Abstract
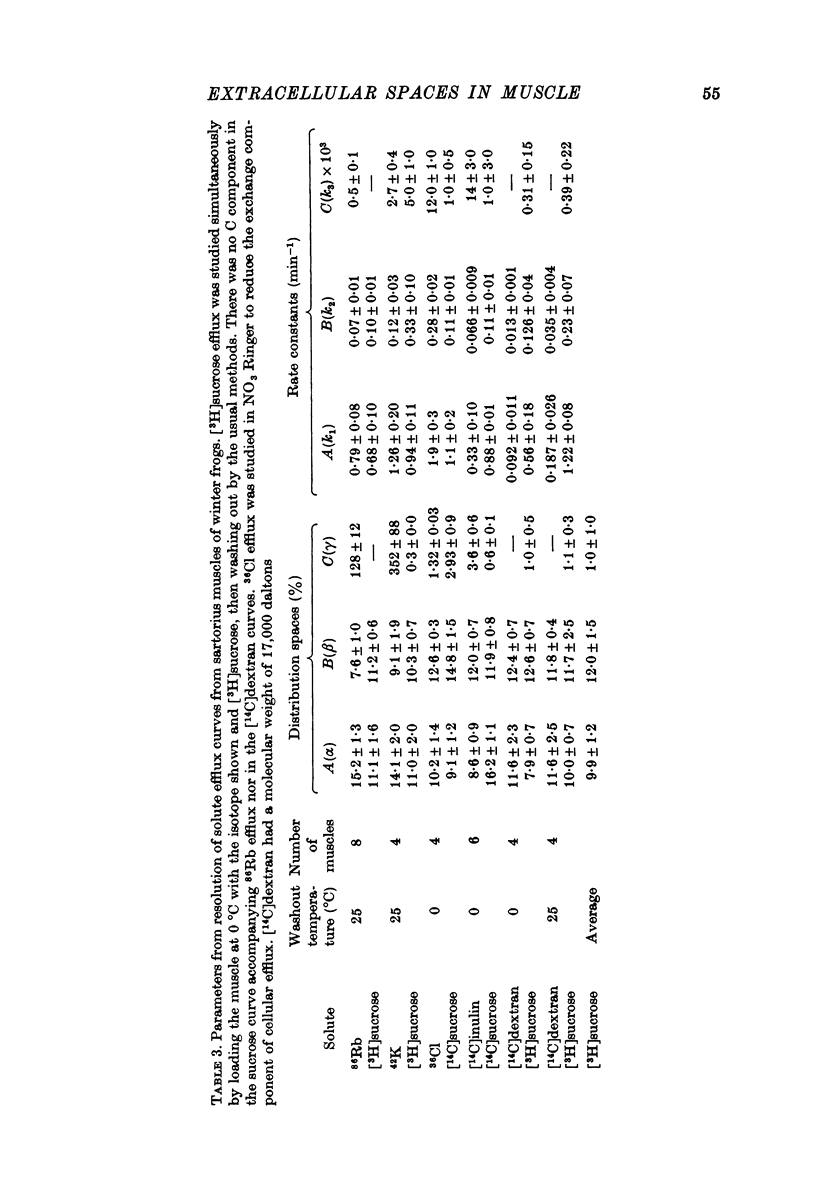
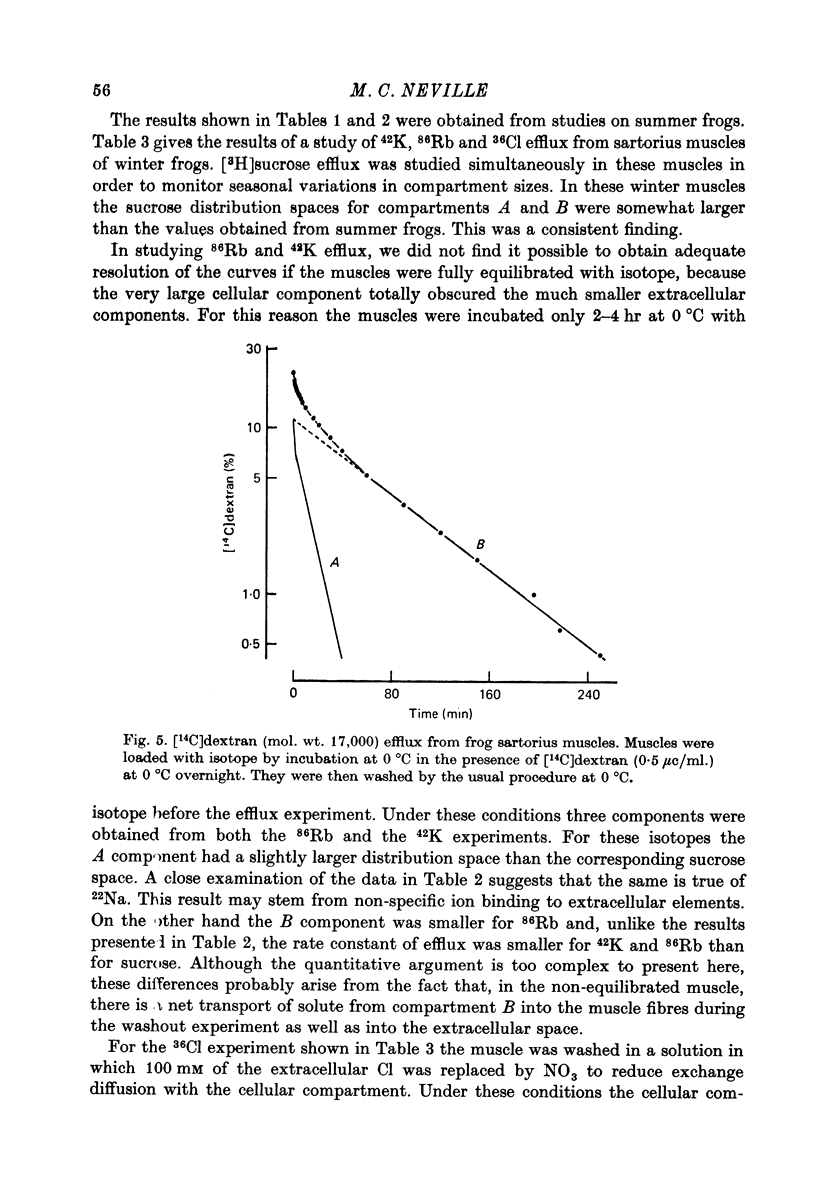
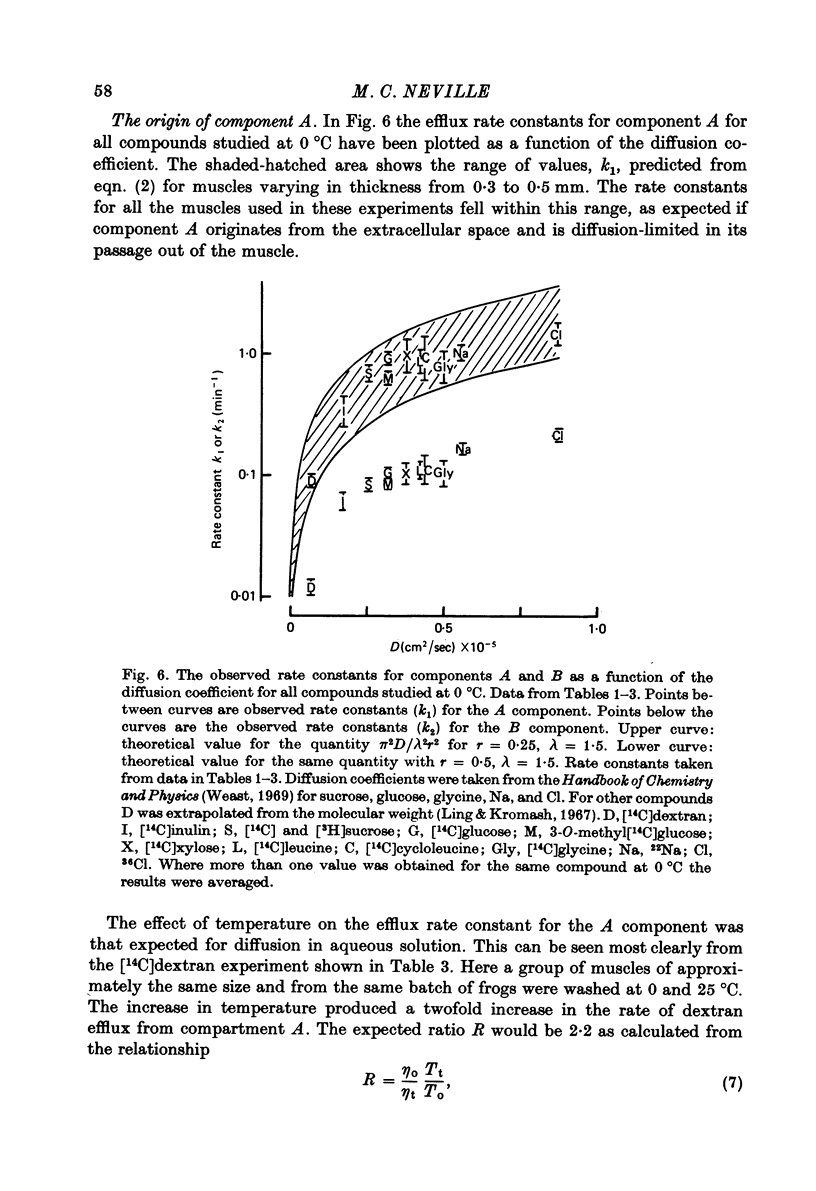
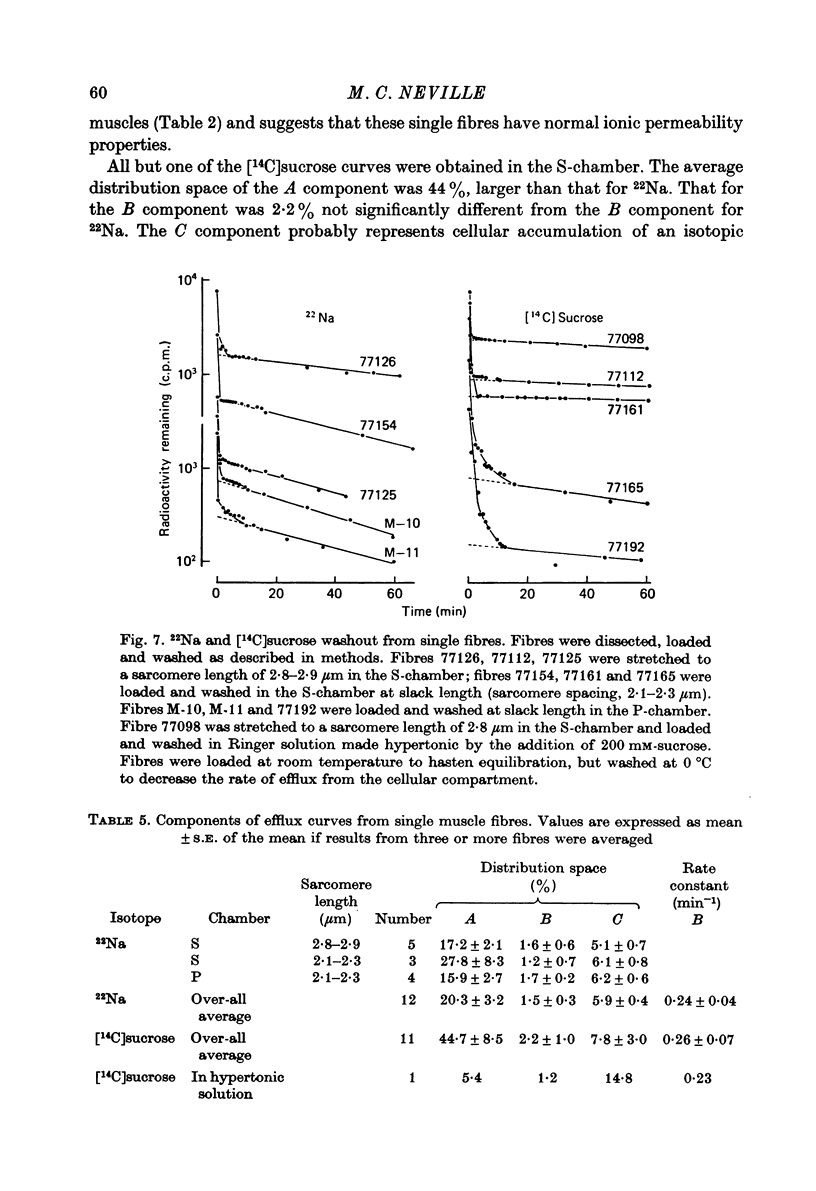
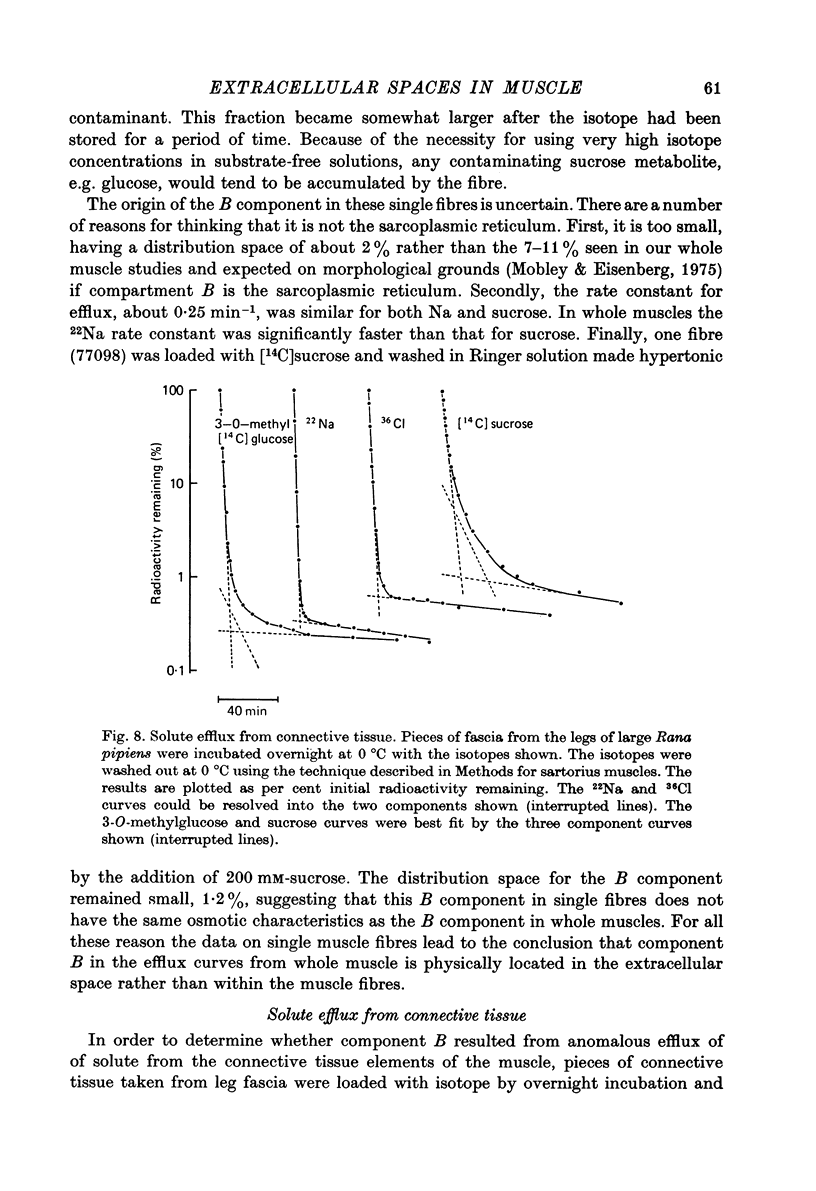
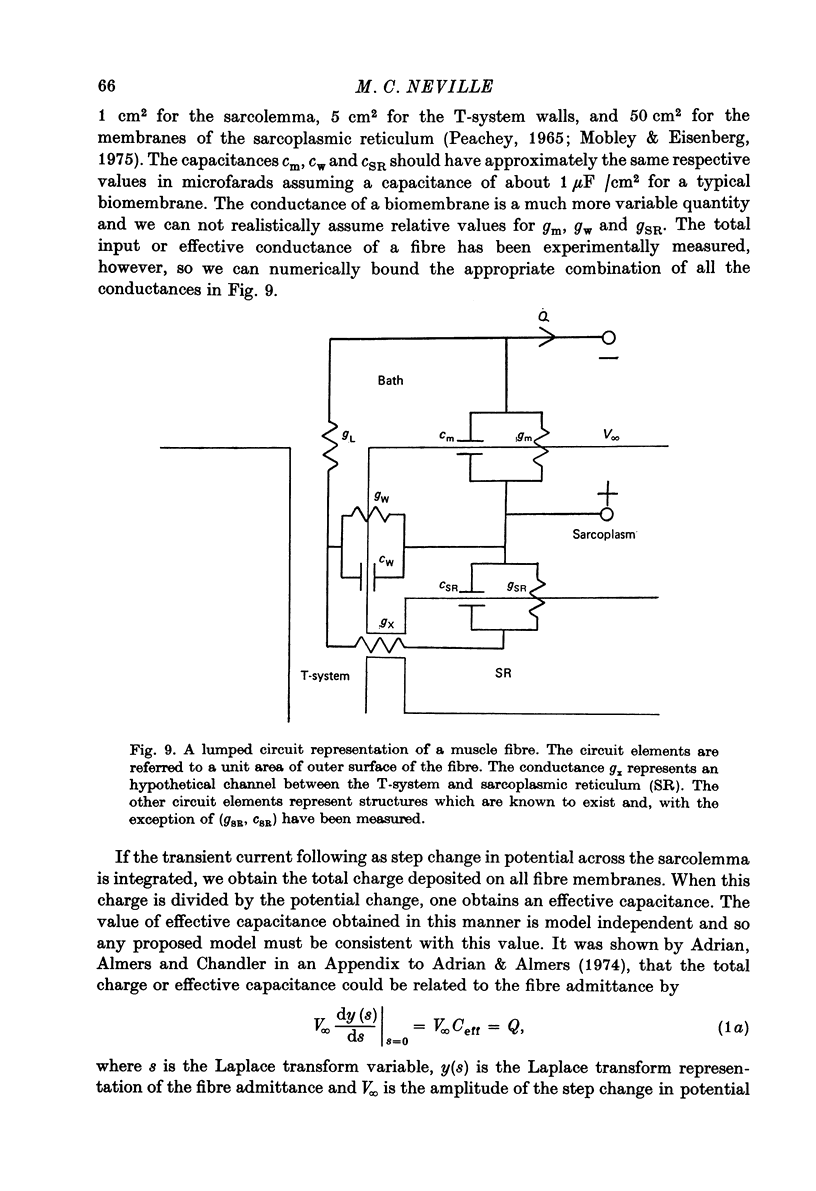
1. Detailed studies of solute efflux from frog sartorius muscle and single muscle fibres were carried out in order to characterize a 'special region' (Harris, 1963) in the extracellular space of muscle and determine whether this 'special region' is the sarcoplasmic reticulum. 2. The efflux of radioactive Na, Cl, glusose, 3-O-methylglucose, xylose, glycine, leucine, cycloleucine, Rb, K, inulin (mol. wt. 5000) and dextran (mol. wt. 17,000) from previously loaded muscles was studied. In all cases except dextran the curve had three components, a rapid (A) component which could be equated with efflux from the extracellular space proper, a slow (C) component representing cellular solute and an intermediate (B) component. The distribution space for the B component was 8% of muscle volume in summer frogs and 12% in winter frogs and appeared to be equal for all compounds studied. We tested the hypothesis that the B component originated from the sarcoplasmic reticulum. 3. The C component was missing from the dextran curves. Both dextran and inulin entered the compartment of origin of the B component (compartment B) to the same extent as small molecules. 