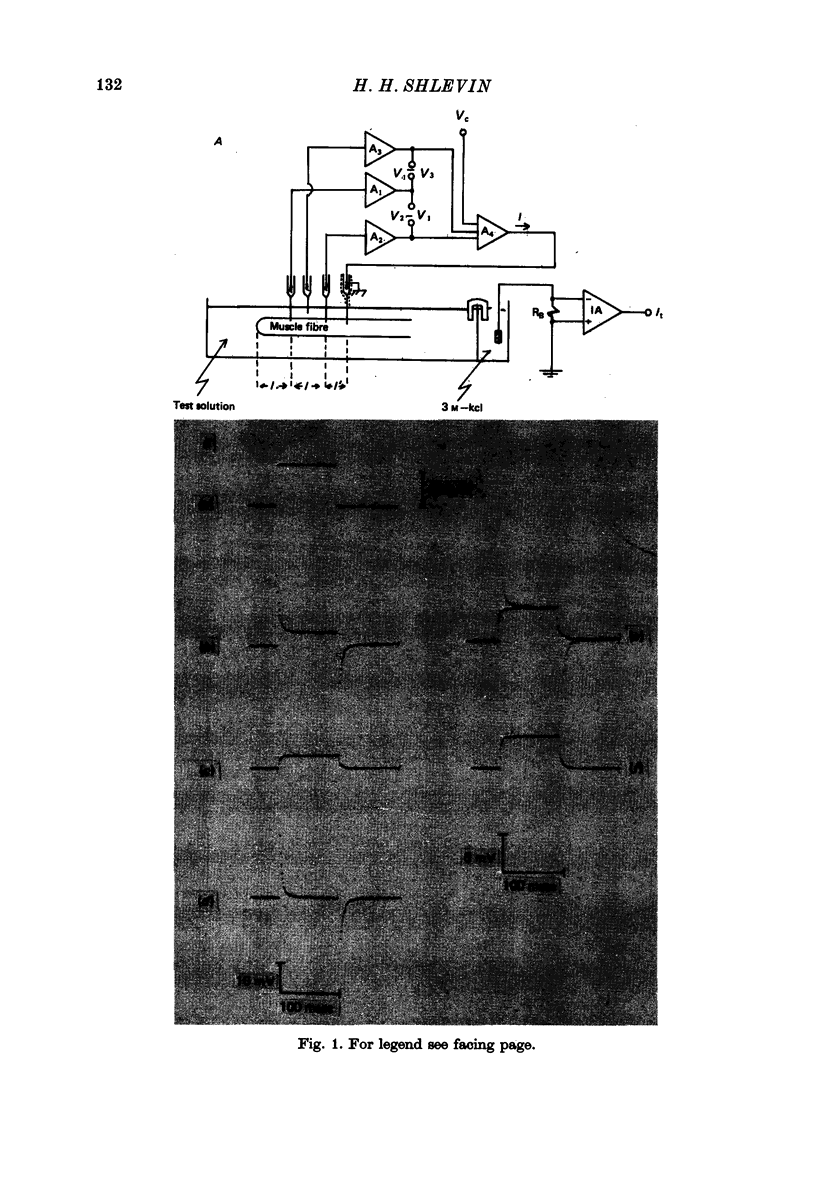
Abstract
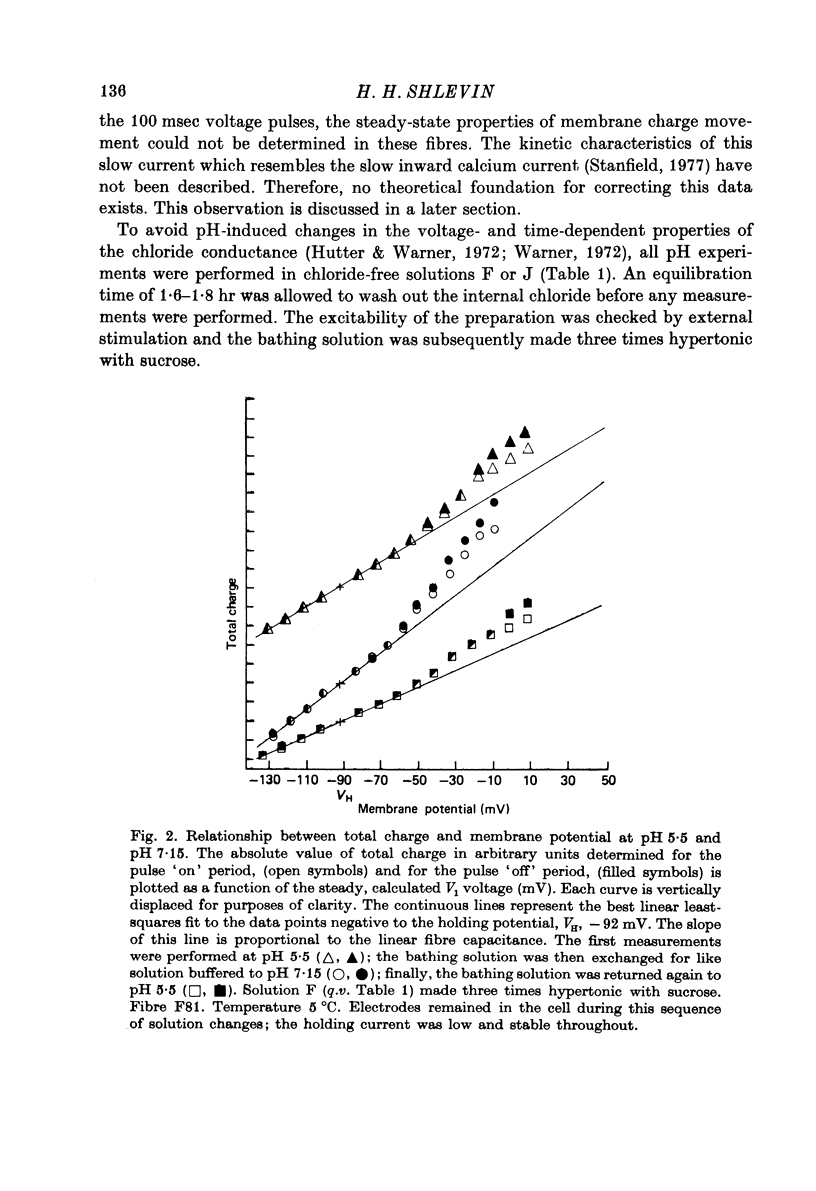
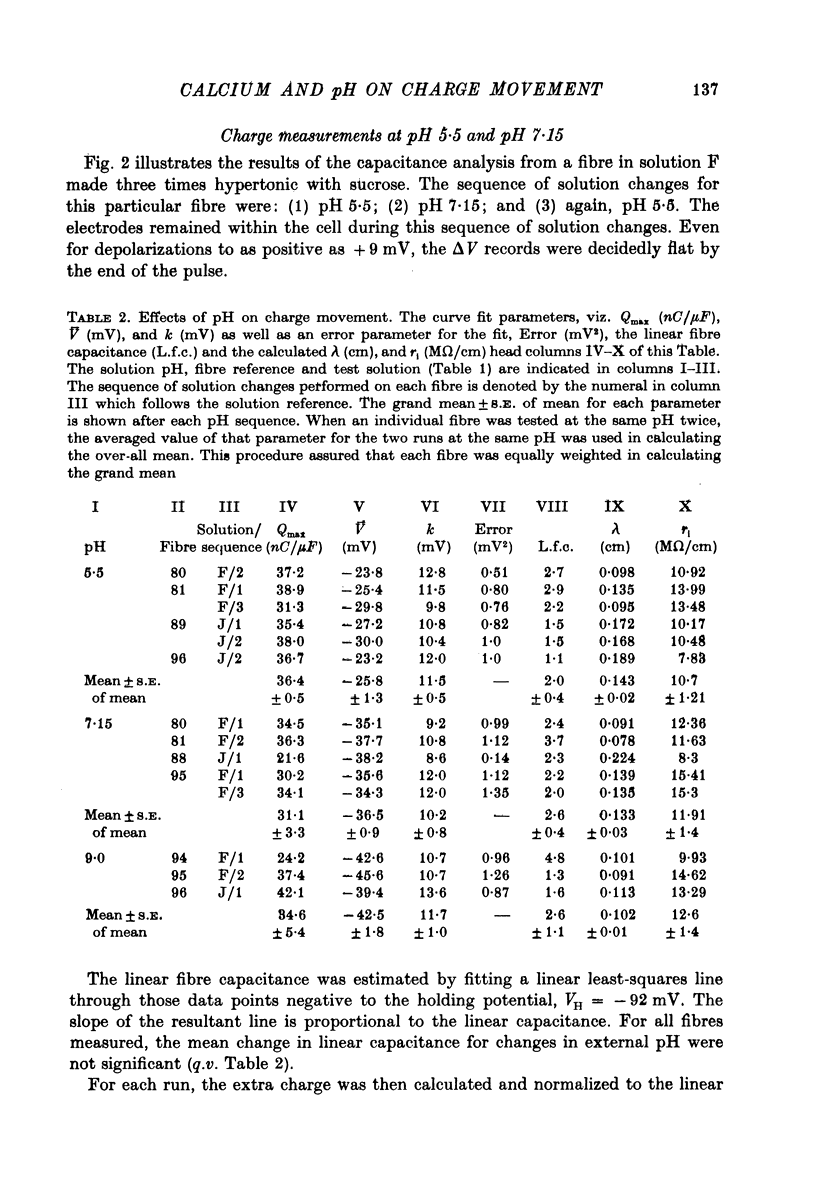
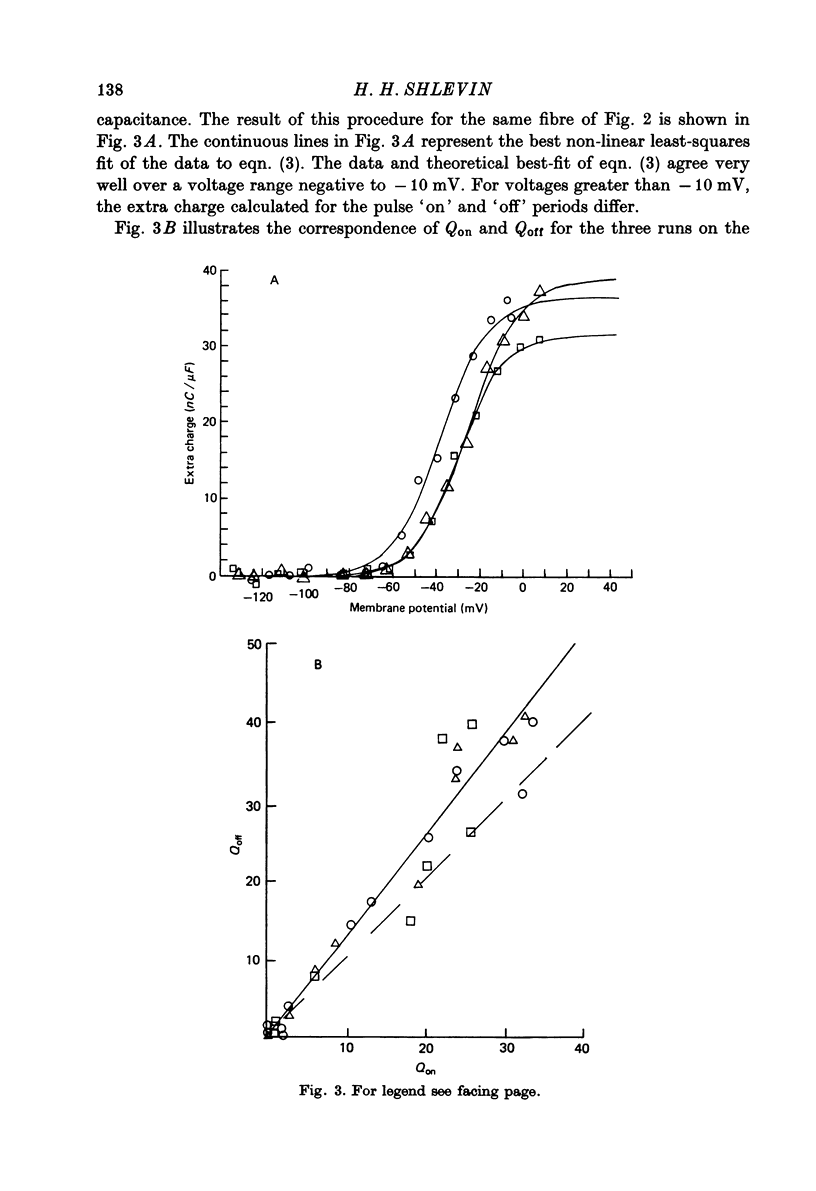
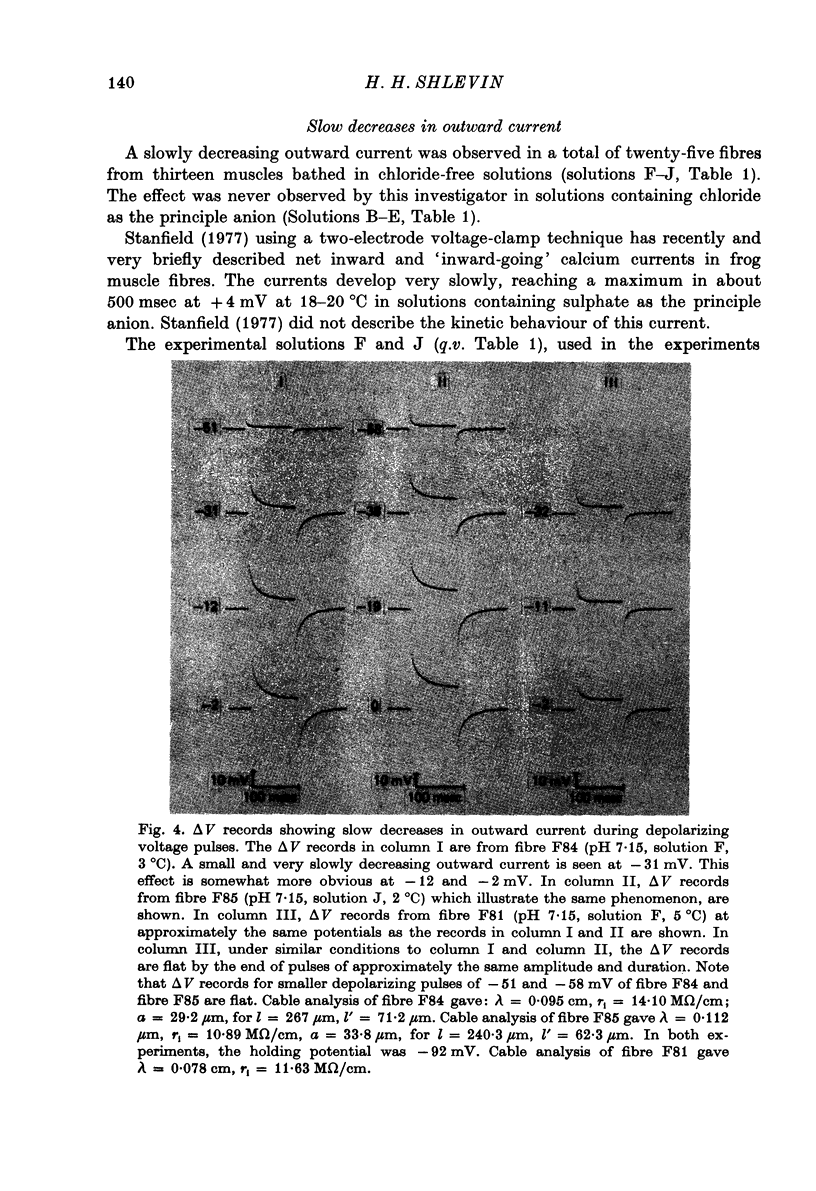
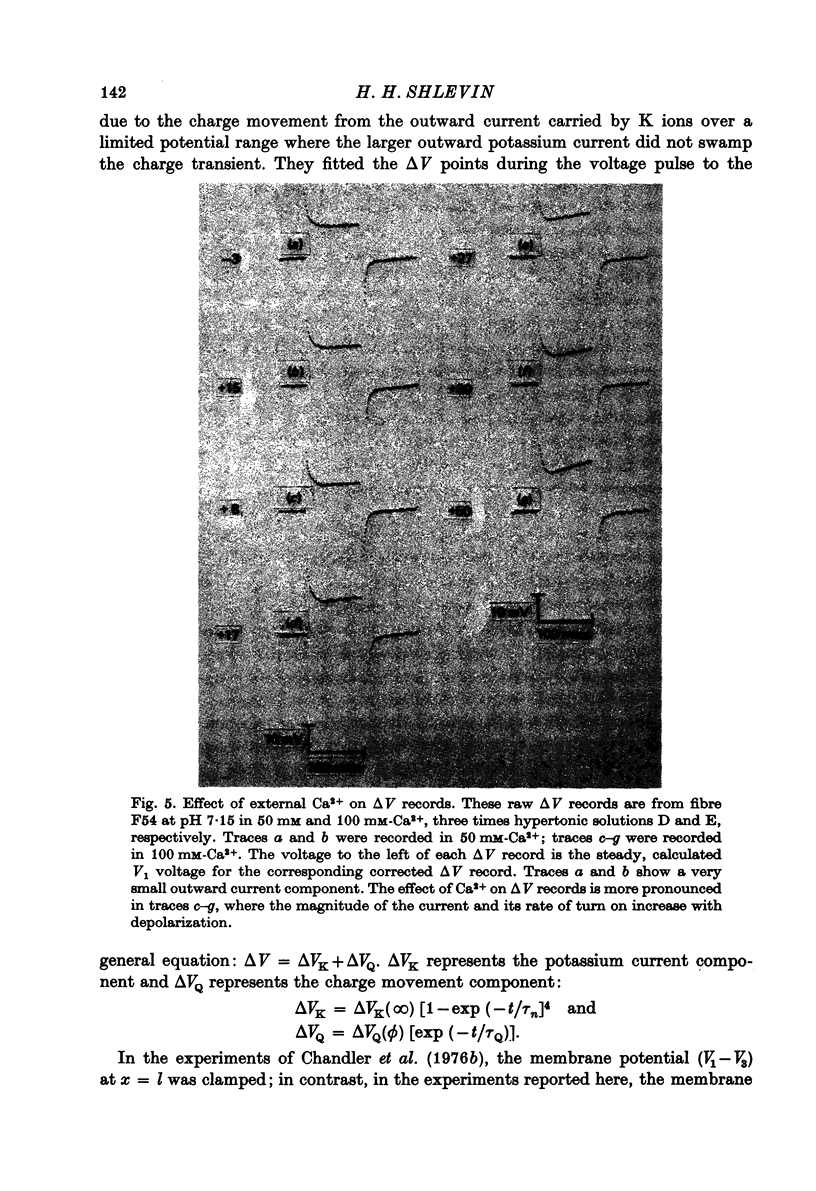
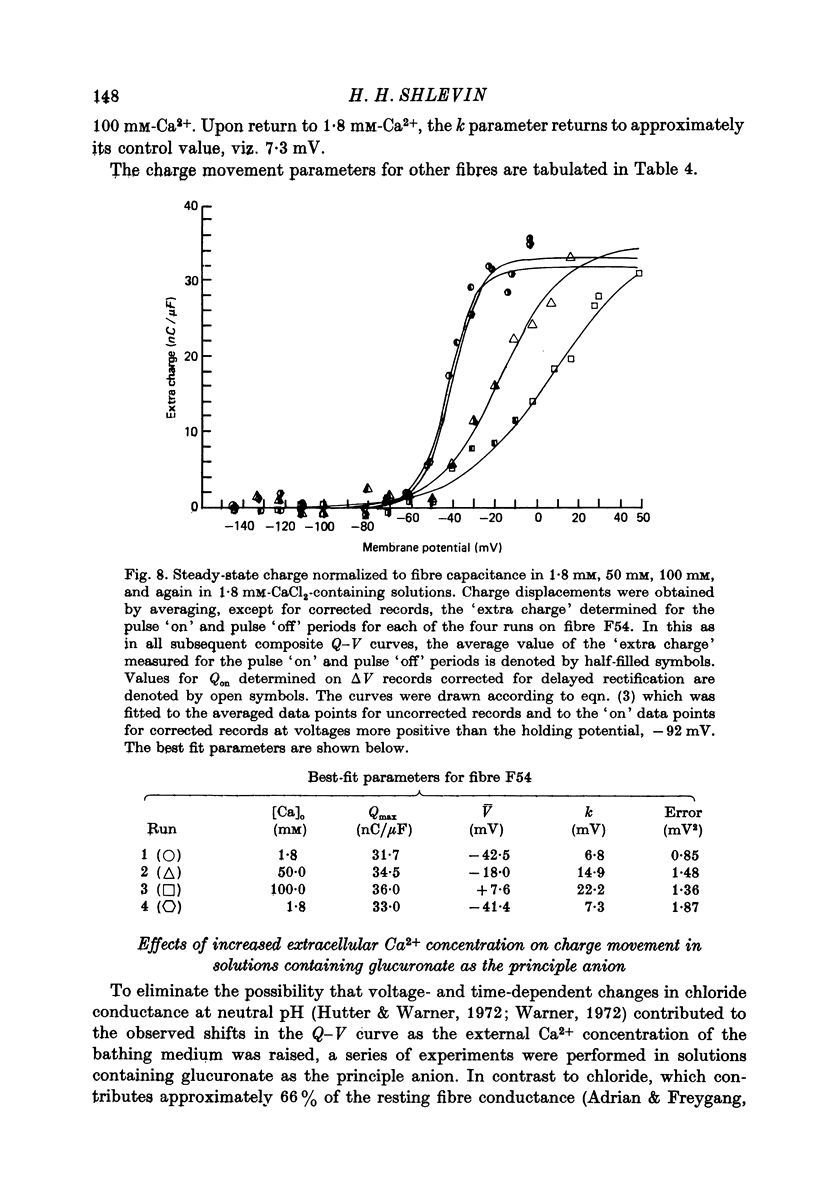
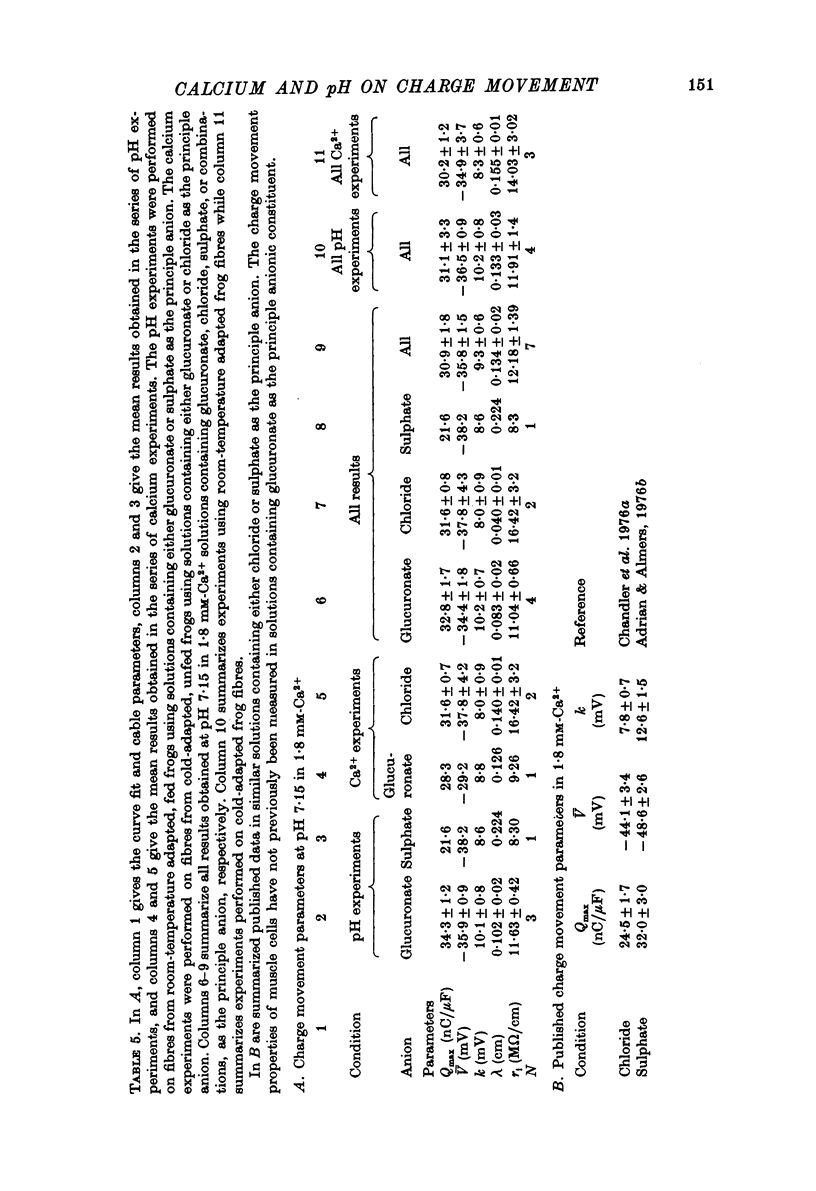
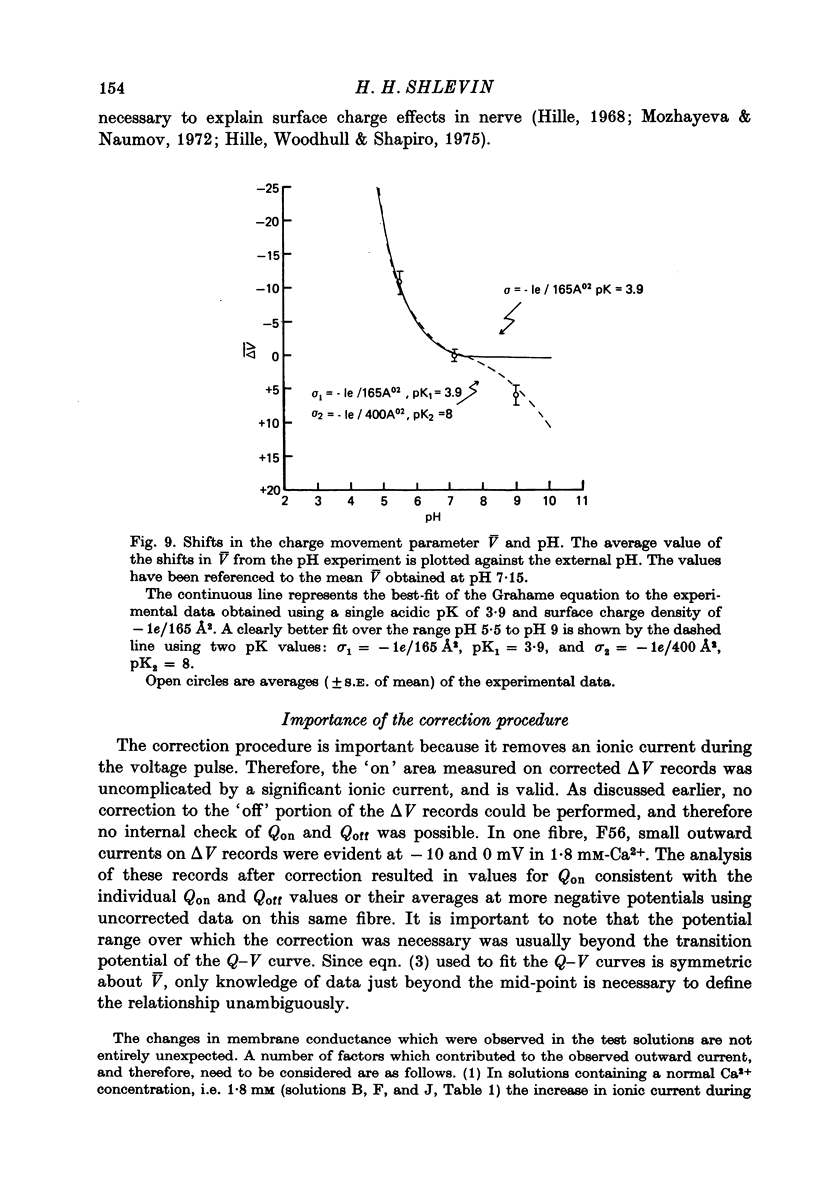
1. The effects of both external Ca2+ (1.8, 25, 50 and 100 mM) and external pH (pH 5.5, 7.15, and 9.0) on the voltage-dependence of charge movement in frog skeletal muscle were examined using the three intracellular micro-electrode voltage-clamp technique. 