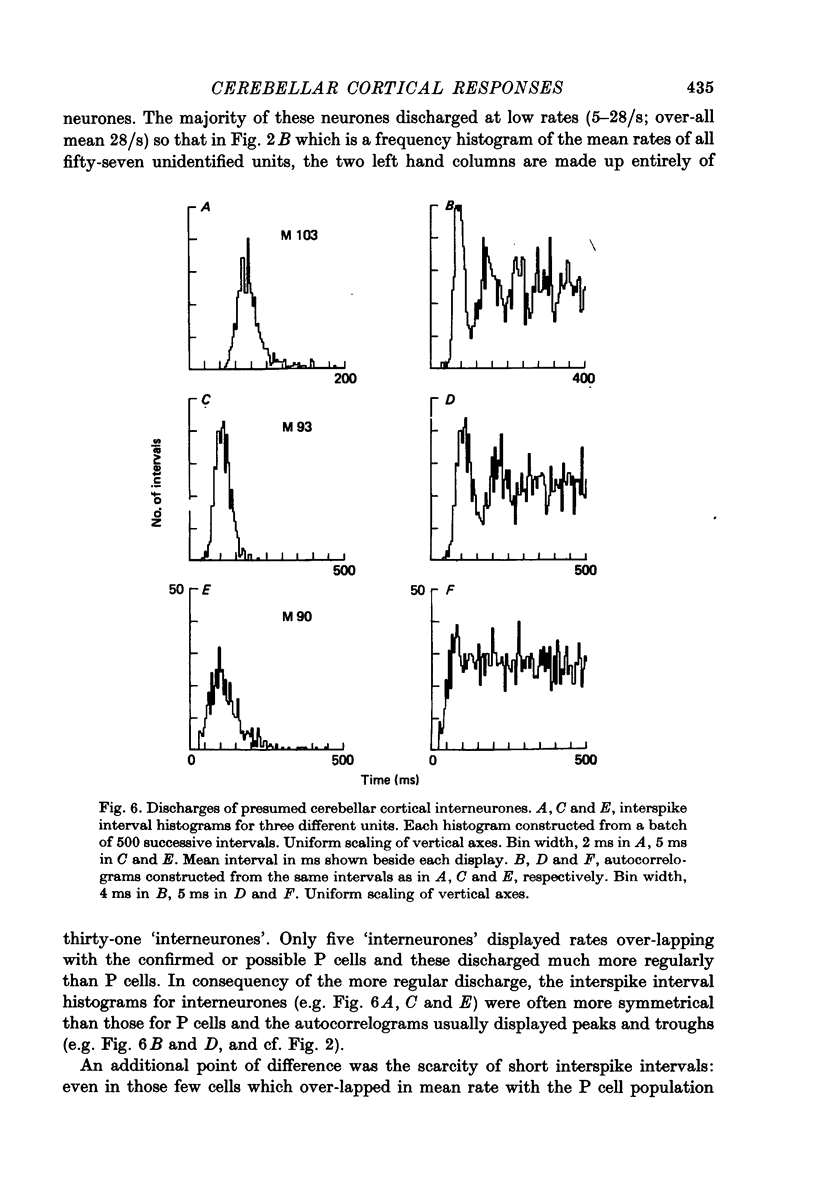
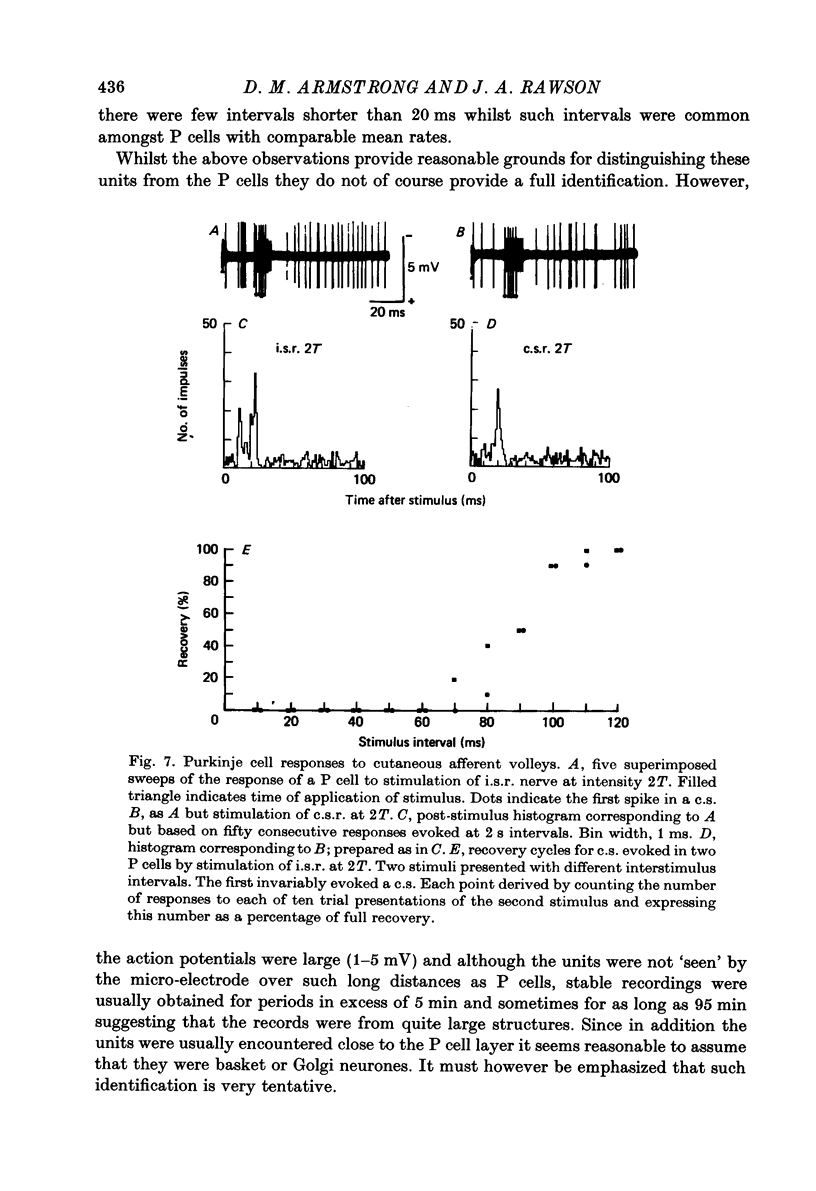


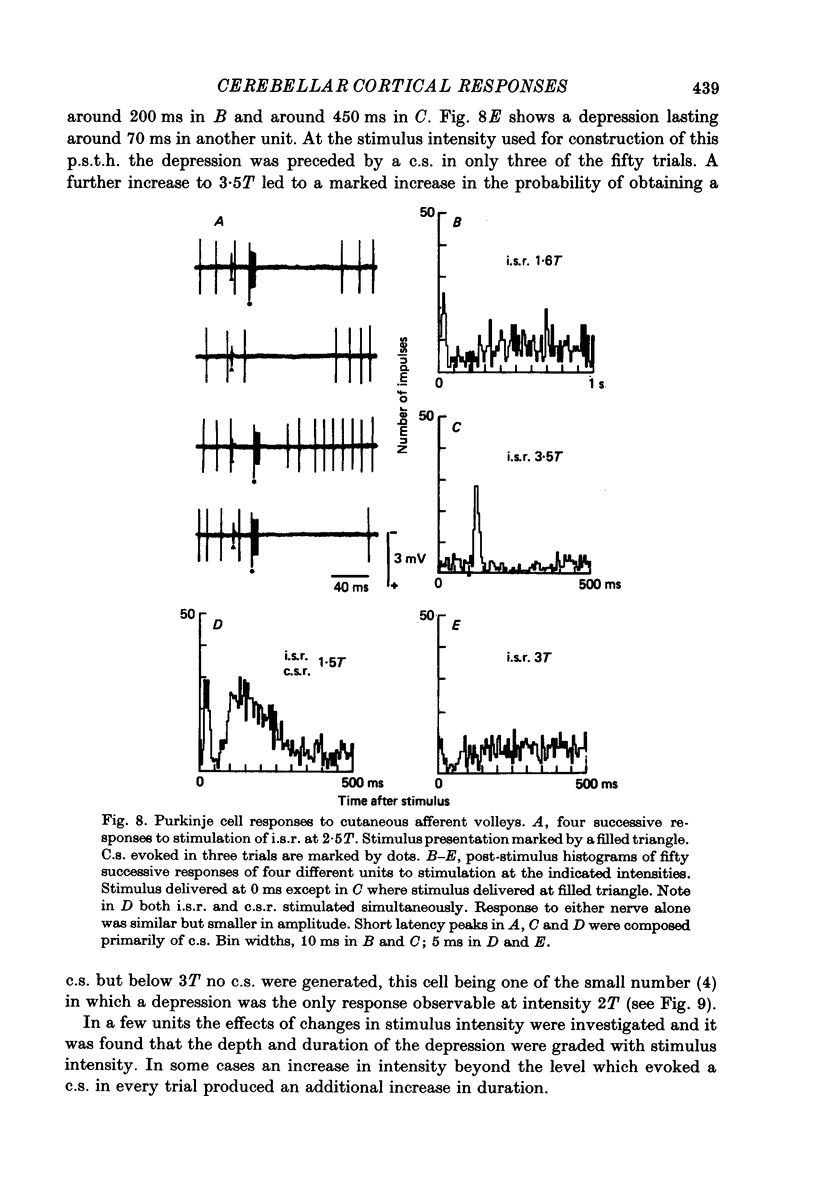







Images in this article
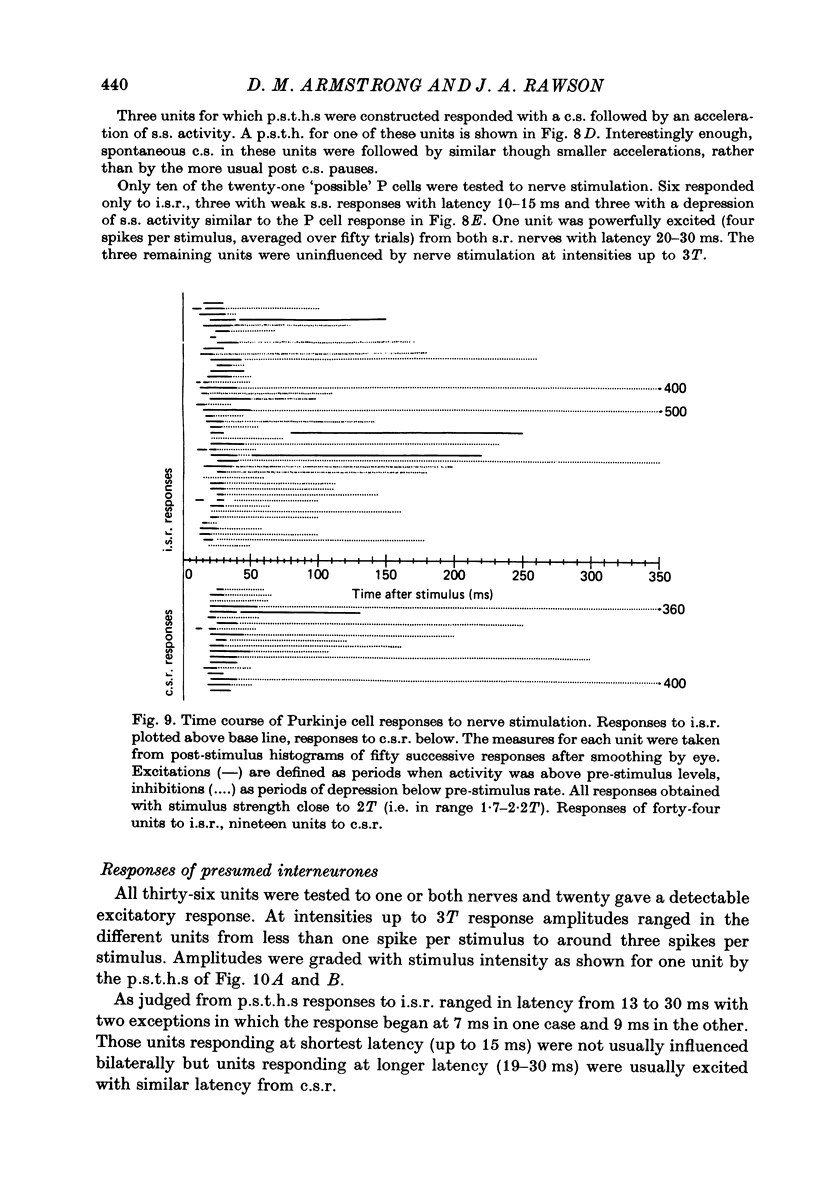
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. M., Cogdell B., Harvey R. Effects of afferent volleys from the limbs on the discharge patterns of interpositus neurones in cats anaesthetized with alpha-chloralose. J Physiol. 1975 Jun;248(2):489–517. doi: 10.1113/jphysiol.1975.sp010985. [DOI] [PMC free article] [PubMed] [Google Scholar]
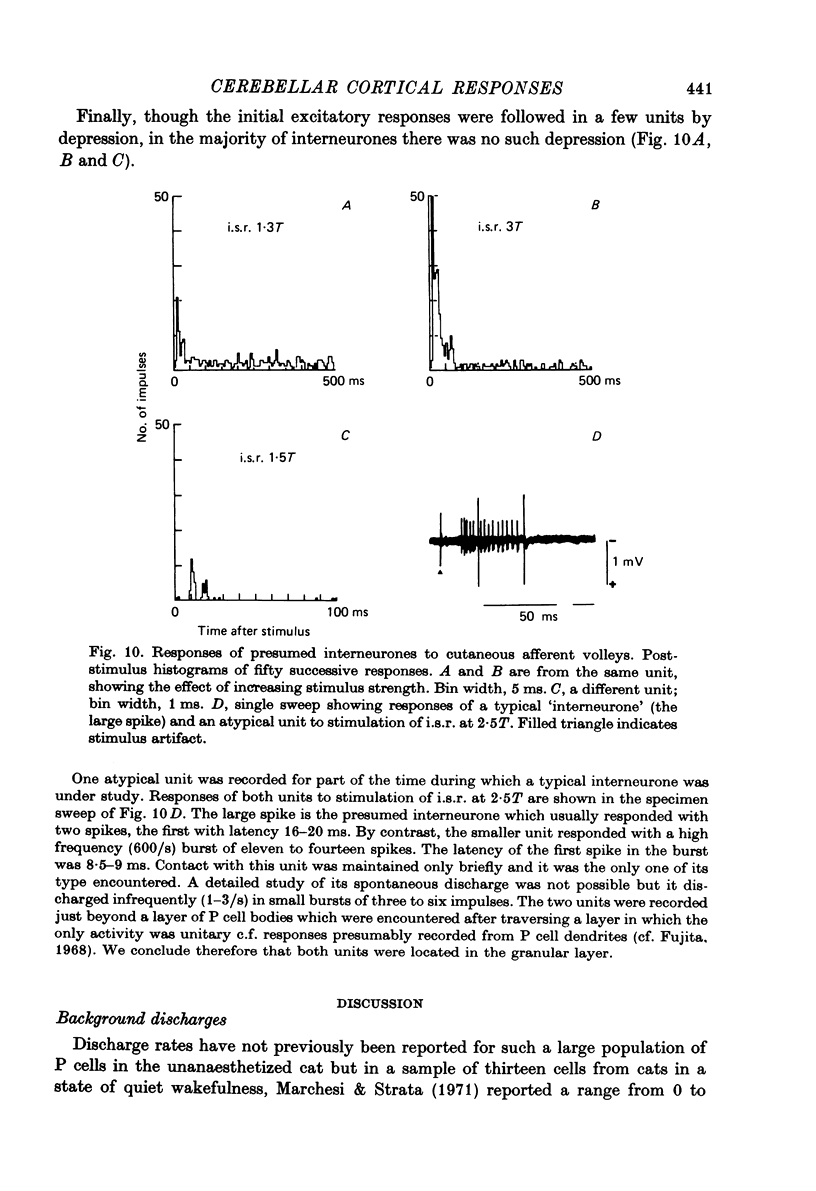
- Armstrong D. M. Functional significance of connections of the inferior olive. Physiol Rev. 1974 Apr;54(2):358–417. doi: 10.1152/physrev.1974.54.2.358. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Harvey R. J., Schild R. F. Spino-olivocerebellar pathways to the posterior lobe of the cat cerebellum. Exp Brain Res. 1973 Aug 31;18(1):1–18. doi: 10.1007/BF00236553. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Rawson J. A. Responses of neurones in nucleus interpositus of the cerebellum to cutaneous nerve volleys in the awake cat. J Physiol. 