4. For all compounds studies, the efflux rate constant for the A component could be predicted from the diffusion coefficient. For the B component the efflux rate constant was 6--10 times slower than that for the A component but was still proportional to the diffusion coefficient for the solute in question. 5. When Na and sucrose efflux from single fibres was followed, a B component was usually observed. The average distribution space for this component was small, averaging 1.5% of fibre volume. There was no difference between the average efflux rate constants for Na and sucrose. 6. In an appendix, the constraints placed on the properties of a hypothetical channel between the sarcoplasmic reticulum and the T-system by the linear electrical parameters of frog skeletal muscle are derived. It is shown that the conductance of such a channel must be less than 0.06 x 10(-3) mohs/cm2 of fibre membrane. 7. The conductance between compartment B and the extracellular space can be calculated from the efflux rate constants for Na, K and Cl. The value obtained was 5 x 10(-3) mhos/cm2 of fibre membrane or 100 times the limiting value for the conductance of the T-SR junction. 8. The finding that there is a B component in the efflux curves for large molecular weight substances like inulin and dextran and the small size of the B component in efflux curves from single muscle fibres indicate that the 'speical region' of the extra-cellular space of frog muscle is not the sarcoplasmic reticulum. This conclusion is confirmed by a calculation of the conductance between the B compartment and the extracellular space. The value obtained is incompatible with predicted electrical properteis of the SR-T-tubule junction...
