Abstract
We report that dissemination of Mycobacterium tuberculosis in the mouse is under host control and precedes the initiation of T-cell immunity. Nine to eleven days after aerosol inoculation, M. tuberculosis disseminates to the pulmonary lymph nodes (LN), where M. tuberculosis-specific T cells are detected 2 to 3 days thereafter. This indicates that the initial spread of bacteria occurs via lymphatic drainage and that the acquired T-cell immune response is generated in the draining LN. Dissemination to peripheral sites, such as the spleen and the liver, occurs 11 to 14 days postinfection and is followed by the appearance of M. tuberculosis-specific T cells in the lung and the spleen. In all cases studied, dissemination to the LN or the spleen preceded activation of M. tuberculosis-specific T cells in that organ. Interestingly, bacteria disseminate earlier from the lungs of resistant C57BL/6 mice than from the lungs of susceptible C3H mice, and consequently, C57BL/6 mice generate an immune response to M. tuberculosis sooner than C3H mice generate an immune response. Thus, instead of spreading infection, early dissemination of M. tuberculosis may aid in the initiation of an appropriate and timely immune response. We hypothesize that this early initiation of immunity following inoculation with M. tuberculosis may contribute to the superior resistance of C57BL/6 mice.
The ability of the host to recognize the presence of pathogens is crucial for the development of the adaptive immune response to an infection. As most organs are unable to generate an adaptive immune response in situ, the current model of T-cell-mediated immunity suggests that microbial antigens must be delivered to the draining lymph nodes (LN) before a T-cell response can be generated. For example, following cutaneous infection with Leishmania major, the trafficking of infected Langerhans cells to the draining LN is critical in the development of T-cell immunity. Although the dissemination of certain enteric and pulmonary pathogens from the initial site of infection occurs within days, other pathogens, such as L. major, can remain undetected for weeks, which significantly delays the onset of immunity (4, 9, 12, 21, 30, 39). If the presence of microbial antigens in the LN is important in activating antigen-specific T cells, then the timing of dissemination to the draining LN may be critical in determining whether the infectious agent or the host immune system ultimately triumphs.
Mycobacterium tuberculosis is a human pulmonary pathogen that poses a unique challenge for the host immune system. In addition to being a facultatively intracellular organism, it has special tropism for macrophages and has developed strategies for survival in these cells, such as inhibiting the fusion of the phagosome with the lysosome (38). This tactic may impair the ability of the immune system to recognize that an infection has taken place, since the processing and expression of bacterial antigens on the surface of infected cells are delayed. Little is known about the early events that follow infection of alveolar macrophages by M. tuberculosis which lead to the initiation of immunity. For example, it is not known whether M. tuberculosis disseminates from the lung as free bacteria or if the trafficking from the lung of infected myeloid cells leads to the spread of M. tuberculosis, as is now appreciated for other pathogens (20, 27, 35). The route of spread, whether hematogenous or via the lymphatics, also remains unknown. Finally, it is not clear what factors influence the movement of bacteria and their antigens from the initial site of infection to the draining LN.
Despite the fact that M. tuberculosis has developed strategies to avoid immune detection, a protective anti-M. tuberculosis response is generated in the majority of infected people. In fact, only 5 to 10% of infected individuals develop clinical disease despite the fact that M. tuberculosis establishes a latent infection, even in asymptomatic people (29). Clinically, it has been observed that impairment of cell-mediated immunity leads to an increased risk of reactivation tuberculous disease. However, tuberculosis also develops in individuals with no obvious impairment of the immune system, and it is currently thought that such individuals may have genetic polymorphisms that alter their susceptibility to disease (1, 8, 11). Proving this in humans can be difficult. However, the use of animal models has clearly established that the host genetic background modulates the generation of protective immunity. For example, differences exist in the survival of inbred mouse strains following inoculation with virulent M. tuberculosis (16, 25). It has been shown that susceptible and resistant mouse strains also differ in their histopathological and immune responses (7, 14, 15, 17, 33, 37). In addition, host genes that influence these survival differences, such as the allelic sst1 locus which in part determines resistance or susceptibility of C57BL/6 (B6) and C3H mice to tuberculosis, are beginning to be identified (10, 19).
It has previously been observed that B6 mice have prolonged survival following aerosol or intravenous inoculation compared to the survival of susceptible C3H mice (17). In the present study, we sought to determine whether the initiation of the immune response to M. tuberculosis differs in these strains. We used an aerosol delivery system to experimentally inoculate mice with M. tuberculosis by the natural route of infection. This infection model allowed us to examine the influence of host factors on the early dissemination of M. tuberculosis from the lung, both locally to the pulmonary lymph node (PLN) and systemically to the liver and the spleen. We found that dissemination initially proceeded via the lymphatics to the draining PLN, where subsequently an antigen-specific response was first detected. Antigen-specific immunity was observed in the LN or the spleen only after bacteria had disseminated to that organ. Our analysis revealed that host genetic factors do influence the rate of initial bacterial dissemination from the lung. Surprisingly, bacteria disseminate from the lungs of resistant B6 mice earlier than from the lungs of susceptible C3H mice. Since this seems to promote earlier initiation of immunity and correlates with enhanced long-term survival, we suggest that effective host immunity requires early development of adaptive immunity.
MATERIALS AND METHODS
Mice.
Female B6 and C3H/HeJ mice that were 6 weeks old were purchased from Jackson Laboratories (Bar Harbor, Maine). B6-SJL-PTprc{a}BoCrTac-Rag2tm1 N10 (B6 RAG−/−), C3H/HeNTac-Rag2tm1 N12 (C3H/HeN RAG−/−), and control C57BL/6Ntac and C3H/HeNTac-MTV (C3H/HeN) mice were originally purchased from Taconic (Germantown, N.Y.) and bred by us. Splenectomized and sham-splenectomized B6 mice were purchased from Taconic. For each experiment, experimental groups of mice were matched for both age (within 1 to 2 weeks) and gender. Mice were housed in a biosafety level 3 facility under specific-pathogen-free conditions at the Animal Biohazard Containment Suite (Dana Farber Cancer Institute, Boston, Mass.) and were used in a protocol approved by the institution.
Bacteria and aerosol infections.