1979 Apr;289:403–423. doi: 10.1113/jphysiol.1979.sp012744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C. C., Grimm R. J. Discharge properties of Purkinje cells recorded on single and double microelectrodes. J Neurophysiol. 1969 Nov;32(6):1044–1055. doi: 10.1152/jn.1969.32.6.1044. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Duggan A. W., Headley P. M., Lodge D. Proceedings: Rhythmical field potentials induced in the inferior olive complex by iontophoretically applied harmaline and other unrelated alkaloids. Br J Pharmacol. 1973 Sep;49(1):174P–175P. [PMC free article] [PubMed] [Google Scholar]
- Bloedel J. R., Roberts W. J. Functional relationship among neurons of the cerebellar cortex in the absence of anesthesia. J Neurophysiol. 1969 Jan;32(1):75–84. doi: 10.1152/jn.1969.32.1.75. [DOI] [PubMed] [Google Scholar]
- Cody F. W., Richardson H. C. Responses of cerebellar interpositus nuclear neurones to trigeminal inputs in the cat [proceedings]. J Physiol. 1978 Apr;277:62P–63P. [PubMed] [Google Scholar]
- Eccles J. C., Faber D. S., Murphy J. T., Sabah N. H., Táboríková H. Afferent volleys in limb nerves influencing impulse discharges in cerebellar cortex. II. In Purkynè cells. Exp Brain Res. 1971 Jul 