Abstract
Mycobacterium tuberculosis upregulates NF-κB binding and interleukin-8 (IL-8) gene expression and secretion in primary human monocytes. Inhibition of tyrosine protein kinases but not of ERK1/2 or p38 mitogen-activated protein kinases downregulates tuberculosis-induced IL-8 secretion. The inhibitor genistein decreased NF-κB nuclear translocation and IL-8 gene transcription in addition to acting on posttranscriptional processing.
Mycobacterium tuberculosis causes about three million deaths annually, and one-third of the world's population has latent tuberculosis (TB) (28). An important mechanism of host defense against TB is granuloma formation, which follows leukocyte influx. Interleukin-8 (IL-8) is one chemokine which appears to have a significant role in regulating leukocyte influx in TB. Elevated IL-8 concentrations are present in bronchoalveolar lavage fluid (4, 14, 29), pleural fluid (34), and plasma (7) of TB patients and in M. tuberculosis-infected human tissue (3, 25). In vivo studies have shown that pretreatment with anti-IL-8 alone inhibits mycobacterial granuloma formation (15). At a cellular level, phagocytosis of M. tuberculosis by monocytic cells is an important stimulus to IL-8 secretion, although other cell types, including respiratory epithelial cells, are also sources of IL-8 in TB (2, 6, 17, 32, 36). IL-8 is involved in attracting neutrophils and T cells and in monocyte recruitment (8, 24). Novel therapeutic approaches targeting IL-8 secretion during inflammation are the subject of ongoing research (35). However, relatively little is known about the mechanisms controlling IL-8 secretion in TB.
IL-8 in monocytes/macrophages is regulated primarily at the level of gene transcription. Many studies have demonstrated that NF-κB mediates the expression of genes involved in the lipopolysaccharide (LPS)-induced proinflammatory response (9), and activation of NF-κB in monocytes is found in TB (2, 21, 22, 31). NF-κB has a pivotal role in the control of IL-8 gene expression (2, 13, 23, 27, 30, 31, 32). Inflammatory stimuli including tumor necrosis factor and LPS, which activate NF-κB, have also been shown to activate upstream signaling pathways involving tyrosine kinases (1, 5, 33) and mitogen-activated protein kinases (MAPK) p38 and p42/44 (ERK) as well as stress-activated protein kinase/JNK (9, 12, 16, 18, 19, 20, 26), and these can further regulate the action of transcription factors (9, 10). However, regulation of such cellular signaling pathways is both cell type and stimulus specific. In the present study, we examined the role of these signal transduction paths in the control of M. tuberculosis-induced, NF-κB-associated upregulation of IL-8 secretion.
Transcriptional upregulation of IL-8 mRNA after stimulation of primary human monocytes by M. tuberculosis was first confirmed by semiquantitative reverse transcription-PCR, as we have described previously (2). Primers used were homologous to the 5′ end of exon 1 and the 3′ end of exon 2 of the IL-8 gene. Adhesion purification of monocytes results in limited transcriptional upregulation of the IL-8 gene (11). Further specific upregulation of IL-8 transcripts due to TB stimulation was detected at 8 h, and peak mRNA levels were detected after 16 h (data not shown), which is consistent with previous data obtained by using Northern analysis (6). In contrast, peak IL-8 mRNA levels were detected 4 h after stimulation by LPS, the positive control. We next analyzed the kinetics of TB-induced NF-κB activation in monocytes by using established methodology (2). TB is known to stimulate translocation of this transcription factor, which is important in the control of IL-8 gene expression (2, 31). Electromobility gel shift assays showed activation of NF-κB within 30 min, peaking at 1 to 2 h and still detectable 24 h after infection; the specificity of transcription factor binding was confirmed by competition experiments (data not shown). Transient upregulation of NF-κB is typical of responses of human monocytes to pathogens, and this pattern is similar to but of longer duration than that found for M. tuberculosis-stimulated respiratory epithelial cells (32).