Virulent M. tuberculosis (Erdman strain) was originally obtained from Barry Bloom (Harvard School of Public Health, Boston, Mass.). The bacteria were passaged through mice, grown once in Middlebrook 7H9 medium supplemented with oleic acid-albumin-dextrose complex (Difco, Detroit, Mich.), and stored at −80°C as described previously (7). Mice were inoculated by the aerosol route by using a nose-only exposure unit (Intox Products, Albuquerque, N.M.). For each experiment, an aliquot was thawed, sonicated, and diluted to a concentration of approximately 4 × 106 CFU per ml in 0.9% NaCl-0.02% Tween 80. Preliminary experiments established that this concentration of M. tuberculosis allowed delivery of 350 ± 200 cells per mouse (mean ± standard deviation), an inoculum which we chose based on its ability to cause progressive lung pathology (28). A 10-ml suspension of M. tuberculosis was loaded into a nebulizer (MiniHEART nebulizer; VORTRAN Medical Technologies, Sacramento, Calif.), and animals were exposed to the bacterial aerosol for 20 min. The titer of the M. tuberculosis suspension was confirmed by plating serial dilutions onto 7H10 agar plates (Remel, Lenexa, Kans.).
CFU determination.
The left lung, the spleen (in some experiments, one-half of the spleen), the left liver lobe, and the PLN were aseptically removed from euthanized animals. Blood was purged from the lungs by perfusing RPMI 1640 through the right ventricle of the heart after the inferior vena cava was severed. The tissue was homogenized in 0.9% NaCl-0.02% Tween 80 with a Mini-Bead Beater-8 (BioSpec Products, Bartlesville, Okla.). Viable mycobacteria were quantitated by plating 10-fold serial dilutions of organ homogenates onto 7H11 Mitchison agar plates (Remel). Colonies were counted after 2 to 3 weeks of incubation at 37°C.
Preparation of cells.
Spleens and LN were dissociated by grinding the tissue between sterile frosted-glass slides. Red blood cells were lysed by treating the cells for 5 min with lysis buffer (0.15 M NaCl, 1 mM KHCO3, 0.1 mM Na EDTA; pH 7.3). Lung tissue removed as described above was minced and digested for 1.5 h at 37°C with 150 U of collagenase type IV (Sigma, St. Louis, Mo.) per ml. The tissue was then pressed through a 60-mesh cell sieve (Sigma) and then a 10-μm-pore-size nylon Falcon cell strainer (Fisher, Houston, Tex.) to remove debris. Red blood cells were lysed as described above. All cells were resuspended in complete medium (RPMI, 10% fetal calf serum, 2% HEPES, 10% l-glutamine, 10% penicillin-streptomycin, 10% essential amino acids, 10% nonessential amino acids, 10% sodium pyruvate, 0.1% β-mercaptoethanol, 0.2% NaOH) for use in in vitro restimulation assays (7).
In vitro restimulation assays.
Splenocytes, lung cells, or LN cells (2.5 × 106 cells/ml) in a volume of 2 ml (splenocytes and lung mononuclear cells) or 0.2 ml (PLN cells) were incubated in complete medium containing 1 μg of concanavalin A per ml, in M. tuberculosis H37Ra sonicate (3) (diluted 1:1,000, 1:5,000, or 1:25,000 in complete medium), or in medium alone for 48 h at 37°C. Culture supernatants were assayed for cytokines by an enzyme-linked immunosorbent assay by using antibody pairs and cytokines from Pharmingen (San Diego, Calif.) (2). At the antigen concentrations used, a clear dose response was observed for both B6 and C3H mononuclear cells. For clarity, only the gamma interferon (IFN-γ) production in response to stimulation with the middle antigen concentration (1:5,000) is presented below. The antigen-dependent IFN-γ production observed in this assay was blocked by antibodies specific for class II major histocompatibility complex or CD4, and no IFN-γ was produced when splenocytes from infected RAG−/− (recombinase-activating gene knockout) mice were used. These observations suggest that cytokine production by CD4+ T cells in response to class II major histocompatibility complex-presented antigens is measured by this assay. In the absence of antigen, no IFN-γ was detected, and all cell populations produced IFN-γ in response to concanavalin A at all time points tested (data not shown).
Histological analysis.
Blood was purged and the lungs were fixed by perfusion with Z-fix (Anatech Ltd., Battle Creek, Mich.) via the right heart ventricle, followed by injection of Z-fix into the lungs via the trachea. Paraffin-embedded 5-μm-thick sections were stained with hematoxylin and eosin. Lung sections stained with hematoxylin and eosin were scanned with a Polaroid SprintScan (Polaroid Corporation, Boston, Mass.), and morphometric analysis was carried out by using the NIH Image software (Scion Inc., Frederick, Md.). Random lung sections from each mouse in a group were analyzed by using a threshold lesion size of 20 pixels2. The percentage of infiltrated lung tissue was calculated for each lung section, and groups of mice were compared by using an unpaired t test.
Statistics.
Two-way analysis of variance (ANOVA) with Bonferroni post tests was used to compare the bacterial burdens in the organs of B6 and C3H mice after log transformation of the individual CFU values. For samples that contained no colonies, the values were transformed by using y = log(y + 1). In some experiments, an unpaired t test was used. Statistical analysis of B6, B6 RAG−/−, C3H, and C3H/HeN RAG−/− mouse strains was carried out by using a one-way ANOVA with a Tukey post test for multiple comparisons. All analyses were done by using the Prism software program (GraphPad, San Diego, Calif.).
RESULTS
Dissemination of M. tuberculosis is modulated by the host genetic background.