2. The two-state model of Schneider & Chandler (1973) was used to describe the voltage distribution of membrane charge. The parameters of this model are: Qmax, the maximum quantity of charge; V, the potential of equal distribution of charge; and k, a constant relating to the steepness of the charge vs. voltage relationship. 3. In 1.8 mM external Ca2+, alterations, in external pH shifted the transition potential, V, from a mean +/- S.E. of mean of -36.5 +/- 0.9 mV at pH 7.15 to -25.8 +/- 1.3 mV at pH 5.5 and to -42.5 +/- 1.8 mV at pH 9.0. These shifts are consistent with surface charge theory. No significant changes in Qmax or k were observed over the range of pH 5.5--9.0. 4. A reasonable fit of surface charge theory to the shifts in V over the range pH 5.5--9.0 could be obtained with surface charge densities and binding constants: sigma 1 = -1 e/165 A2, pK1 = 3.9 and sigma 2 = -1 e/400 A2, pK2 = 8. 5. However, at pH 7.15, both V and k changed with increasing external Ca2+ concentration. V shifted from -34.9 +/- 3.7 mV in 1.8 mM-Ca2+ to -13.8 +/- 5.1 mV, -19.3 +/- 3.6 mV and 3.3 +/- 9.3 mV in 25, 50 and 100 mM-Ca2+ respectively. k increased from 8.3 +/- 0.6 mV in 1.8 mM-Ca2+ to 15.3 +/- 1.4 mV, 14.6 +/- 1.6 mV and 20.0 +/- 2.9 mV in 25, 50 and 100 mM-Ca2+. Changes in k reflect decreases in the apparent charged particle valence from approximately 3 in 1.8 mM-Ca2+ to approximately 1.2 in 100 mM-Ca2+. As the external Ca2+ concentration was raised, Qmax was at least as large as that measured in 1.8 mM-Ca2+. The 43% decrease in the apparent valence of the charged groups cannot be explained by simple surface charge theory and may reflect a specific interaction between external Ca2+ and the charged groups. 6. Shifts in V with alterations in external pH and Ca2+ concentration are consistent with the effects of these agents on the contraction threshold of muscle fibres. This observation lends further support to the hypothesis that the charge movement is involved in gating muscle contraction and that the charged particles respond to changes in the electric field across the muscle cell membrane. 7. No difference was observed in the charge movement parameters of fibres from both room-temperature and cold-adapted frog tested at 2--5 degrees C in 1.8 mM-Ca2+ at pH 7.15.