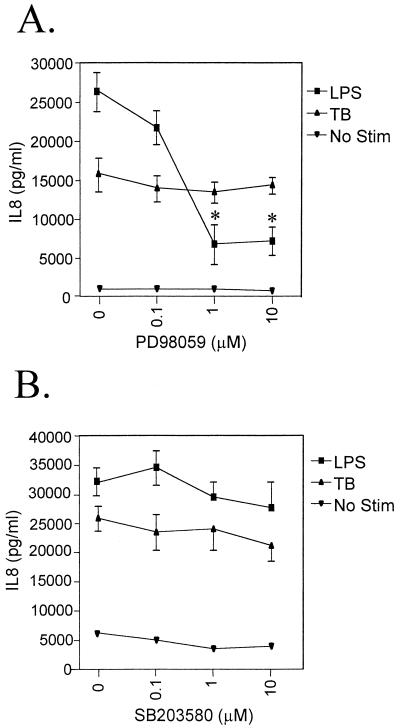
The role of MAPK pathways in control of IL-8 gene expression was investigated by pretreating monocytes for 1 h with either the established ERK kinase-specific inhibitor PD98059 or the p38 kinase-specific inhibitor SB203580 before infection with TB (Fig. 1). Neither inhibitor had any effect on monocyte viability (data not shown). Pretreatment of cultures with PD98059 did not alter TB-induced IL-8 secretion, although there was a dose-dependent inhibition of LPS-induced IL-8 secretion, as has been observed previously (20). Inhibition of the p38 MAPK path by SB203580 did not inhibit TB- or LPS-induced IL-8 secretion. The activity of this compound was confirmed since it inhibited IL-8 production induced by zymosan. LPS is known to activate the p38 path, and our data contrast with a report by Manthey et al. in which blocking this pathway was found to inhibit IL-8 mRNA accumulation (19). Such a difference may reflect the fact that they used a different inhibitor, SB202190, and that in that study monocytes were purified by elutriation. TB induces p38 MAPK activation in neutrophils, which leads to the generation of reactive oxygen intermediates that may contribute to mycobacterial killing (26). Although we did not find that p38 MAPK was involved in the control of IL-8 secretion, our data do not exclude other roles for p38 in TB.
FIG. 1.
Effect of MAPK inhibitors on IL-8 secretion by human monocytes stimulated with either LPS or TB or left unstimulated under control conditions. Cells were cultured in the presence of 0.1, 1, and 10 μM PD98059 (A) and SB203580 (B). Data are presented as the means ± standard errors of the mean of the results of at least three experiments. ∗, P < 0.05 versus untreated control.
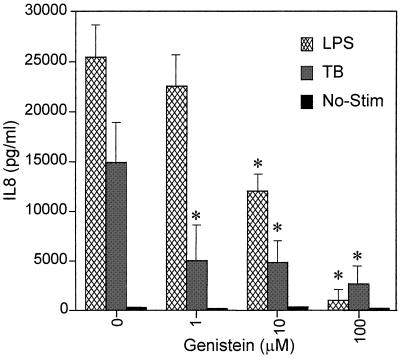
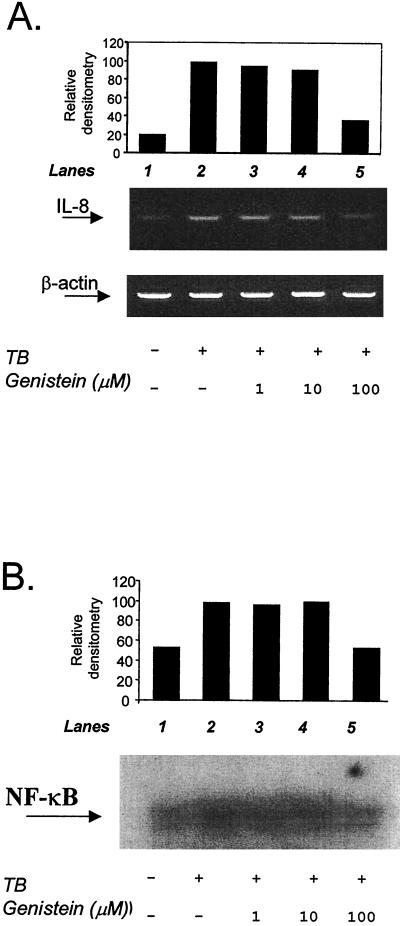
The effects of broad-spectrum protein tyrosine kinase inhibition were next investigated, since MAPK inhibition did not affect TB-induced IL-8 secretion. Cultures were pretreated with 1, 10, or 100 μM genistein (Fig. 2). Genistein did not alter monocyte viability, and in a dose-dependent manner, it significantly inhibited both TB- and LPS-dependent IL-8 secretion. Genistein at 100 μM but not at lower concentrations inhibited IL-8 mRNA accumulation (Fig. 3A), suggesting that tyrosine kinase acts at least in part on transcription. Consequently, we investigated the effect of tyrosine kinase inhibition on NF-κB binding and observed a partial downregulation in TB-infected monocytes pretreated with the highest dose of genistein (Fig. 3B), which contrasts with the effects of tumor necrosis factor stimulation, where inhibition of NF-κB binding is critical (1). We attribute the basal NF-κB activity level seen in Fig. 3B, lane 1, to the purification procedure. Since lower concentrations of genistein also inhibit IL-8 secretion, it is probable that protein tyrosine kinases have effects on posttranscriptional regulatory pathways in addition to regulating NF-κB-dependent IL-8 gene expression.