26;13(1):36–53. [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Rosén I., Scheid P., Táboríková H. Temporal patterns of responses of interpositus neurons to peripheral afferent stimulation. J Neurophysiol. 1974 Nov;37(6):1424–1437. doi: 10.1152/jn.1974.37.6.1424. [DOI] [PubMed] [Google Scholar]
- Eccles J. C. The cerebellum as a computer: patterns in space and time. J Physiol. 1973 Feb;229(1):1–32. doi: 10.1113/jphysiol.1973.sp010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y. Activity of dendrites of single Purkinje cells and its relationship to so-called inactivation response in rabbit cerebellum. J Neurophysiol. 1968 Mar;31(2):131–141. doi: 10.1152/jn.1968.31.2.131. [DOI] [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G. Excitatory and inhibitory processes acting upon individual Purkinje cells of the cerebellum in cats. J Physiol. 1956 Sep 27;133(3):520–547. doi: 10.1113/jphysiol.1956.sp005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P. F. A theory of memory that explains the function and structure of the cerebellum. Brain Res. 1974 Apr 12;70(1):1–18. doi: 10.1016/0006-8993(74)90208-x. [DOI] [PubMed] [Google Scholar]
- Gilbert P. F., Thach W. T. Purkinje cell activity during motor learning. Brain Res. 1977 Jun 10;128(2):309–328. doi: 10.1016/0006-8993(77)90997-0. [DOI] [PubMed] [Google Scholar]
- Gordon M., Rubia F. J., Strata P. The effect of pentothal on the activity evoked in the cerebellar cortex. Exp Brain Res. 1973 Mar 29;17(1):50–62. doi: 10.1007/BF00234563. [DOI] [PubMed] [Google Scholar]
- Harvey R. J., Porter R., Rawson J. A. The natural discharges of Purkinje cells in paravermal regions of lobules V and VI of the monkey's cerebellum. J Physiol. 1977 Oct;271(2):515–536. doi: 10.1113/jphysiol.1977.sp012012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson J. A., McCarley R. W. Spontaneous discharge rates of cat cerebellar Purkinje cells in sleep and waking. Electroencephalogr Clin Neurophysiol. 1972 Nov;33(5):457–469. doi: 10.1016/0013-4694(72)90210-6. [DOI] [PubMed] [Google Scholar]
- Larson B., Miller S., Oscarsson O. A spinocerebellar climbing fibre path activated by the flexor reflex afferents from all four limbs. J Physiol. 1969 Aug;203(3):641–649. doi: 10.1113/jphysiol.1969.sp008883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham A., Paul D. H. Spontaneous activity of cerebellar Purkinje cells and their responses to impulses in climbing fibres. J Physiol. 