The controlled destruction of tissue by freezing is today widely practised in medicine. Terms for it include cryotherapy, cryocautery, cryocongelation and cryogenic surgery, but cryosurgery (literally, cold handiwork) seems most appropriate. Cryosurgery is a cheap, easy, and safe treatment suitable for both hospital and office based practice. Its major advantage is excellent cosmetic results with minimal scarring.
The benefits of cold have been appreciated for many thousands of years. The ancient Egyptians, and later Hippocrates, were aware of the analgesic and anti-inflammatory properties of cold. Over the past 200 years cold treatment has evolved from generalized application such as hydrotherapy (Figure 1) to specific, focal destruction of tissue—today's cryosurgery.
Figure 1.

A man self-administering hydrotherapy. Wellcome Library, London
THE BEGINNINGS OF CRYOSURGERY
James Arnott (1797-1883), an English physician, published on the use of cold between 1819 and 18791,2. He was the senior physician of Brighton Infirmary but moved to London on winning fame. His brother, a scientist, had already gained fame and fortune as inventor of the slow combustion stove. Arnott was the first person to use extreme cold locally for the destruction of tissue. He used a mixture of salt and crushed ice (`two parts finely pounded ice and one part of chloride of sodium'1) for palliation of tumours, with resultant reduction of pain and local haemorrhage. He stated that a very low temperature will arrest every inflammation which is near enough to the surface to be accessible to its influence1. He designed his own equipment, consisting of a waterproof cushion applied to the skin, two long flexible tubes to convey water to and from the affected part and a reservoir for the ice/water mixture and a sump. He exhibited this at the Great Exhibition of London in 1851 and won a prize medal for his effort2. (The Great Exhibition was a showcase for the Empire's scientific prowess not unlike the Millennium Dome but with considerably more style.) Arnott treated breast cancer, uterine cancers and some skin cancers. Although palliation was his main aim he recognized the potential of cold for curing cancer, stating that the cases he had seen `are therefore, by no means unfavourable to the supposition of the curability of cancer by congelation'. He advocated cold treatment for acne, neuralgia and headaches, achieving temperatures of -24°C. In addition he recognized the analgesic `benumbing' effect of cold, recommending the use of cold to anaesthetize skin before operation. He was concerned about the safety of the new anaesthetic agents that were being introduced and advocated the use of cold as an alternative. This was to become a lifelong crusade that was ultimately unsuccessful, but his contribution to the development of cryosurgery was crucial.
FIRST USE OF REFRIGERANTS
Salt/ice mixtures were not capable of reducing tissue temperatures sufficiently to treat tumours effectively. It was not until refrigerants came into use that lower tissue temperatures could be achieved. In the late 1800s, at a time of tremendous scientific advance, there was an interest in liquefying gases. Cailletet, on Christmas Eve 1877, demonstrated at the French Academy of Science that oxygen and carbon monoxide could be liquefied under high pressure3. Pictet also demonstrated the liquefaction of oxygen but used a mechanical refrigeration cascade4. Von Linde was responsible for the first commercial production of liquid air in 1895, which led the way to its widespread introduction.
LIQUID AIR AND LIQUID OXYGEN
Campbell White, of New York, was the first person to employ refrigerants for medical use. He reported his success in 1899, advocating liquid air for the treatment of a large range of conditions including lupus erythematosus, herpes zoster, chancroid, naevi, warts, varicose leg ulcers, carbuncles and epitheliomas5,6. He recognized `the efficiency of liquid air in the treatment of carcinoma' and enthusiastically stated `I can truly say today that I believe that epithelioma, treated early in its existence by liquid air, will always be cured6'.
Whitehouse7 reviewed the effects of liquid air on normal skin, finding it to be especially useful for epitheliomata, lupus erythematosus and vascular naevi. He stated that liquid air `outranks some of the remedies on which we have placed great reliance7'. He treated recurrences of epitheliomata after radiotherapy and found liquid air to be more successful than repeat radiotherapy. Bowen and Towle8 reported the successful use of liquid air for vascular lesions in 1907. Liquid oxygen had a limited vogue in the 1920s and 1930s. It has similar properties to liquid air, achieving temperatures of -182.9°C, but was chiefly used for acne.