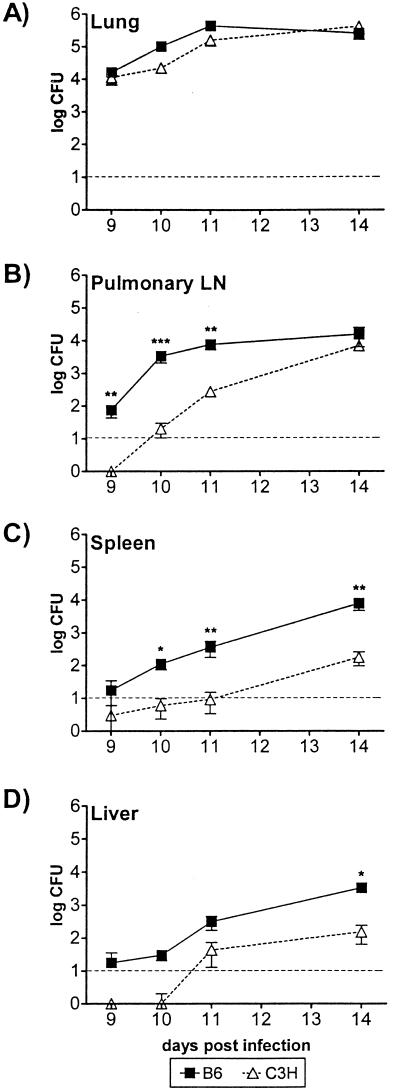
To determine how host genetic background influences dissemination of M. tuberculosis from the lung, B6 and C3H/HeJ mice were infected aerogenically with approximately 350 CFU of virulent M. tuberculosis by using a nose-only aerosol exposure system. Under these conditions, the C3H/HeJ mice succumbed to the infection early after aerosol infection (median survival time, 65 ± 36 days [mean ± standard deviation; six experiments]), while B6 mice survived more than 220 days (three experiments). At various times after infection, four to six mice per strain were sacrificed, and the viable bacteria in different organs were quantitated. The bacterial burden in the lungs of C3H/HeJ mice ultimately surpassed that of B6 mice (data not shown). However, during the first 3 weeks following infection, the numbers of CFU in the lungs recovered from both strains of mice were nearly identical in all experiments performed, indicating that bacterial replication was similar for the two strains in the early phase of infection (Fig. 1A and see Fig. 6C; also data not shown). Extrapulmonary bacteria appeared in the draining PLN by day 9 postinfection in B6 mice and by day 11 in the majority of C3H/HeJ mice (Fig. 1B). Thus, viable M. tuberculosis emigrated from the lungs earlier in resistant animals. Dissemination to the local PLN was followed in 1 to 3 days by systemic dissemination to other organs. Again, this dissemination occurred earlier in B6 mice than in C3H/HeJ mice (Fig. 1C and D). For example, M. tuberculosis was detected by day 10 in the spleens of B6 mice, but it was not detected until day 14 in the spleens of C3H/HeJ mice (Fig. 1C and D). Fourteen days following infection, 10- to 100-fold more bacteria were typically found in the spleens of B6 mice than in the spleens of C3H/HeJ mice. M. tuberculosis was not detected in the axillary LN, which do not drain the lungs, until systemic dissemination had occurred (data not shown). These observations indicate that bacteria disseminate via the lymphatics to the draining PLN and then spread hematogenously to other solid organs, such as the spleen and the liver. Dissemination, both local and systemic, occurred earlier in B6 mice, suggesting that there is a delay in emigration of bacteria from the lungs in C3H/HeJ mice.
FIG. 1.
Dissemination of M. tuberculosis following aerosol inoculation. B6 and C3H/HeJ mice were sacrificed at various times after infection, and the numbers of M. tuberculosis CFU in the lungs (A), PLN (B), spleens (C), and livers (D) were determined. The symbols indicate means, and the error bars indicate the standard deviations based on four to six mice per time point per group. The dashed line indicates the limit of detection of M. tuberculosis (10 CFU). A two-way ANOVA with Bonferroni post tests was used to test for statistically significant differences between the groups of mice, and the P values are indicated as follows: one asterisk, P < 0.05; two asterisks, P < 0.01; and three asterisks, P < 0.001. The data are representative of the data obtained in three to five separate experiments.
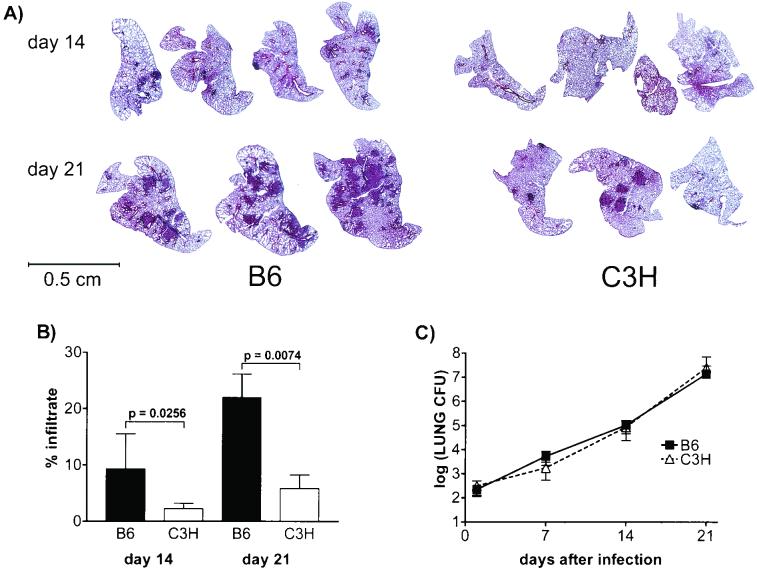
FIG. 6.
Gross pathology of the lung and image analysis. (A) Lung sections from B6 and C3H/HeJ mice stained with hematoxylin and eosin. Three or four representative lung sections per time point are shown. (B) Percentages of infiltrated lung determined as described in Materials and Methods. Six mice were analyzed per strain per time point, and the data for the B6 and C3H/HeJ mouse strains were compared by using an unpaired t test. (C) M. tuberculosis CFU from the left lung of each mouse in panel A were quantitated. Two-way ANOVA with Bonferroni post tests did not reveal a statistically significant difference in the number of lung CFU between the two strains.
Dissemination is independent of B and T lymphocytes but is dependent on host factors.