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. Internal chloride concentration and chloride efflux of frog muscle. J Physiol. 1961 May;156:623–632. doi: 10.1113/jphysiol.1961.sp006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Almers W. Membrane capacity measurements on frog skeletal muscle in media of low ion content. J Physiol. 1974 Mar;237(3):573–605. doi: 10.1113/jphysiol.1974.sp010499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I., Davey D. F. Osmotic responses demonstrating the extracellular character of the sarcoplasmic reticulum. J Physiol. 1969 May;202(1):171–188. doi: 10.1113/jphysiol.1969.sp008802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J., Kane F., O'reilly H. L. Volume of interfibre spaces in frog muscle and the calculation of concentrations in the fibre water. J Physiol. 1941 Jun 30;99(4):401–414. doi: 10.1113/jphysiol.1941.sp003911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R., Creese R., Dixon W. J., Massey F. J., Taylor D. B. Movement of labelled decamethonium in muscle fibres of the rat. J Physiol. 1977 Nov;272(2):283–294. doi: 10.1113/jphysiol.1977.sp012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. Effects of glycerol treatment and maintained depolarization on charge movement in skeletal muscle. J Physiol. 1976 Jan;254(2):285–316. doi: 10.1113/jphysiol.1976.sp011233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L. Contractile activation in skeletal muscle. Prog Biophys Mol Biol. 1975;29(2):197–224. doi: 10.1016/0079-6107(76)90023-7. [DOI] [PubMed] [Google Scholar]