FIG. 2.
Effect of inhibition of protein tyrosine kinases by 1, 10, or 100 mM genistein on IL-8 secretion from human monocytes stimulated with either LPS or M. tuberculosis (TB). Data are presented as means ± standard errors of the mean of the results of at least three experiments. ∗, P < 0.05 versus control without drug.
FIG. 3.
(A) Effects of protein tyrosine kinase inhibition on IL-8 gene transcription. The graph shows relative IL-8 mRNA expression corrected for total mRNA by using the housekeeping gene β-actin. mRNA bands were quantified by densitometry with NIH Image 1.58. (B) Nuclear binding of the transcription factor NF-κB in TB-infected human monocytes, as detected by electromobility gel shift analysis. All data are representative of the results of three independent experiments.
In summary, we have investigated some of the cellular mechanisms by which M. tuberculosis stimulates primary human monocytes to secrete IL-8 at high concentrations. Soon after phagocytosis of M. tuberculosis, there is an activation of NF-κB which persists over 24 h and has kinetics consistent with those of IL-8 gene expression. Unexpectedly, we demonstrated that neither the ERK1/2 nor the p38 component of the MAPK signaling pathways, which are activated in TB, regulates this process. In contrast, protein tyrosine kinases are shown to be critical regulators of IL-8 in TB, acting pre- and posttranscriptionally.
Acknowledgments
This work was supported by a grant from the British Lung Foundation (BLF).
Editor: R. N. Moore
REFERENCES
- 1.Abu-Amer, Y., F. P. Ross, K. P. McHugh, A. Livolsi, J. F. Peyron, and S. L. Teitelbaum. 1998. Tumor necrosis factor-α activation of nuclear transcription factor-κB in marrow macrophages is mediated by c-Src tyrosine phosphorylation of IκBα. J. Biol. Chem. 273:29417-29423. [DOI] [PubMed] [Google Scholar]
- 2.Ameixa, C., and J. S. Friedland. 2001. Down-regulation of interleukin-8 secretion from Mycobacterium tuberculosis-infected monocytes by interleukin-4 and -10 but not by interleukin-13. Infect. Immun. 69:2470-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergeron, A., M. Bonay, M. Kambouchner, D. Lecossier, M. Riquet, P. Soler, A. Hance, and A. Tazi. 1997. Cytokine patterns in tuberculous and sarcoid granulomas: correlations with histopathologic features of the granulomatous response. J. Immunol. 159:3034-3043. [PubMed] [Google Scholar]
- 4.Casarini, M., F. Ameglio, L. Alemanno, P. Zangrilli, P. Mattia, G. Paone, A. Bisetti, and S. Giosue. 1999. Cytokine levels correlate with a radiologic score in active pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 159:143-148. [DOI] [PubMed] [Google Scholar]
- 5.Dackiw, A. P., G. Grinstein, G. F. Brisseau, I. D. McGilvray, A. B. Nathens, J. A. McGuire, R. Romanek, P. Y. Cheung, and O. D. Rotstein. 1997. The role of tyrosine phosphorylation in lipopolysaccharide- and zymosan-induced procoagulant activity and tissue factor expression in macrophages. Infect. Immun. 65:2362-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedland, J. S., D. G. Remick, R. Shattock, and G. E. Griffin. 1992. Secretion of interleukin-8 following phagocytosis of Mycobacterium tuberculosis by human monocyte cell line. Eur. J. Immunol. 22:1373-1378. [DOI] [PubMed] [Google Scholar]