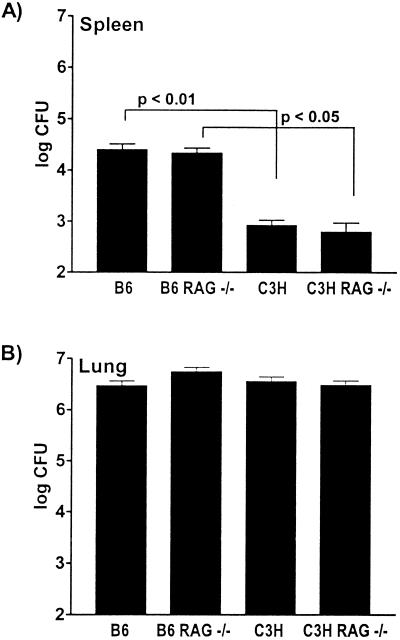
M. tuberculosis is an intracellular pathogen that infects macrophages, and under certain conditions it is able to survive by evading detection by the immune system. We entertained the hypothesis that the killing of infected macrophages by T cells could induce extracellular release of bacteria and lead to bacterial dissemination. Furthermore, the formation of inflammatory lesions would provide the bacteria with access to lymphatics and blood vessels. Alternatively, the adaptive immune response may play no role in inducing dissemination of bacteria. To test whether dissemination of M. tuberculosis from the lung was T and B cell independent, we inoculated B6, C3H/HeN, B6 RAG−/−, and C3H/HeN RAG−/− mice by the aerosol route and measured the bacterial load in the spleen 14 days postinfection. We found that B6 and B6 RAG−/− mice had similar numbers of CFU in their spleens (Fig. 2A). C3H/HeN and C3H/HeN RAG−/− mice also had comparable numbers of CFU in their spleens. As usual, all mice with the C3H/HeN background had reduced numbers of CFU in their spleens compared with the numbers in animals with the B6 background. Similar results were obtained for the liver (data not shown). All mice had similar numbers of CFU in their lungs at this time point (Fig. 2B). Therefore, the timing of dissemination of bacteria from the lungs is determined by innate host factors and occurs independent of B and T cells.
FIG. 2.
Dissemination is similar in RAG+/+ and RAG−/− mice. B6, B6 RAG−/−, C3H/HeN, and C3H/HeN RAG−/− mice were sacrificed at 14 days postinfection. The numbers of M. tuberculosis CFU in the spleens (A) and lungs (B) were determined. The bars and error bars indicate the means and standard deviations, respectively, based on six mice per group. The number of lung CFU did not vary significantly among the four different strains of mice. The number of splenic CFU did not differ significantly between the B6 and B6 RAG−/− mice or between the C3H/HeN and C3H/HeN RAG−/− mice. Significant differences were detected between B6 and C3H/HeN mice and between B6 RAG−/− and C3H/HeN RAG−/− mice, as indicated. All analyses were carried out by using the one-way ANOVA with the Tukey posttest for multiple comparisons. The data are representative of the data obtained in four experiments.
Dissemination precedes the acquired immune response.
The surprising finding that M. tuberculosis disseminates from the lungs of B6 mice earlier than from the lungs of C3H mice was difficult to reconcile with the superior resistance of B6 mice, unless dissemination has a previously unrecognized benefit to the host. We hypothesized that early bacterial dissemination may be beneficial if it leads to more rapid initiation of the adaptive immune response. To test this hypothesis, we studied the relationship between the dissemination of M. tuberculosis to the spleen and the development of a splenic immune response. At various times following infection, spleens were removed and divided in half. One half was used to determine the bacterial load, and the other half was used to prepare splenocytes for an in vitro restimulation assay. It was not possible to carry out these assays in parallel for the PLN because of their small size.
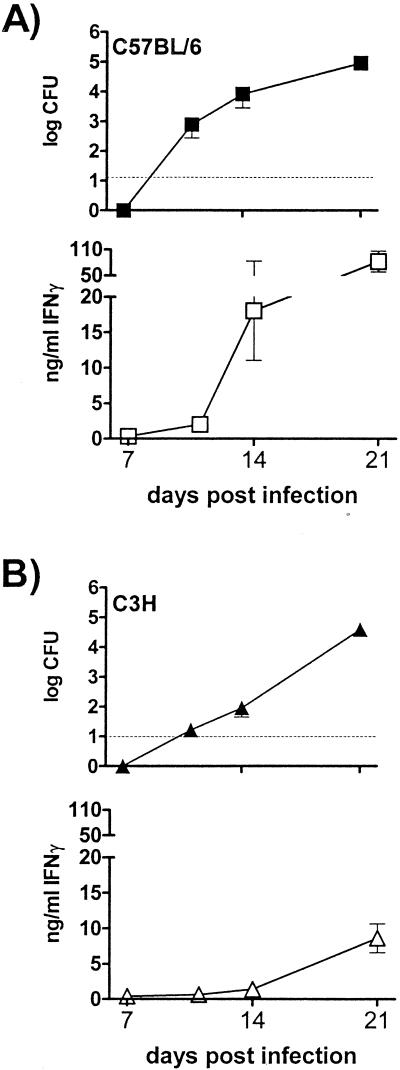
We found that a splenic immune response was never detected in the absence of splenic CFU in more than 250 mice tested for both B6 and C3H mice (data not shown). M. tuberculosis began to appear in the spleens of B6 mice 11 days postinfection; however, an antigen-specific immune response by B6 splenocytes was not detected until 14 days postinfection (Fig. 3A). In contrast, dissemination to the spleens of C3H/HeJ mice did not occur until day 14, and a splenic antigen-specific immune response was not detected until 19 to 21 days postinfection (Fig. 3B). Interestingly, B6 splenocytes produced 10-fold more IFN-γ than C3H/HeJ splenocytes at 21 days postinfection. B6 splenocytes also made more interleukin-12 (IL-12) and tumor necrosis factor alpha than C3H/HeJ splenocytes (data not shown). Splenocytes from the two strains made similar amounts of IL-10 and did not produce IL-2 or IL-4 (data not shown). Thus, the early bacterial dissemination that was observed in B6 mice correlated with the early initiation of systemic antimycobacterial immunity, while the delay in dissemination in C3H mice was coupled with delayed activation of antigen-specific T cells.
FIG. 3.
Dissemination of M. tuberculosis precedes development of an acquired immune response. Spleens were removed from B6 mice (A) and C3H/HeJ mice (B) at 11, 14, and 21 days after infection. One half of each spleen was used to determine the number of bacterial CFU (solid symbols), and the other half was used in in vitro restimulation assays to determine IFN-γ production in response to M. tuberculosis antigen (open symbols). The dashed line indicates the limit of CFU detection (10 CFU). The amounts of IFN-γ produced after 48 h of stimulation with a 1:5,000 dilution of M. tuberculosis sonicate are shown. The error bars indicate the standard deviations based on six mice per group.
The adaptive immune response is initiated in the PLN.