1971 Feb;213(1):135–156. doi: 10.1113/jphysiol.1971.sp009373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R., Baker R., Sotelo C. Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol. 1974 May;37(3):560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- Mano N. Changes of simple and complex spike activity of cerebellar purkinje cells with sleep and waking. Science. 1970 Dec 18;170(3964):1325–1327. doi: 10.1126/science.170.3964.1325. [DOI] [PubMed] [Google Scholar]
- Marchesi G. F., Strata P. Mossy and climbing fiber activity during phasic and tonic phenomena of sleep. Pflugers Arch. 1971;323(3):219–240. doi: 10.1007/BF00586385. [DOI] [PubMed] [Google Scholar]
- Martinez F. E., Crill W. E., Kennedy T. T. Electrogenesis of cerebellar Purkinje cell responses in cats. J Neurophysiol. 1971 May;34(3):348–356. doi: 10.1152/jn.1971.34.3.348. [DOI] [PubMed] [Google Scholar]
- Murphy J. T., Sabah N. H. Cerebellar Purkinje cell responses to afferent inputs. I. Climbing fiber activation. Brain Res. 1971 Feb 5;25(3):449–467. doi: 10.1016/0006-8993(71)90454-9. [DOI] [PubMed] [Google Scholar]
- Murphy J. T., Sabah N. H. Spontaneous firing of cerebellar Purkinje cells in decerebrate and barbiturate anesthetized cats. Brain Res. 1970 Feb 3;17(3):515–519. doi: 10.1016/0006-8993(70)90259-3. [DOI] [PubMed] [Google Scholar]
- Oscarsson O. Termination and functional organization of the dorsal spino-olivocerebellar path. J Physiol. 1969 Jan;200(1):129–149. doi: 10.1113/jphysiol.1969.sp008685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach W. T. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J Neurophysiol. 1968 Sep;31(5):785–797. doi: 10.1152/jn.1968.31.5.785. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. II. Purkinje cell output and input. J Neurophysiol. 1970 Jul;33(4):537–547. doi: 10.1152/jn.1970.33.4.537. [DOI] [PubMed] [Google Scholar]
- Van Gilder J. C., O'Leary J. L. Effect of Nembutal anesthesia upon Purkinje cell activation in the cat. Electroencephalogr Clin Neurophysiol. 1971 Mar;30(3):173–188. doi: 10.1016/0013-4694(71)90052-6. [DOI] [PubMed] [Google Scholar]
- de Montigny C., Lamarre Y. Rhythmic activity induced by harmaline in the olivo-cerebello-bulbar system of the cat. Brain Res. 1973 Apr 13;53(1):81–95. doi: 10.1016/0006-8993(73)90768-3. [DOI] [PubMed] [Google Scholar]