- 7.Friedland, J. S., J. C. Hartley, C. G. Hartley, R. J. Shattock, and G. E. Griffin. 1995. Inhibition of ex vivo proinflammatory cytokine secretion in fatal Mycobacterium tuberculosis infection. Clin. Exp. Immunol. 100:233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerszten, R. E., E. A. Garcia-Zepeda, Y. C. Lim, M. Yoshida, H. A. Ding, M. A. Gimbrone, Jr., A. D. Luster, F. W. Luscinskas, and A. Rosenzweig. 1999. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 398:718-723. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh, S. 1999. Regulation of inducible gene expression by the transcription factor NF-kappaB. Immunol. Res. 19:183-189. [DOI] [PubMed] [Google Scholar]
- 10.Hambleton, J., S. L. Weinstein, L. Lem, and A. L. DeFranco. 1996. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc. Natl. Acad. Sci. USA 93:2774-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasahara, K., R. M. Strieter, S. W. Chensue, T. J. Standiford, and S. L. Kunkel. 1991. Mononuclear cell adherence induces neutrophil chemotactic factor/interleukin-8 gene expression. J. Leukoc. Biol. 50:287-295. [DOI] [PubMed] [Google Scholar]
- 12.Krause, A., H. Holtmann, S. Eickemeier, R. Winzen, M. Szamel, K. Resch, J. Saklatvala, and M. Kracht. 1998. Stress-activated protein kinase/Jun N-terminal kinase is required for interleukin (IL)-1-induced IL-6 and IL-8 gene expression in the human epidermal carcinoma cell line KB. J. Biol. Chem. 273:23681-23689. [DOI] [PubMed] [Google Scholar]
- 13.Kunsch, C., and C. A. Rosen. 1993. NF-κB subunit-specific regulation of the interleukin-8 promoter. Mol. Cell. Biol. 13:6137-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurashima, K., N. Mukaida, M. Fujimura, M. Yasui, Y. Nakazumi, and T. Matsuda. 1997. Elevated chemokine levels in bronchoalveolar lavage fluid of tuberculosis patients. Am. J. Respir. Crit. Care Med. 155:1474-1477. [DOI] [PubMed] [Google Scholar]
- 15.Larsen, C. G., M. K. Thomsen, B. Gesser, P. D. Thomsen, B. W. Deleuran, J. Nowak, V. Skodt, H. K. Thomsen, M. Deleuran, K. Thestrup-Pedersen, et al. 1995. The delayed-type hypersensitivity reaction is dependent on IL-8. Inhibition of a tuberculin skin reaction by an anti-IL-8 monoclonal antibody. J. Immunol. 155:2151-2157. [PubMed] [Google Scholar]
- 16.Lee, J. C., J. T. Laydon, P. C. McDonnell, T. F. Gallagher, S. Kumar, D. Green, D. McNulty, M. J. Blumenthal, J. R. Heys, S. W. Landvatter, et al. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372:739-746. [DOI] [PubMed] [Google Scholar]
- 17.Lin, Y., M. Zhang, and P. F. Barnes. 1998. Chemokine production by a human alveolar epithelial cell line in response to Mycobacterium tuberculosis. Infect. Immun. 66:1121-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu, M. K., P. Herrera-Velit, R. W. Brownsey, and N. E. Reiner. 1994. CD14-dependent activation of protein kinase C and mitogen-activated protein kinases (p42 and p44) in human monocytes treated with bacterial lipopolysaccharide. J. Immunol. 153:2642-2652. [PubMed] [Google Scholar]
- 19.Manthey, C. L., S. W. Wang, S. D. Kinney, and Z. Yao. 1998. SB202190, a selective inhibitor of p38 mitogen-activated protein kinase, is a powerful regulator of LPS-induced mRNAs in monocytes. J. Leukoc. Biol. 64:409-417. [DOI] [PubMed] [Google Scholar]
- 20.Marie, C., S. Roman-Roman, and G. Rawadi. 1999. Involvement of mitogen-activated protein kinase pathways in interleukin-8 production by human monocytes and polymorphonuclear cells stimulated with lipopolysaccharide or Mycoplasma fermentans membrane lipoproteins. Infect. Immun. 67:688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendez-Samperio, P., J. Palma, and A. Vazquez. 2002. Signals involved in mycobacteria-induced CXCL-8 production by human monocytes. J. Interferon Cytokine Res. 22:189-197. [DOI] [PubMed] [Google Scholar]