CARBONIC ACID SNOW
Around the time that liquid air was being investigated, William Pusey9 of Chicago popularized the use of carbon dioxide snow (or carbonic acid snow) in preference to a salt and ice mixture. He advocated carbon dioxide snow because of its easy availability (thanks to its use by manufacturers of mineral waters). Liquid air was very difficult to obtain at this time. The liquid carbon dioxide gas was supplied in steel cylinders under pressure. When the gas was allowed to escape, rapid expansion caused a fall in temperature (the Joule—Thompson effect) and a fine snow was formed. The snow was easily compressed into various shapes, known as pencils, suitable for different treatments. Pusey's first reported case9 was the treatment of a large black hairy naevus on a young girl's face. Impressive before-and-after photographs showed the successful depigmentation of the lesion. This was one of the first demonstrations of the extraordinary sensitivity of melanocytes to cold. He successfully treated other naevi, warts and lupus erythematosus. Pusey stated of carbon dioxide snow that `we have found a destructive application whose action can be accurately gauged and is therefore controllable'. He recognized the low scarring potential of cryosurgery although he attributed this to regeneration of residual epidermal cells rather than to collagen's resistance to cold.
Hall-Edwards, of Birmingham, first described his carbon dioxide collection model in The Lancet in 191110. Hall-Edward's monograph, written later in 1913, detailed the uses of carbon dioxide and methods of collection11 (Figure 2). His contribution to cryosurgery was all the more remarkable because he was a respected radiotherapist in charge of much of the Midlands. He would have been well aware of the place of cryosurgery in relation to X-ray use. He detailed many conditions in which treatment was effective but was particularly struck by its efficacy in rodent ulcers. At the same time Cranston-Low, a physician in the Edinburgh skin department, was likewise promoting the use of carbon dioxide snow12. He observed that `thrombosis, direct injury to tissues, and the inflammatory exudate probably all act together' to produce the effects of freezing.
Figure 2.
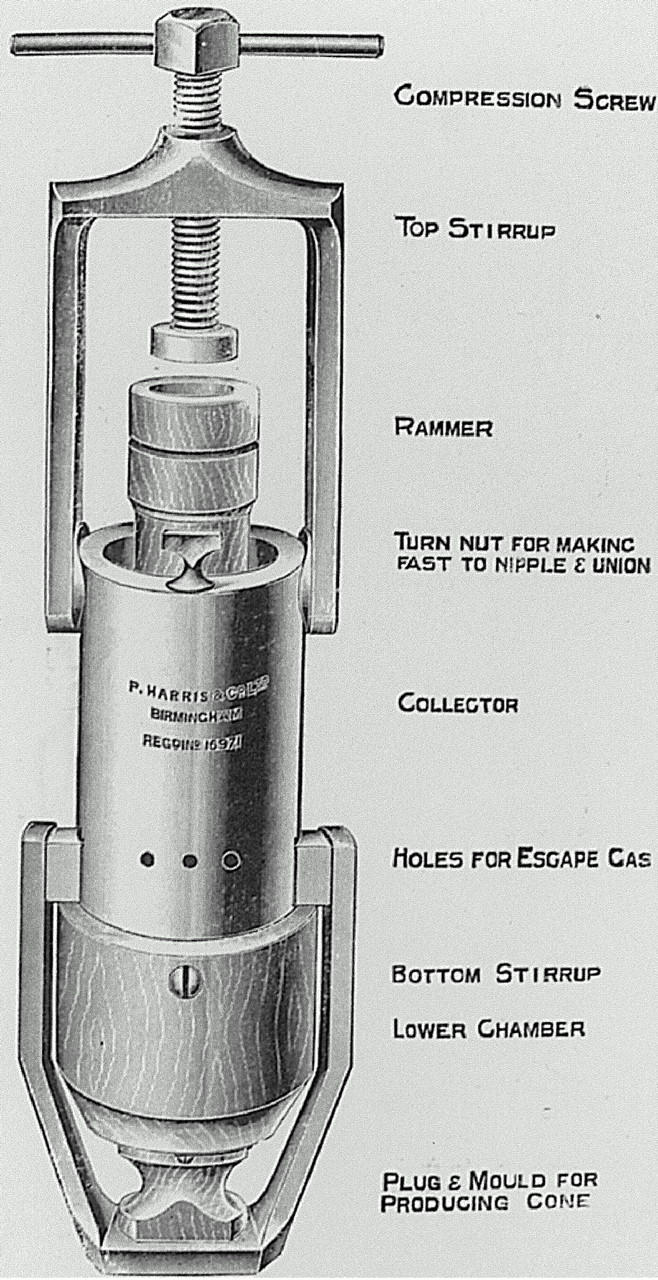
Hall-Edward's carbon dioxide snow collector and compressor
Solid carbon dioxide applied directly to the skin cannot get the surface temperature below -79°C. This is insufficient for deeper freezing of tissue necessary for treatment of malignancies, when a temperature of -50°C at a tissue depth of 3 mm is required. Nevertheless it proved very successful for a wide variety of benign skin conditions and remained popular until the 1960s. Carbon dioxide slush, a mixture of carbon dioxide and acetone, was used extensively for acne. As the use of carbon dioxide snow became more widespread so did the range of conditions treated. De Quervain reported the successful use of carbonic snow for bladder papillomas and bladder cancers in 191713.