Full text
PDF







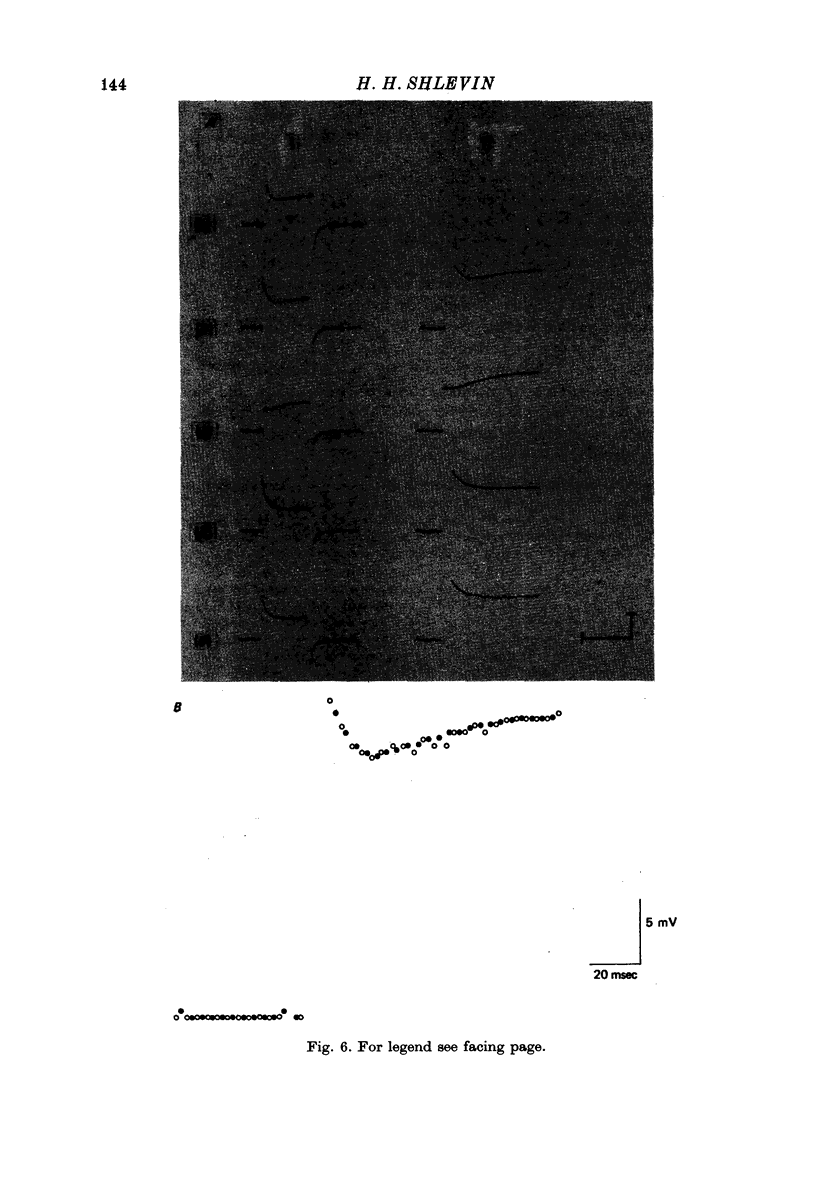
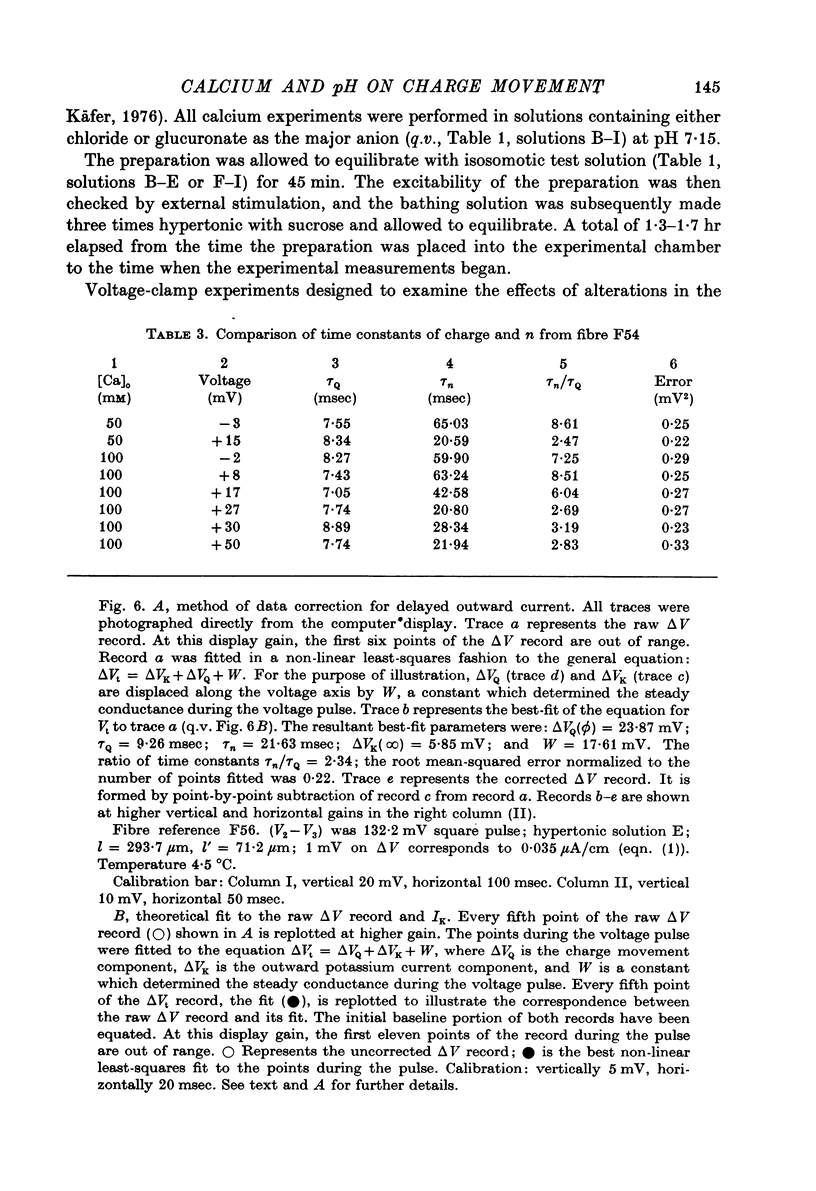
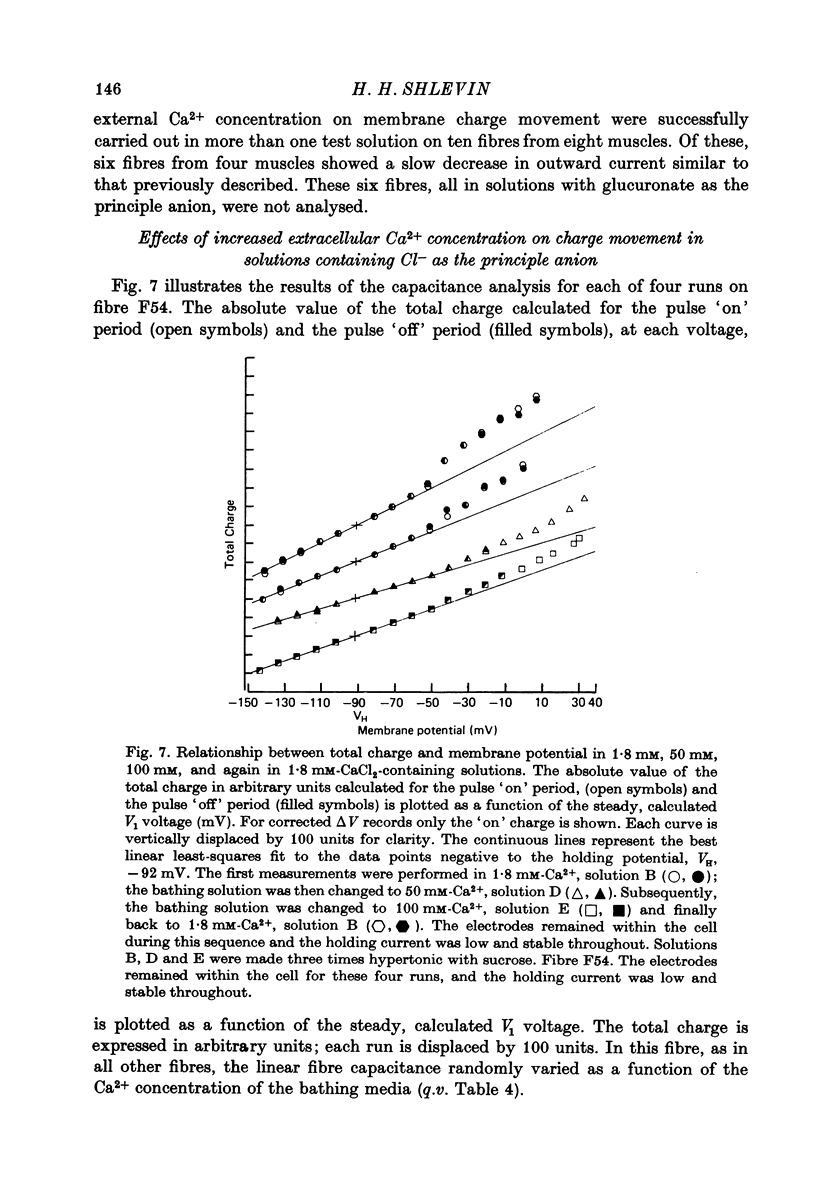
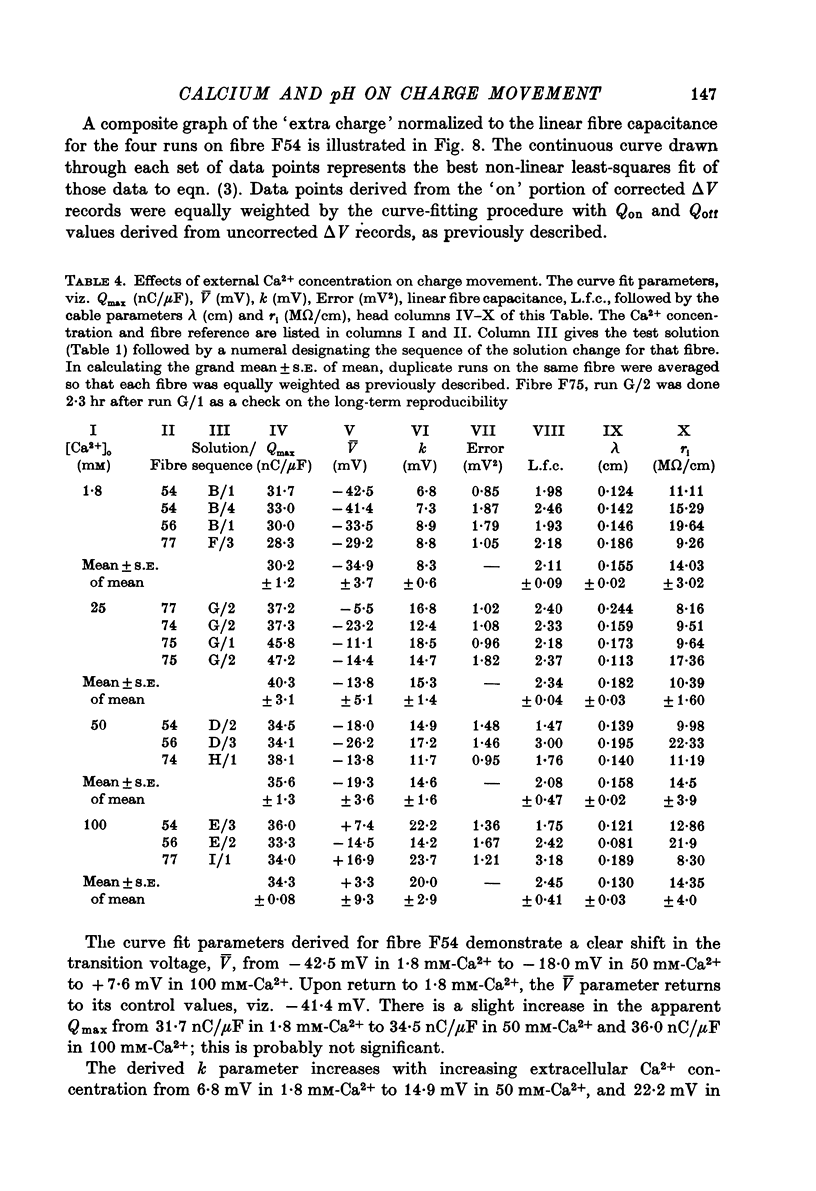








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Almers W. Charge movement in the membrane of striated muscle. J Physiol. 1976 Jan;254(2):339–360. doi: 10.1113/jphysiol.1976.sp011235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Almers W. The voltage dependence of membrane capacity. J Physiol. 1976 Jan;254(2):317–338. doi: 10.1113/jphysiol.1976.sp011234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Slow changes in potassium permeability in skeletal muscle. J Physiol. 1970 Jul;208(3):645–668. doi: 10.1113/jphysiol.1970.sp009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. The kinetics of mechanical activation in frog muscle. J Physiol. 1969 Sep;204(1):207–230. doi: 10.1113/jphysiol.1969.sp008909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in skeletal muscle fibres. J Physiol. 1966 Oct;186(2):51P–52P. [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Costantin L. L., Peachey L. D. Radial spread of contraction in frog muscle fibres. J Physiol. 1969 Sep;204(1):231–257. doi: 10.1113/jphysiol.1969.sp008910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Freygang W. H. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962 Aug;163(1):61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973 Apr 13;242(5398):459–461. doi: 10.1038/242459a0. [DOI] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. A large birefringence signal preceding contraction in single twitch fibres of the frog. J Physiol. 1977 Jan;264(1):141–162. doi: 10.1113/jphysiol.1977.sp011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. Birefringence signals from surface and t-system membranes of frog single muscle fibres. J Physiol. 1977 Jan;264(1):199–213. doi: 10.1113/jphysiol.1977.sp011663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. The optical properties of birefringence signals from single muscle fibres. J Physiol. 1977 Jan;264(1):163–198. doi: 10.1113/jphysiol.1977.sp011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Kinetic properties and inactivation of the gating currents of sodium channels in squid axon. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):449–458. doi: 10.1098/rstb.1975.0022. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Horowicz P. Fluorescence intensity changes associated with contractile activation in frog muscle stained with Nile Blue A. J Physiol. 1975 Apr;246(3):709–735. doi: 10.1113/jphysiol.1975.sp010912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. A non-linear voltage dependent charge movement in frog skeletal muscle. J Physiol. 1976 Jan;254(2):245–283. doi: 10.1113/jphysiol.1976.sp011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. Effects of glycerol treatment and maintained depolarization on charge movement in skeletal muscle. J Physiol. 1976 Jan;254(2):285–316. doi: 10.1113/jphysiol.1976.sp011233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L. The effect o f calcium on contraction and conductance thresholds in frog skeletal muscle. J Physiol. 