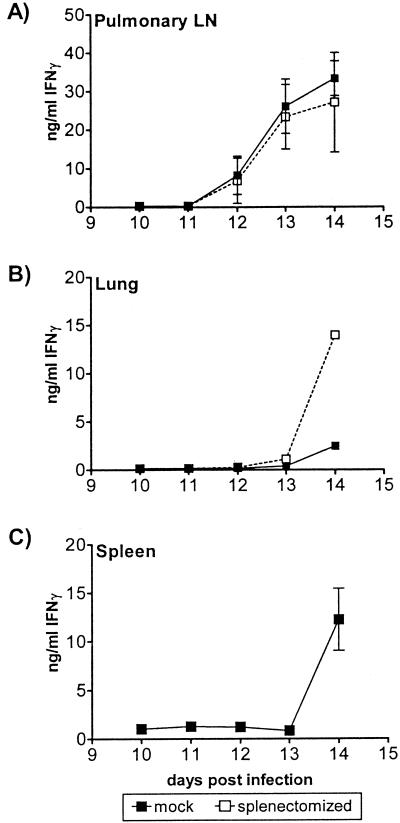
Although development of the splenic immune response is a reasonable surrogate for systemic immunity, T-cell immunity is thought to be initiated in the draining LN. Since mycobacteria first disseminate to the PLN, we sought to establish whether generation of the immune response follows the same pattern in the PLN as it does in the spleen. A recall response to M. tuberculosis antigen was first detected in the B6 PLN beginning on day 12, 3 to 4 days after the appearance of bacteria in these nodes (Fig. 4A). In contrast, the immune response in the spleen and the lung was not detected until day 14 (Fig. 4B and C). Interestingly, the antigen-induced IFN-γ responses of both B6 lung mononuclear cells and splenocytes occurred simultaneously. No antigen-specific T cells were detected in the peripheral axillary LN (data not shown). These findings strongly suggest that initiation of the immune response occurs in the draining PLN.
FIG. 4.
Recall response to M. tuberculosis antigen by splenectomized or mock-splenectomized mice. At various times after infection, cell suspensions were prepared from the PLN (A), lungs (B), or spleens (C) of B6 mice which had been splenectomized or mock splenectomized prior to inoculation. The amounts of IFN-γ produced after 48 h of stimulation with a 1:5,000 dilution of M. tuberculosis sonicate are shown. The error bars indicate the standard deviations based on three to six mice per group. The spleens and LN were assayed individually. The lung mononuclear cells were pooled; thus, there are no error bars in panel B. The data are representative of the data obtained in two experiments.
We examined the role of the spleen in generating an antigen-specific immune response to M. tuberculosis. Splenectomized and mock-splenectomized B6 mice were infected, and at various times the recall responses of lung, PLN, and spleen mononuclear cells (in mice that had spleens) to M. tuberculosis antigen were measured. The kinetics of the IFN-γ response in the PLN were similar in splenectomized and intact mice, and the responses were detected by day 12, 2 days earlier than the responses in the lungs and spleens were detected (Fig. 4). Interestingly, although the kinetics of antigen-induced IFN-γ production by lung mononuclear cells from splenectomized mice was similar to that in the sham group, the splenectomized group made more IFN-γ in response to M. tuberculosis sonicate (Fig. 4B). This finding is consistent with immune cells from the PLN entering the vascular circulation before homing to the lung. The spleen may trap antigen-specific lymphocytes, possibly due to the presence of antigen or infected cells. In the absence of the spleen, perhaps more antigen-specific T cells are able to home directly to the lung parenchymal tissue.
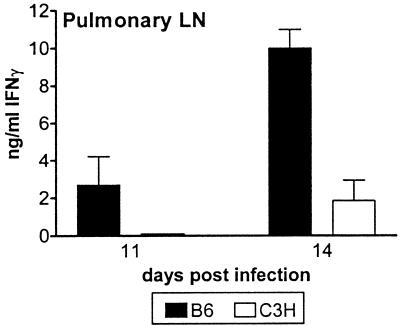
The delay in detection of systemic antimycobacterial immunity observed for C3H mice was most likely secondary to a delay in initiation of the immune response in the PLN. However, other possibilities included a delay in the emigration of T cells from the LN or an intrinsic defect in the initiation of the adaptive immune response. To distinguish these possibilities, the PLN recall responses in B6 and C3H/HeJ mice were compared. PLN cells from B6 mice made IFN-γ in response to exogenously added M. tuberculosis antigen both earlier and to a greater extent than cells from C3H/HeJ PLN (Fig. 5). Thus, as in the spleen, the IFN-γ response in the PLN is delayed in C3H/HeJ mice compared to the IFN-γ response in the PLN of B6 mice, and this delay is likely to be a result of slower dissemination of live M. tuberculosis from the lung to the LN and not an intrinsic defect in initiation of the immune response.
FIG. 5.
An antigen-specific response can be detected earlier in B6 PLN than in C3H/HeJ PLN following infection with M. tuberculosis. PLN cells were prepared from B6 and C3H/HeJ mice 11 and 14 days postinfection and stimulated in vitro with M. tuberculosis antigen. The IFN-γ levels shown are the mean responses of cells to a 1:5,000 dilution of H37Ra sonicate, and the error bars indicate the standard deviations based on four mice per group. The groups of mice were compared to each other by using an unpaired t test, and P = 0.003 at day 14. The data are representative of the data obtained in two experiments.
Dissemination correlates with the appearance of pulmonary lesions.
The cellular mechanisms of dissemination are unknown. Even the basic question of whether the bacteria disseminate in an intracellular or extracellular form remains unanswered. Our data suggest that bacteria initially emigrate from the lungs via the lymphatics, since bacteria were detected in the draining PLN sooner than in other organs. Lung tissue was examined at various times after aerosol inoculation to ascertain pathological correlates of dissemination. No lesions were identified by light microscopy in the lungs of B6 and C3H/HeJ mice 7 days following infection, and at this time little or no dissemination was observed (Fig. 1; also data not shown). By day 14, when B6 lung cells were just beginning to be able to make antigen-induced IFN-γ, the lungs in both strains of mice looked relatively healthy, with few lesions (Fig. 6A). The pulmonary lesions that were present contained predominantly macrophages and neutrophils and few lymphocytes (data not shown). Morphometric analysis revealed that the lung lesions in B6 mice were larger than those in C3H/HeJ mice (Fig. 6B). Interestingly, the pulmonary lesions in the lungs of infected congenic RAG−/− mice at this time looked quite similar to those in the lungs of RAG+/+ mice (data not shown). The difference in lesion size was more apparent by day 21 (Fig. 6A). The pathological differences reflect variation between the B6 and C3H/HeJ host responses since the numbers of mycobacteria present in the lungs were virtually identical in B6 and C3H/HeJ mice at these times (Fig. 1A and 6C). Ultimately, the lesions in B6 mice evolved into granulomas containing lymphocytic infiltrates, whereas the premorbid lesions in C3H/HeJ mice consisted of areas of granulomatous inflammation punctuated by necrosis (data not shown). Although we cannot discriminate whether the formation of inflammatory lesions leads to dissemination or is a consequence of dissemination, the two processes are temporally linked and are a dramatic example of how allelic differences influence the host response to pathogens.