DEBATE ON THE BEST REFRIGERANT
The debate on the best cryogen to use persisted for much of the first half of the twentieth century. Reviewing the uses of liquid air and carbon dioxide snow in 1910, Gold14 concluded `there is no hesitancy in saying liquid air is far preferable' although he acknowledged the difficulty in obtaining liquid air at that time. In 1929 Irvine and Turnacliffe15 similarly favoured liquid air and oxygen over carbon dioxide snow, but the controversy continued into the 1960s. Irvine and Turnacliffe15 reported liquid air treatment of seborrhoeic keratoses, senile keratoses, lichen simplex, poison ivy dermatitis and herpes zoster. They found liquid oxygen very useful for warts, declaring that `it offers a practically sure, quick and painless method for removal of all types of warts, including the plantar type'. The debate was at times acrimonious. Pusey, an ardent advocate of carbon dioxide snow, said in reply to Irvine's paper, `I do not want to throw any question on Dr Irvine's results with liquid air but for any method of treatment to convince me of its adequacy in warts, I want about 10,000 cases'15.
APPLICATION AND STORAGE
Refrigerants were generally applied either by painting directly onto the skin or by use of cotton wool twisted around a piece of cane that had been dipped into liquid air. Some ingenious devices were developed including Campbell White's roller for treatment of erysipelas6. Grimmett16 highlighted the limitations of a cotton wool applicator, showing that the depth of freeze was insufficient to treat tumours. Whitehouse (1864-1938), a New York dermatologist, developed a spray in 1907 which allowed much lower minimum temperatures7. His simple design consisted of two glass tubes inserted into a cork stopper of a laboratory wash bottle, operated by finger control. Whitehouse used his spray to treat skin lesions including cancers but abandoned it because of the difficulty in limiting the area of the spray. The great advantage of liquid air over salt/ice mixtures was the lower temperatures that could be achieved, allowing tumours to be treated, but a disadvantage was the difficulty in obtaining and transporting it. Sir James Dewar solved the problems of transportation and storage by inventing a flask made of two walls of glass with a vacuum between11. Even today the containers used for refrigerants have much the same design.
LIQUID NITROGEN
Allington is generally thought to have been first to use liquid nitrogen, in 195017. He recognized that the properties of liquid nitrogen were very similar to those of liquid air and oxygen. After the Second World War, liquid nitrogen became freely available and was preferable to liquid oxygen with its explosive potential. He used a cotton swab for treating various benign lesions but poor heat transfer between swab and skin meant this method was insufficient for tumour treatment.