1968 Mar;195(1):119–132. doi: 10.1113/jphysiol.1968.sp008450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L. The role of sodium current in the radial spread of contraction in frog muscle fibers. J Gen Physiol. 1970 Jun;55(6):703–715. doi: 10.1085/jgp.55.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DYDYNSKA M., WILKIE D. R. THE OSMOTIC PROPERTIES OF STRIATED MUSCLE FIBERS IN HYPERTONIC SOLUTIONS. J Physiol. 1963 Nov;169:312–329. doi: 10.1113/jphysiol.1963.sp007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty A. F., Gage P. W. Electrical properties of toad sartorius muscle fibres in summer and winter. J Physiol. 1973 May;230(3):619–641. doi: 10.1113/jphysiol.1973.sp010208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörrscheidt-Käfer M., Lüttgau H. C. Proceedings: The effect of lanthanum ions on mechanical threshold and potassium contractures in frog skeletal muscle fibres. J Physiol. 1974 Oct;242(2):101P–102P. [PubMed] [Google Scholar]
- Dörrscheidt-Käfer M. The action of Ca2+ , Mg2+ and H+ on the contraction threshold of frog skeletal muscle: Evidence for surface charges controlling electro-mechanical coupling. Pflugers Arch. 1976 Mar 11;362(1):33–41. doi: 10.1007/BF00588678. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969 Nov;2(4):351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Falk G. Predicted delays in the activation of the contractile system. Biophys J. 1968 May;8(5):608–625. doi: 10.1016/S0006-3495(68)86511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C. Membrane particles and transmission at the triad. Fed Proc. 1975 Apr;34(5):1382–1389. [PubMed] [Google Scholar]
- Franzini-Armstrong C. STUDIES OF THE TRIAD : I. Structure of the Junction in Frog Twitch Fibers. J Cell Biol. 1970 Nov 1;47(2):488–499. doi: 10.1083/jcb.47.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C. Studies of the triad. II. Penetration of tracers into the junctional gap. J Cell Biol. 1971 Apr;49(1):196–203. doi: 10.1083/jcb.49.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. I., Hutter O. F. Proceedings: The actions of 4-aminopyridine on the delayed potassium current in skeletal muscle fibres. J Physiol. 1975 Nov;252(2):70P–71P. [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., TAYLOR R. E. Local activation of striated muscle fibres. J Physiol. 1958 Dec 30;144(3):426–441. doi: 10.1113/jphysiol.1958.sp006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Pharmacological modifications of the sodium channels of frog nerve. J Gen Physiol. 1968 Feb;51(2):199–219. doi: 10.1085/jgp.51.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Woodhull A. M., Shapiro B. I. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. The pH sensitivity of the chloride conductance of frog skeletal muscle. J Physiol. 1967 Apr;189(3):403–425. doi: 10.1113/jphysiol.1967.sp008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. The voltage dependence of the chloride conductance of frog muscle. J Physiol. 1972 Dec;227(1):275–290. doi: 10.1113/jphysiol.1972.sp010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y., Stanfield P. R. Actions of some cations on the electrical properties and mechanical threshold of frog sartorius muscle fibers. J Gen Physiol. 1970 May;55(5):620–639. doi: 10.1085/jgp.55.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács L., Schneider M. F. Increased optical transparency associated with excitation--contraction coupling in voltage-clamped cut skeletal muscle fibres. Nature. 1977 Feb 10;265(5594):556–560. doi: 10.1038/265556a0. [DOI] [PubMed] [Google Scholar]