- DESMEDT J. E. Electrical activity and intracellular sodium concentration in frog muscle. J Physiol. 1953 Jul;121(1):191–205. doi: 10.1113/jphysiol.1953.sp004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DYDYNSKA M., WILKIE D. R. THE OSMOTIC PROPERTIES OF STRIATED MUSCLE FIBERS IN HYPERTONIC SOLUTIONS. J Physiol. 1963 Nov;169:312–329. doi: 10.1113/jphysiol.1963.sp007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell R. B., Sciacca R., Lieberman K., Case D. B., Cannon P. J. A weighted least-squares technique for the analysis of kinetic data and its application to the study of renal xenon washout in dogs and man. Circ Res. 1973 Jan;32(1):71–84. doi: 10.1161/01.res.32.1.71. [DOI] [PubMed] [Google Scholar]
- Eisenberg B., Eisenberg R. S. Selective disruption of the sarcotubular system in frog sartorius muscle. A quantitative study with exogenous peroxidase as a marker. J Cell Biol. 1968 Nov;39(2):451–467. doi: 10.1083/jcb.39.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. S., Gage P. W. Ionic conductances of the surface and transverse tubular membranes of frog sartorius fibers. J Gen Physiol. 1969 Mar;53(3):279–297. doi: 10.1085/jgp.53.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Entry of fluorescent dyes into the sarcotubular system of the frog muscle. J Physiol. 