The contribution of Dr Irving S Cooper to cryosurgery was immense18,19. An American neurosurgeon based in New York, in 1913 he designed a liquid nitrogen probe that was capable of achieving temperatures of -196°C. With it he treated Parkinson's disease and other movement disorders by freezing the thalamus, in addition to previously inoperable brain tumours. Although Cooper was controversial in his lifetime because of his showmanship, his work led to an explosion of interest in liquid nitrogen and its eventual acceptance as a standard treatment in many specialties. More general use of cryosurgery was facilitated by the development of devices suitable for office based practice. Torre20 developed a liquid nitrogen spray in 1965 and Zacarian a hand-held device, the Kryospray, in 196720. Zacarian popularized the use of this equipment21. Zacarian's spray allowed one-handed operation with trigger type control, and interchangeable tips permitted variations in spray diameter. Zacarian also developed copper probes that allowed tissue-freezing to depths of up to 7 mm. His contributions to cryosurgery equipment, understanding of the science of the cryolesion and the published work on cryosurgery was very great. Amoils22 developed a liquid nitrogen probe that achieved cooling by expansion. He performed cataract extraction (cryoextraction) successfully but cooling was slow and temperatures were not low enough for tumour work. This system is still widely used in gynaecology and ophthalmology. The use of liquid nitrogen spread through different specialties21. Rand performed a transphenoidal hypophysectomy with liquid nitrogen, Gage treated oral cancers and Cahan performed cryosurgery of the uterus with a liquid nitrogen probe. The use of liquid nitrogen in Great Britain took off when Zacarian donated the first hand-held liquid nitrogen spray to the Oxford dermatology department in the 1970s. This centre became the focus of cryosurgical research in Britain.
CRYOBIOLOGY
The past 50 years have seen great advances in knowledge of the biological effects of freezing. Almost all research has concerned the effects of liquid nitrogen. The development of temperature probes that can be inserted into skin has allowed measurement of tissue temperatures during freezing. An accurate picture of the shape and depth of iceball formation with different lengths of freeze has been built up, allowing development of guidelines for freezing times (best established for cutaneous lesions23). For malignant lesions freezing times are longer than for benign lesions since destruction of all malignant cells is vital. Tissue temperatures must be below -50°C for adequate treatment of tumours. A 30-second spot freeze, counted 30 seconds after an iceball formation, is capable of achieving a tissue temperature of -50°C in the centre of the ice ball and is usually the minimum time necessary for tumour work3. Other research has concentrated on determining the sensitivity of individual cell types to freezing. Melanocytes are most sensitive, hence the depigmentation of skin often seen after cutaneous cryosurgery. Collagen is the most resilient tissue, and indeed preservation of the normal structure of collagen bundles is observed on electron microscopy even after the deep freezes necessary for tumour work. This explains why there is so little scarring24. Cartilage necrosis is extremely rare, so cryosurgery is particularly suitable in areas where maintenance of elasticity and function are important—such as the ear, around the eyes and the nose25.
WHICH REFRIGERANT?
Liquid nitrogen is by far the most popular cryogen in current use. Its popularity is due to the low temperatures achievable (-197°C), which make it suitable for both benign and malignant lesions. Its effects are predictable and well documented. Carbon dioxide still enjoys some popularity because of its easy storage but is really only suitable for the occasional user and for treatment of benign lesions. Nitrous oxide is favoured by many gynaecologists and oral surgeons. Storage presents no problems but the large cylinders required are not easily portable. Only a probe method is suitable because spraying results in formation of solid crystals of nitrous oxide. A lowest temperature of -89°C makes nitrous oxide unsuitable for malignant lesions. Freons (fluorinated hydrocarbons with a low boiling point) have been used in dermatological practice since 1955 when Wilson advocated their use for firming skin before dermabrasion27. Freon 12 has been used for acne pits and is especially useful when a large surface area needs to be treated. The major advantages of freons are their portability and easy storage but their disadvantages are insufficiently low temperatures for tumour work, potential toxicity in inhaled air and their role in depleting the ozone layer. Currently a spray-on non-fluorinated hydrocarbon can be prescribed (the Histofreezer, Thames Laboratories UK) but this is unlikely to achieve temperatures low enough to be highly effective.