- LUETTGAU H. C. THE ACTION OF CALCIUM IONS ON POTASSIUM CONTRACTURES OF SINGLE MUSCLE FIBRES. J Physiol. 1963 Oct;168:679–697. doi: 10.1113/jphysiol.1963.sp007215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H., Pichon Y. Proceedings: Effects of 4-aminopyridine on the potassium current in internally perfused giant axons of the squid. J Physiol. 1975 Sep;251(1):60P–62P. [PubMed] [Google Scholar]
- Mozhayeva G. N., Naumov A. P. Tetraethylammonium ion inhibition of potassium conductance of the nodal membrane. Biochim Biophys Acta. 1972 Dec 1;290(1):248–255. doi: 10.1016/0005-2736(72)90067-3. [DOI] [PubMed] [Google Scholar]
- Rojas E., Keynes R. D. On the relation between displacement currents and activation of the sodium conductance in the squid giant axon. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):459–482. doi: 10.1098/rstb.1975.0023. [DOI] [PubMed] [Google Scholar]
- Sandow A. Excitation-contraction coupling in skeletal muscle. Pharmacol Rev. 1965 Sep;17(3):265–320. [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Effects of membrane potential on the capacitance of skeletal muscle fibers. J Gen Physiol. 1976 Feb;67(2):125–163. doi: 10.1085/jgp.67.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. A calcium dependent inward current in frog skeletal muscle fibres. Pflugers Arch. 1977 Apr 25;368(3):267–270. doi: 10.1007/BF00585206. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. The differential effects of tetraethylammonium and zinc ions on the resting conductance of frog skeletal muscle. J Physiol. 1970 Jul;209(1):231–256. doi: 10.1113/jphysiol.1970.sp009164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of the tetraethylammonium ion on the delayed currents of frog skeletal muscle. J Physiol. 1970 Jul;209(1):209–229. doi: 10.1113/jphysiol.1970.sp009163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TASAKI I., POLLEY E. H., ORREGO F. Action potentials from individual elements in cat geniculate and striate cortex. J Neurophysiol. 1954 Sep;17(5):454–474. doi: 10.1152/jn.1954.17.5.454. [DOI] [PubMed] [Google Scholar]
- Ulbricht W., Wagner H. H. The influence of pH on equilibrium effects of tetrodotoxin on myelinated nerve fibres of Rana esculenta. J Physiol. 1975 Oct;252(1):159–184. doi: 10.1113/jphysiol.1975.sp011139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdiosera R., Clausen C., Eisenberg R. S. Measurement of the impedance of frog skeletal muscle fibers. Biophys J. 1974 Apr;14(4):295–315. doi: 10.1016/S0006-3495(74)85917-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner A. E. Kinetic properties of the chloride conductance of frog muscle. J Physiol. 1972 Dec;227(1):291–312. doi: 10.1113/jphysiol.1972.sp010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winegrad S. Intracellular calcium movements of frog skeletal muscle during recovery from tetanus. J Gen Physiol. 1968 Jan;51(1):65–83. doi: 10.1085/jgp.51.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]