DISCUSSION
In human tuberculosis, little is known about dissemination of M. tuberculosis from the lung or the initiation of antimycobacterial immunity following infection. It is likely that M. tuberculosis disseminates from the lung parenchyma to the draining LN via the lymphatics based on the observation that the Gohn complex, a calcified granuloma in the lung periphery that is associated with similar lesions in the hilar LN, occurs in both symptomatic and asymptomatic people. However, the Gohn complex is a pathological finding, and when it develops during the course of infection is uncertain. To better understand the early events following M. tuberculosis infection in humans, we used a mouse model of M. tuberculosis infection to study bacterial dissemination and its role in the development of immunity in resistant B6 and susceptible C3H mice following experimental inoculation with virulent M. tuberculosis by the aerosol route. Two counterintuitive observations were made. First, bacteria disseminate from the lungs earlier in the resistant B6 mice than in the susceptible C3H mice. Second, pulmonary lesions develop more rapidly in B6 mice than in C3H mice. We were surprised that early dissemination and lesion formation are associated with resistance instead of progression of disease since at later stages of infection we have observed more bacteria and more tissue damage in the lungs of the susceptible mice. However, our study of the kinetics of antituberculous immunity following infection reconciled this discrepancy. We found that initiation of T-cell-mediated immunity occurs only after dissemination of live M. tuberculosis to the pulmonary LN has taken place. The earlier initiation of the adaptive immune response to M. tuberculosis may ultimately enable B6 mice to better contain the spread of bacteria within the lungs and other organs.
Although such results have not been previously obtained for mice, similar observations were made in the 1950s and 1960s by Lurie, who used rabbit strains bred for susceptibility or resistance to tuberculosis (13). Lurie noted that dissemination from the lungs to the draining LN was greater in resistant rabbits than in sensitive rabbits. The resistant rabbits also had earlier “allergic sensitization and antibody formation” and developed earlier pulmonary inflammatory lesions than the susceptible rabbits (13). Lurie attributed these findings to retention of more bacteria within the lungs of resistant rabbits upon infection and believed that resistant rabbits had a greater innate phagocytic capacity than susceptible rabbits. We do not believe that this is the case in our system because B6 and C3H mice have identical bacterial loads in the lungs at the time that dissemination takes place.
It is not known how and in what context M. tuberculosis moves from the lung to the local LN. Since lymphatics are absent from the alveolar walls, we thought that the appearance of inflammatory lung lesions would correlate with dissemination of bacteria from the lung. However, no lesions were observed at day 7, and few lesions were observed at day 14, although bacteria were detected as early as day 9 in B6 PLN. These observations argue that dissemination is not a consequence of tissue damage. Presumably, infected macrophages or dendritic cells could carry viable bacteria via the lymphatics to the draining LN, although M. tuberculosis has been reported to cross the bronchial epithelium via M cells under certain circumstances (31). The mycobacterial protein heparin binding hemagglutinin A (HbhA), which mediates the attachment of M. tuberculosis to epithelial cells and macrophages, is important in facilitating dissemination to the spleen (18, 22, 24). The absence of B cells has been shown to delay the dissemination of CDC1551 (a clinical isolate of M. tuberculosis) to the spleen and the liver in experiments performed with mice that lack mature B cells (BKO), although it has been reported by other workers using strain Erdman that the ultimate outcome of infection is not altered in these mice (5, 32). As in our studies, the delay in dissemination in BKO mice was coupled with a delay in the development of pulmonary lesions. Interestingly, our observations with the RAG−/− mice, which do not have B or T cells, differ, as we saw no effect of the RAG gene on early dissemination or granuloma formation in both B6 and C3H mice. Thus, we argue that components of the acquired immune response, such as mature B and T cells, do not play a role in early dissemination. Although it is difficult to reconcile our results with the results of the BKO mouse study, there may be differences in mice that have both B and T cells missing rather than mature B cells alone. In addition, different strains of M. tuberculosis were used in the two studies, and this may have affected the outcome. The observed association of the timing of dissemination with pulmonary inflammation, however, is in agreement with our findings.
Resistance to tuberculosis is multifactorial and is controlled by both environmental and genetic factors, many of which ultimately affect the efficiency of the host immune response to infection. Allelic differences between B6 and C3H mice have been identified in the nramp1 gene and the supersusceptibility 1 (sst1) locus. Both are important in modulating susceptibility to mycobacterial infections; however, the mechanisms are unknown (10, 36). Our preliminary data suggest that neither the sst1 locus nor the nramp1 gene controls dissemination (unpublished observations). Our results with RAG−/− mice suggest that control of dissemination lies in the interaction of M. tuberculosis with innate components of the immune system. The toll-like receptors are a family of proteins that have a role in innate immunity (26, 34). Although the C3H/HeJ substrain has a defective tlr4 gene, we did not find that this gene affected the survival of C3H mice in an intravenous inoculation model of tuberculosis (7). Similarly, the tlr4 gene does not appear to affect dissemination (compare Fig. 1 and 2, which show results obtained with C3H/HeJ and C3H/HeN mice, respectively). Thus, although the specific genes remain to be identified, it is evident that the host genetic background modulates dissemination, possibly as a consequence of differences in cell trafficking, infectibility of host cells, host cell control of bacteria, or host cell survival.