DERMATOLOGICAL CRYOSURGERY
Cryosurgery is now indispensable in a dermatology department. Benign lesions amenable to treatment include viral warts, seborrhoeic keratoses, molluscum contagiosum, spider angiomata and digital myxoid cysts23. The efficacy of treatment of viral warts is approximately 75% if lesions are treated every two to three weeks, in line with other methods of treatment28. However, when cryosurgery is contemplated for benign lesions it is especially important to consider the possible side-effects of pain, blistering and hypopigmentation.
Cryosurgery is highly effective for premalignant solar keratoses and Bowen's disease. Cure rates after cryosurgery of Bowen's disease are comparable with those of excision, curettage and cautery29. Basal cell carcinomas are commonly treated by cryosurgery and the cure rates also compare very favourably with those of surgical treatments, in carefully selected patients. Other tumours that can be effectively treated are squamous cell carcinomas and lentigo maligna23.
THE WIDER APPLICATION OF CRYOSURGERY
Many other specialties have embraced and refined the technique of cryosurgery. Eye surgeons have used it extensively. The first report of retinal tears treated by freezing came from Bietti30 in 1933, and when Bellowes reviewed cryotherapy of ocular diseases in 1966 he included cryoextraction of cataracts and treatments for glaucoma and tumours30. Cryosurgery still has an important place in modern ophthalmological practice, particularly for eyelash ablation in trichiasis31, treatment of retinopathy of prematurity32 and retinal detachment.
In gynaecology the use of cold treatment goes back as far as 1883, when Openchowski13 treated chronic cervicitis with cold water irrigation. Temple Fay33, in Philadelphia, applied both local and general cold treatment for cervical tumours during the 1940s and in 1964 Cahan34 developed the liquid nitrogen probe for the treatment of uterine fibroids and cervical neoplasia. There has been some interest in cryosurgical treatment of cervical intraepithelial neoplasia but this is losing favour. Cryosurgery of vulval intraepithelial neoplasia is followed by early recurrence and is not to be recommended35. Palliation of surgically unresectable vulval squamous cell tumours can be very beneficial, with reduction of pain and tumour size36.
General surgeons have used freezing as an adjunct to surgery. Allen37, in 1938, recognized that limbs packed in ice for 3 hours could be subsequently amputated without an anaesthetic agent, and as recently as 1985 a review article37 described the use of freezing to delay an otherwise urgent amputation and allow more time for stabilization of a critically ill patient. A recent study of palliative treatment for primary rectal carcinoma showed complete relief of symptoms in 62%38. Patients selected were unable to undergo surgical treatment either because of prohibitive operative risk or because of unresectable tumour. Cryosurgery has been shown to be an effective treatment for haemorrhoids39 and may be a useful alternative to surgical haemorrhoidectomy in countries where health resources are limited. Unresectable tracheobronchial carcinomas have been managed by cryosurgery40, with haemoptysis alleviated in over 90%.
Other areas of current interest include a nephronsparing treatment option for kidney cancers41 and cryosurgical treatment of prostatic cancer42. In prostate cancer, impotence and incontinence are less frequent with cryosurgery than with radical prostatectomy or radiotherapy. Further studies will be necessary to assess longterm cure rates. Hepatic cryosurgery for either metastatic carcinoma or primary hepatocellular carcinoma, via cryoprobe, gives results similar to those of surgical resection. The major advantage is the ability to treat widespread lesions, whereas surgical resection is limited to isolated or small foci of tumour43. Also, cryosurgery has been used for bone tumours for 30 years and still has a role44.
After nearly two centuries, the technique of cryotherapy remains widely applicable. At a time when surgical excision is in the ascendant this simple method, with its cosmetic and functional benefits, should not be neglected.