1966 Jul;185(1):224–238. doi: 10.1113/jphysiol.1966.sp007983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS E. J. Distribution and movement of muscle chloride. J Physiol. 1963 Apr;166:87–109. doi: 10.1113/jphysiol.1963.sp007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL D. K. THE SPACE ACCESSIBLE TO ALBUMIN WITHIN THE STRIATED MUSCLE FIBRE OF THE TOAD. J Physiol. 1964 Dec;175:275–294. doi: 10.1113/jphysiol.1964.sp007517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Movements of Na and K in single muscle fibres. J Physiol. 1959 Mar 3;145(2):405–432. doi: 10.1113/jphysiol.1959.sp006150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON J. A. Kinetics of release of radioactive sodium, sulfate and sucrose from the frog sartorius muscle. Am J Physiol. 1955 May;181(2):263–268. doi: 10.1152/ajplegacy.1955.181.2.263. [DOI] [PubMed] [Google Scholar]
- Kirby A. C., Lindley B. D., Picken J. R. Calcium content and exchange in frog skeletal muscle. J Physiol. 1975 Dec;253(1):37–52. doi: 10.1113/jphysiol.1975.sp011178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulczycky S., Mainwood G. W. Evidence for a functional connection between the sarcoplasmic reticulum and the extracellular space in frog sartorius muscle. Can J Physiol Pharmacol. 1972 Feb;50(2):87–98. doi: 10.1139/y72-015. [DOI] [PubMed] [Google Scholar]
- Ling G. N. Cell membrane and cell permeability. Ann N Y Acad Sci. 1966 Jul 14;137(2):837–859. doi: 10.1111/j.1749-6632.1966.tb50204.x. [DOI] [PubMed] [Google Scholar]