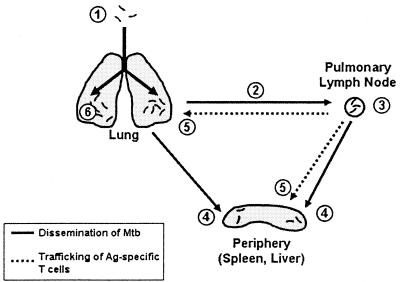
Our data suggest how dissemination, initiation of T-cell-mediated immunity, and trafficking of immune lymphocytes are interrelated (Fig. 7). Following aerosol inoculation and initial infection of the lung (1), M. tuberculosis disseminates via lymphatic drainage to the local PLN by day 9 in B6 mice and by day 11 in C3H mice (2). Two to three days after dissemination to the PLN, antigen-specific T cells can be detected there (3). During this time, M. tuberculosis can be detected in the spleen and the liver by day 10 in B6 mice and by day 14 in C3H mice (4). Whether the bacteria that are hematogenously spread originate from the lung or the LN is unknown. It is possible that increased vascular permeability and tissue damage within pulmonary inflammatory lesions allow M. tuberculosis or M. tuberculosis-infected cells to penetrate blood vessels and enter the systemic circulation. Alternatively, M. tuberculosis could enter the circulatory system either through the efferent lymphatics or through venous drainage of the PLN. By day 14 in B6 mice and by day 19 in C3H mice, antigen-specific T cells are detected in the spleen and the lung (5). It is possible that these T cells are activated in the PLN and traffic to the lungs and the spleen. In B6 mice, small pulmonary inflammatory lesions start to develop around this time, and by day 21 they are quite pronounced (6).
FIG. 7.
Model of extrapulmonary dissemination and initiation of T-cell immunity. Mtb, M. tuberculosis; Ag, antigen.
Although the timing of this process is delayed in C3H mice compared to B6 mice, dissemination always precedes the development of the T-cell response in both strains of mice. Thus, we believe that dissemination of M. tuberculosis from the lung is essential in the generation of T-cell-mediated immunity. Although intravenous infection does not convert C3H mice to a resistant phenotype, there is evidence from other studies that an earlier immune response reduces the severity of disease. When the same number of bacteria is seeded into the lung, infection by the aerosol route is more lethal than intravenous infection (23). This effect has been attributed to the more rapid induction of protective immunity in the intravenously infected animals (6). In the context of these two studies, our observations suggest that it is the delay in dissemination following aerosol inoculation that leads to the observed difference in mortality between intravenously and aerogenically infected animals.
We do not yet know whether the earlier dissemination and initiation of immunity observed in B6 mice contributes to their superior resistance. However, the comparison of B6 and C3H mice illustrates how host genes can affect dissemination and, in turn, how dissemination strongly correlates with the development of the immune response. Based on these results, we hypothesize that early bacterial dissemination may enhance host resistance, as it leads to earlier induction of the adaptive immune response.
Acknowledgments
This work was supported by National Institutes of Health grant HL64540 and by an award from the Potts Memorial Foundation to S.M.B. A.A.C. was supported by grant T32 AI 07306.
We thank Steve Jean, Linda Callahan, and the staff of the Animal Biohazard Containment Suite at the Dana Farber Cancer Institute for their help in facilitating the experiments.
Editor: R. N. Moore
REFERENCES
- 1.Abel, L., and J. L. Casanova. 2000. Genetic predisposition to clinical tuberculosis: bridging the gap between simple and complex inheritance. Am. J. Hum. Genet. 67:274-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behar, S. M., T. A. Podrebarac, C. J. Roy, C. R.Wang, and M. B. Brenner. 1999. Diverse TCRs recognize murine CD1. J. Immunol. 162:161-167. [PubMed] [Google Scholar]
- 3.Behar, S. M., S. A. Porcelli, E. M. Beckman, and M. B. Brenner. 1995. A pathway of costimulation that prevents anergy in CD28- T cells: B7-independent costimulation of CD1-restricted T cells. J. Exp. Med. 182:2007-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belkaid, Y., S. Mendez, R. Lira, N. Kadambi, G. Milon, and D. Sacks. 2000. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165:969-977. [DOI] [PubMed] [Google Scholar]
- 5.Bosio, C. M., D. Gardner, and K. L. Elkins. 2000. Infection of B cell-deficient mice with CDC 1551, a clinical isolate of Mycobacterium tuberculosis: delay in dissemination and development of lung pathology. J. Immunol. 164:6417-6425. [DOI] [PubMed] [Google Scholar]
- 6.Cardona, P. J., A. Cooper, M. Luquin, A. Ariza, F. Filipo, I. M Orme, and V. Ausina. 1999. The intravenous model of murine tuberculosis is less pathogenic than the aerogenic model owing to a more rapid induction of systemic immunity. Scand. J. Immunol. 49:362-366. [DOI] [PubMed] [Google Scholar]
- 7.Chackerian, A. A., T. V. Perera, and S. M. Behar. 2001. Gamma interferon-producing CD4+ T lymphocytes in the lung correlate with resistance to infection with Mycobacterium tuberculosis. Infect. Immun. 69:2666-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill, A. V. 1998. The immunogenetics of human infectious diseases. Annu. Rev. Immunol. 16:593-617. [DOI] [PubMed] [Google Scholar]
- 9.Kitajima, T., K. Ariizumi, P. R. Bergstresser, and A. Takashima. 1996. A novel mechanism of glucocorticoid-induced immune suppression: the inhibiton of T cell-mediated terminal maturation of a murine dendritic cell line. J. Clin. Investig. 98:142-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kramnik, I., W. F. Dietrich, P. Demant, and B. R. Bloom. 2000. Genetic control of resistance to experimental infection with virulent Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 97:8560-8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin, M., and M. Newport. 2000. Inherited predisposition to mycobacterial infection: historical considerations. Microbes Infect. 2:1549-1552. [DOI] [PubMed] [Google Scholar]
- 12.Lira, R., M. Doherty, G. Modi, and D. Sacks. 2000. Evolution of lesion formation, parasitic load, immune response, and reservoir potential in C57BL/6 mice following high- and low-dose challenge with Leishmania major. Infect. Immun. 68:5176-5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lurie, M. B. 1965. Resistance to tuberculosis; experimental studies in native and acquired defensive mechanisms. Harvard University Press, Boston, Mass.