References
- 1.Arnott J. On the Treatment of Cancer by the Regulated Application of an Anaesthetic Temperature. London: Churchill, 1851
- 2.Bird H. James Arnott, MD (Aberdeen), 1797-1883, a pioneer in refrigeration. Anaesthesia 1949;4: 10-17 [Google Scholar]
- 3.Cailletet L. Recherches sur la liquéfaction des gaz. Ann Chemie Physique 1878;15: 132-44 [Google Scholar]
- 4.Pictet R. Mémoire sur la liquéfaction de l'oxygène. Am Chemie Physique 1878;13: 145-227 [Google Scholar]
- 5.White AC. Liquid air: its application in medicine and surgery. Med Rec 1899;56: 109-12 [Google Scholar]
- 6.White AC. Possibilities of liquid air to the physician. JAMA 1901;36: 426-9 [Google Scholar]
- 7.Whitehouse H. Liquid air in dermatology: its indications and limitations. JAMA 1907;49: 371-7 [Google Scholar]
- 8.Bowen JT, Towle HP. Liquid air in dermatology. Med Surg J 1907;157: 561 [Google Scholar]
- 9.Pusey W. The use of carbon dioxide snow in the treatment of naevi and other lesions of the skin. JAMA 1935;49: 1354-6 [Google Scholar]
- 10.Edwards JH. The therapeutic effects of carbon dioxide snow: methods of collecting and application. Lancet 1911;ii: 87-90 [Google Scholar]
- 11.Hall-Edwards J. Carbon Dioxide Snow: its Therapeutic Uses. London: Simpkin, Marshall, Hamilton, Kent, 1913
- 12.Cranston-Low R. Carbonic Acid Snow as a Therapeutic Agent in the Treatment of Disease of the Skin. Edinburgh/London: William Green, 1911
- 13.Bracco D. The historic development of cryosurgery. Clin Dermatol 190;8: 1-4 [DOI] [PubMed]
- 14.Gold J. Liquid air and carbonic acid snow: therapeutic results obtained by dermatologists. NY Med J 1910;92: 1276-7 [Google Scholar]
- 15.Irvine H, Turnacliffe D. Liquid oxygen in dermatology. Arch Dermatol Syphilol 1929;19: 270-80 [Google Scholar]
- 16.Grimmett R. Liquid nitrogen therapy. Histologic observations. Arch Dermatol 1961;83: 563-7 [DOI] [PubMed] [Google Scholar]
- 17.Allington H. Liquid nitrogen in the treatment of skin diseases. Calif Med 1950;72: 153-5 [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper IS. Cryogenic surgery. A new method of destruction or extirpation of benign or malignant tissues. N Engl J Med 1963;263: 741-9 [DOI] [PubMed] [Google Scholar]
- 19.Das K, Benzil DL, Rovit RL. Irving S Cooper (1922-1985): a pioneer in functional neurosurgery. J Neurosurg 1998;89: 865-73 [DOI] [PubMed] [Google Scholar]
- 20.Zacarian S. Cryogenics: the cryolesion and the pathogenesis of cryonecrosis. In: Zacarian SA, ed. Cryosurgery for Skin Cancer and Cutaneous Disorders. St Louis: Mosby, 1985; 1-30
- 21.Kuflik EG, Gage AA. History. In: Kuflik EG, Gage AA, eds Cryosurgical Treatment for Skin Cancer. New York: Igaku-Shoin 1990: 1-13
- 22.Amoils SP. The Joule Thomson cryoprobe. Arch Ophthalmol 1967;78: 201-7 [DOI] [PubMed] [Google Scholar]
- 23.Dawber R, Colver G, Jackson A. Cutaneous Cryosurgery: Principles and Practice. London: Martin Dunitz, 1997
- 24.Shepherd JP, Dawber RPR. Wound healing and scarring after cryosurgery. Cryobiology 1984;21: 157-69 [DOI] [PubMed] [Google Scholar]
- 25.Dawber RPR. Cold kills! Clin Exp Dermatol 1988;13: 137-50 [DOI] [PubMed] [Google Scholar]