- Ling G. N., Kromash M. H. The extracellular space of voluntary muscle tissues. J Gen Physiol. 1967 Jan;50(3):677–694. doi: 10.1085/jgp.50.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R. L., Mann J. E., Jr, Sperelakis N. Derivation of general equations describing tracer diffusion in any two-compartment tissue with application to ionic diffusion in cylindrical muscle bundles. J Theor Biol. 1974 May;45(1):107–130. doi: 10.1016/0022-5193(74)90046-0. [DOI] [PubMed] [Google Scholar]
- Mathias R. T., Eisenberg R. S., Valdiosera R. Electrical properties of frog skeletal muscle fibers interpreted with a mesh model of the tubular system. Biophys J. 1977 Jan;17(1):57–93. doi: 10.1016/S0006-3495(77)85627-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley B. A., Eisenberg B. R. Sizes of components in frog skeletal muscle measured by methods of stereology. J Gen Physiol. 1975 Jul;66(1):31–45. doi: 10.1085/jgp.66.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHARA H. T., OZAND P. Studies of tissue permeability. IX. The effect of insulin on the penetration of 3-methylglucose-H3 in frog muscle. J Biol Chem. 1963 Jan;238:40–49. [PubMed] [Google Scholar]
- Neville M. C. Solute concentration gradients in frog muscles at 0 degree C: active transport or adsorption? Science. 1972 Apr 21;176(4032):302–303. doi: 10.1126/science.176.4032.302. [DOI] [PubMed] [Google Scholar]