- 22.Mendez-Samperio, P., J. Palma, and A. Vazquez. 2001. Roles of intracellular calcium and NF-kappaB in the Bacillus Calmette-Guerin-induced secretion of interleukin-8 from human monocytes. Cell. Immunol. 211:113-122. [DOI] [PubMed] [Google Scholar]
- 23.Mukaida, N., Y. Mahe, and K. Matsushima. 1990. Cooperative interaction of nuclear factor κB and cis-regulatory binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J. Biol. Chem. 265:21128-21133. [PubMed] [Google Scholar]
- 24.Mukaida, N., A. Harada, and K. Matsushima. 1998. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 9:9-23. [DOI] [PubMed] [Google Scholar]
- 25.Pace, E., M. Gjomarkaj, M. Melis, M. Profita, M. Spatafora, A. M. Vignola, G. Bonsignore, and C. H. Mody. 1999. Interleukin-8 induces lymphocyte chemotaxis into the pleural space. Role of pleural macrophages. Am. J. Respir. Crit. Care Med. 159:1592-1599. [DOI] [PubMed] [Google Scholar]
- 26.Perskvist, N., L. Zheng, and O. Stendahl. 2000. Activation of human neutrophils by Mycobacterium tuberculosis H37Ra involves phospholipase C gamma 2, Shc adapter protein, and p38 mitogen-activated protein kinase. J. Immunol. 164:959-965. [DOI] [PubMed] [Google Scholar]
- 27.Ragno, S., M. Romano, S. Howell, D. J. Pappin, P. J. Jenner, and M. J. Colston. 2001. Changes in gene expression in macrophages infected with Mycobacterium tuberculosis: a combined transcriptomic and proteomic approach. Immunology 104:99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rusch-Gerdes, S. 1999. Epidemiology of resistant tuberculosis in Europe. Infection 27(Suppl. 2):S17-S18. [DOI] [PubMed] [Google Scholar]
- 29.Sadek, M. I., E. Sada, Z. Toossi, S. K. Schwander, and E. A. Rich. 1998. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am. J. Respir. Cell Mol. Biol. 19:513-521. [DOI] [PubMed] [Google Scholar]
- 30.Stein, B., and A. S. Baldwin, Jr. 1993. Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-κB. Mol. Cell. Biol. 13:7191-7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toossi, Z., B. D. Hamilton, M. H. Phillips, L. E. Averill, J. J. Elner, and A. Salvekar. 1997. Regulation of nuclear factor-κB and its inhibitor IκB-α/MAD-3 in monocytes by Mycobacterium tuberculosis and during human tuberculosis. J. Immunol. 159:4109-4116. [PubMed] [Google Scholar]
- 32.Wickremasinghe, M., L. H. Thomas, and J. S. Friedland. 1999. Pulmonary epithelial cells are a source of IL-8 in the response to Mycobacterium tuberculosis: essential role of IL-1 from infected monocytes in a NF-κB-dependent network. J. Immunol. 163:3936-3947. [PubMed] [Google Scholar]
- 33.Williams, L. M., and A. J. Ridley. 2000. Lipopolysaccharide induces actin reorganization and tyrosine phosphorylation of Pyk2 and paxillin in monocytes and macrophages. J. Immunol. 164:2028-2036. [DOI] [PubMed] [Google Scholar]
- 34.Yamada, Y., A. Nakamura, M. Hosoda, T. Kato, T. Asano, K. Tonegawa, and M. Itoh. 2001. Cytokines in pleural liquid for diagnosis of tuberculous pleurisy. Respir. Med. 95:577-581. [DOI] [PubMed] [Google Scholar]
- 35.Yang, X. D., J. R. Corvalan, P. Wang, C. M. Roy, and C. G. Davis. 1999. Fully human anti-interleukin-8 monoclonal antibodies: potential therapeutics for the treatment of inflammatory diseases stages. J. Leukoc. Biol. 66:401-410. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, Y., M. Broser, H. Cohen, M. Bodkin, K. Law, J. Reibman, and W. N. Rom. 1995. Enhanced interleukin-8 release and gene expression in macrophages after exposure to Mycobacterium tuberculosis and its components. J. Clin. Investig. 95:586-592. [DOI] [PMC free article] [PubMed] [Google Scholar]