- 26.Dawber RPR, Colver G, Jackson A. Historic and scientific basis of cryosurgery. In: Cutaneous Cryosurgery. Principles and Practice. London: Martin Dunitz, 1997: 15-26
- 27.Wilson JW, Ayers SW, Luikart R. Dichlorotetrafluorethane for surgical planning. Arch Dermatol 1955;71: 253 [Google Scholar]
- 28.Bunney MH, Nolan MW, Williams DA. An assessment of methods of treating viral warts by comparative treatment trials based on a standard design. Br J Dermatol 1976;94: 667-9 [DOI] [PubMed] [Google Scholar]
- 29.Ball S, Dawber RPR. Treatment of Bowen's disease with particular emphasis on the problem of lower leg lesions. Austr J Dermatol 1998;39: 63-8 [DOI] [PubMed] [Google Scholar]
- 30.Bellowes JG. Cryotherapy of Ocular Diseases. Philadelphia: Lippincott, 1966
- 31.Bartley GB, Bullock JD, Olsen TG, et al. An experimental study to compare methods of eyelash ablation. Ophthalmology 1987;94: 1286-9 [DOI] [PubMed] [Google Scholar]
- 32.Anderson CC, Phelps DL. Peripheral retinal ablation for threshold retinopathy of prematurity in preterm infants. Cochrane Database Syst Rev 2000;2: CD001693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gage AA. History of cryosurgery. Semin Surg Oncol 1998;14: 99-109 [DOI] [PubMed] [Google Scholar]
- 34.Cahan WG. Cryosurgery of the uterus: description of technique and potential application. Am J Obstet Gynecol 1964;88: 410-14 [DOI] [PubMed] [Google Scholar]
- 35.Marren P, Dawber RPR, Wojnarowska F. Failure of cryosurgery to eradicate vulval intraepithelial neoplasia: a pilot study. J Eur Acad Dermatol Venerol 1993;2: 247-51 [Google Scholar]
- 36.Almeido Gongalves JC. Cryovulvectomy—a new surgical technique for advanced cancer. Skin Cancer 1986;1: 17-32 [Google Scholar]
- 37.Hunsaker R, Schwartz J, Keasy B, et al. Dry ice cryoamputation: a 12 year experience. J Vasc Surg 1985;2: 812-16 [DOI] [PubMed] [Google Scholar]
- 38.Meijer S, Rahusen FD, Van der Plas LG. Palliative cryosurgery for rectal carcinoma. Int J Colorect Dis 1999;14: 177-80 [DOI] [PubMed] [Google Scholar]
- 39.Dennison AR, Paraskevopoulos JA, Kerrigan DD, et al. New thoughts on the aetiology of haemorrhoids and the development of non-operative methods for their management. Minerva Chir 1996;51: 209-16 [PubMed] [Google Scholar]
- 40.Maiwand MO. The role of cryosurgery in palliation of tracheobronchial carcinoma. Eur J Cardio-Thorac Surg 1999;15: 764-8 [DOI] [PubMed] [Google Scholar]
- 41.Gill IS, Novick AC. Renal cryosurgery. Urology 1999;54: 215-9 [DOI] [PubMed] [Google Scholar]
- 42.Badalament RA, Bahn DK, Kim H. Patient-reported complications after cryoablation therapy for prostatic cancer. Urology 1999;54: 295-9 [DOI] [PubMed] [Google Scholar]
- 43.Saliken JC, McKinnon G, Gray RR, et al. Liver cryosurgery with curative intent: can we realize the promise? Can Assoc Radiologists J 1999;50: 295-7 [PubMed] [Google Scholar]
- 44.Bickels J, Meller I, Shmookler BM, et al. The role and biology of cryosurgery in the treatment of bone tumours. A review. Acta Orthop Scand 1999;70: 308-15 [DOI] [PubMed] [Google Scholar]