- Neville M. C., White S. Extracellular space of frog skeletal muscle in vivo and in vitro: relation to proton magnetic resonance relaxation times. J Physiol. 1979 Mar;288:71–83. [PMC free article] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- Revel J. P., Karnovsky M. J. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967 Jun;33(3):C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogus E., Zierler K. L. Sodium and water contents of sarcoplasm and sarcoplasmic reticulum in rat skeletal muscle: effects of anisotonic media, ouabain and external sodium. J Physiol. 1973 Sep;233(2):227–270. doi: 10.1113/jphysiol.1973.sp010307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio R., Sperelakis N. Penetration of horseradish peroxidase into the terminal cisternae of frog skeletal muscle fibers and blockade of caffeine contracture by Ca ++ depletion. Z Zellforsch Mikrosk Anat. 1972;124(1):57–71. [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Effects of membrane potential on the capacitance of skeletal muscle fibers. J Gen Physiol. 1976 Feb;67(2):125–163. doi: 10.1085/jgp.67.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin R. A., Beaugé L. A. An analysis of the leakages of sodium ions into and potassium ions out of striated muscle cells. J Gen Physiol. 1973 Feb;61(2):222–250. doi: 10.1085/jgp.61.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Shuman H., Somlyo A. P. Composition of sarcoplasmic reticulum in situ by electron probe X-ray microanalysis. Nature. 1977 Aug 11;268(5620):556–558. doi: 10.1038/268556a0. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Shuman H., Somlyo A. P. Elemental distribution in striated muscle and the effects of hypertonicity. Electron probe analysis of cryo sections. J Cell Biol. 1977 Sep;74(3):828–857. doi: 10.1083/jcb.74.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperelakis N., Valle R., Orozco C., Martínez-Palomo A., Rubio R. Electromechanical uncoupling of frog skeletal muscle by possible change in sarcoplasmic reticulum content. Am J Physiol. 1973 Oct;225(4):793–800. doi: 10.1152/ajplegacy.1973.225.4.793. [DOI] [PubMed] [Google Scholar]
- TASKER P., SIMON S. E., JOHNSTONE B. M., SHANKLY K. H., SHAW F. H. The dimensions of the extracellular space in sartorius muscle. J Gen Physiol. 1959 Sep;43:39–53. doi: 10.1085/jgp.43.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova N. A., Nikol'skii N. N., Troshin A. S. Raspredelenie sakharov mezhdu portniazhnymi myshtsami liagushki i sredoi. Tsitologiia. 1967 Jun;9(6):658–665. [PubMed] [Google Scholar]
- Vinogradova N. A. Raspredelenie inulina, sakharozy i fruktozy v portniazhnykh myshtsakh liagushki. Tsitologiia. 1967 Jul;9(7):781–790. [PubMed] [Google Scholar]
- Vinogradova N. A. Raspredelenie nepronikaiushchikh sakharov v portniazhnykh myshtsakh liagushki v usloviiakh giopo- i gipertonii. Tsitologiia. 1968 Jul;10(7):831–838. [PubMed] [Google Scholar]
- Winegrad S. The intracellular site of calcium activaton of contraction in frog skeletal muscle. J Gen Physiol. 1970 Jan;55(1):77–88. doi: 10.1085/jgp.55.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]