- 14.Lyadova, I., V. Yeremeev, K. Majorov, B. Nikonenko, S. Khaidukov, T. Kondratieva, N. Kobets, and A. Apt. 1998. An ex vivo study of T lymphocytes recovered from the lungs of I/St mice infected with and susceptible to Mycobacterium tuberculosis. Infect. Immun. 66:4981-4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyadova, I. V., E. B. Eruslanov, S. V. Khaidukov, V. V. Yeremeev, K. B. Majorov, A. V. Pichugin, B. V. Nikonenko, T. K. Kondratieva, and A. S. Apt. 2000. Comparative analysis of T lymphocytes recovered from the lungs of mice genetically susceptible, resistant, and hyperresistant to Mycobacterium tuberculosis-triggered disease. J. Immunol. 165:5921-5931. [DOI] [PubMed] [Google Scholar]
- 16.Lynch, C. J., C. H. Pierce-Chase, and R. Dubos. 1965. A genetic study of susceptibility to experimental tuberculosis in mice infected with mammalian tubercle bacilli. J. Exp. Med. 121:1051-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medina, E., and R. J. North. 1998. Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility complex haplotype and Nramp1 genotype. Immunology 93:270-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menozzi, F. D., J. H. Rouse, M. Alavi, M. Laude-Sharp, J. Muller, R. Bischoff, M. J. Brennan, and C. Locht. 1996. Identification of a heparin-binding hemagglutinin present in mycobacteria. J. Exp. Med. 184:993-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitsos, L. M., L. R. Cardon, A. Fortin, L. Ryan, R. LaCourse, R. J. North, and P. Gros. 2000. Genetic control of susceptibility to infection with Mycobacterium tuberculosis in mice. Genes Immun. 1:467-477. [DOI] [PubMed] [Google Scholar]
- 20.Moll, H., H. Fuchs, C. Blank, and M. Rollinghoff. 1993. Langerhans cells transport Leishmania major from the infected skin to the draining lymph node for presentation to antigen-specific T cells. Eur. J. Immunol. 23:1595-1601. [DOI] [PubMed] [Google Scholar]
- 21.Moore, T. A., B. B. Moore, M. W. Newstead, and T. J. Standiford. 2000. Gamma delta-T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J. Immunol. 165:2643-2650. [DOI] [PubMed] [Google Scholar]
- 22.Mueller-Ortiz, S. L., A. R. Wanger, and S. J. Norris. 2001. Mycobacterial protein HbhA binds human complement component C3. Infect. Immun. 69:7501-7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.North, R. J., R. LaCourse, and L. Ryan. 1999. Vaccinated mice remain more susceptible to Mycobacterium tuberculosis infection initiated via the respiratory route than via the intravenous route. Infect. Immun. 67:2010-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pethe, K., S. Alonso, F. Biet, G. Delogu, M. J. Brennan, C. Locht, and F. D. Menozzi. 2001. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature 412:190-194. [DOI] [PubMed] [Google Scholar]
- 25.Pierce, C., R. Dubos, and G. Middlebrook. 1947. Infection of mice with mammalian tubercle bacilli grown in Tween-albumin liquid medium. J. Exp. Med. 86:159-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 27.Pron, B., C. Boumaila, F. Jaubert, P. Berche, G. Milon, F. Geissmann, and J. L. Gaillard. 2001. Dendritic cells are early cellular targets of Listeria monocytogenes after intestinal delivery and are involved in bacterial spread in the host. Cell Microbiol. 3:331-340. [DOI] [PubMed] [Google Scholar]
- 28.Saunders, B. M., A. A. Frank, A. M. Cooper, and I. M. Orme. 1998. Role of gamma delta T cells in immunopathology of pulmonary Mycobacterium avium infection in mice. Infect. Immun. 66:5508-5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Small, P. M., and P. I. Fujiwara. 2001. Management of tuberculosis in the United States. N. Engl. J. Med. 345:189-200. [DOI] [PubMed] [Google Scholar]
- 30.Sumyuen, M. H., Y. J. Garin, and F. Derouin. 1995. Early kinetics of Toxoplasma gondii infection in mice infected orally with cysts of an avirulent strain. J. Parasitol. 81:327-329. [PubMed] [Google Scholar]
- 31.Teitelbaum, R., W. Schubert, L. Gunther, Y. Kress, F. Macaluso, J. W. Pollard, D. N. McMurray, and B. R. Bloom. 1999. The M cell as a portal of entry to the lung for the bacterial pathogen Mycobacterium tuberculosis. Immunity 10:641-650. [DOI] [PubMed] [Google Scholar]
- 32.Turner, J., A. A. Frank, J. V. Brooks, M. Gonzalez-Juarrero, and I. M. Orme. 2001. The progression of chronic tuberculosis in the mouse does not require the participation of B lymphocytes or interleukin-4. Exp. Gerontol. 36:537-545. [DOI] [PubMed] [Google Scholar]
- 33.Turner, J., M. Gonzalez-Juarrero, B. M. Saunders, J. V. Brooks, P. Marietta, D. L. Ellis, A. A. Frank, A. M. Cooper, and I. M. Orme. 2001. Immunological basis for reactivation of tuberculosis in mice. Infect. Immun. 69:3264-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 35.Vazquez-Torres, A., J. Jones-Carson, A. J. Baumler, S. Falkow, R. Valdivia, W. Brown, M. Le, R. Berggren, W. T. Parks, and F. C. Fang. 1999. Extraintestinal dissemination of salmonella by CD18-expressing phagocytes. Nature 401:804-808. [DOI] [PubMed] [Google Scholar]
- 36.Vidal, S., M. L. Tremblay, G. Govoni, S. Gauthier, G. Sebastiani, D. Malo, E. Skamene, M. Olivier, S. Jothy, and P. Gros. 1995. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J. Exp. Med. 182:655-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wakeham, J., J. Wang, and Z. Xing. 2000. Genetically determined disparate innate and adaptive cell-mediated immune responses to pulmonary Mycobacterium bovis BCG infection in C57BL/6 and BALB/c mice. Infect. Immun. 68:6946-6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu, S., A. Cooper, S. Sturgill-Koszycki, T. van Heyningen, D. Chatterjee, I. Orme, P. Allen, and D. G. Russell. 1994. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J. Immunol. 153:2568-2578. [PubMed] [Google Scholar]
- 39.Yang, Z. P., C. C. Kuo, and J. T. Grayston. 1995. Systemic dissemination of Chlamydia pneumoniae following intranasal inoculation in mice. J. Infect. Dis. 171:736-738. [DOI] [PubMed] [Google Scholar]