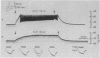
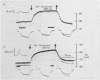
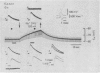
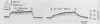Abstract
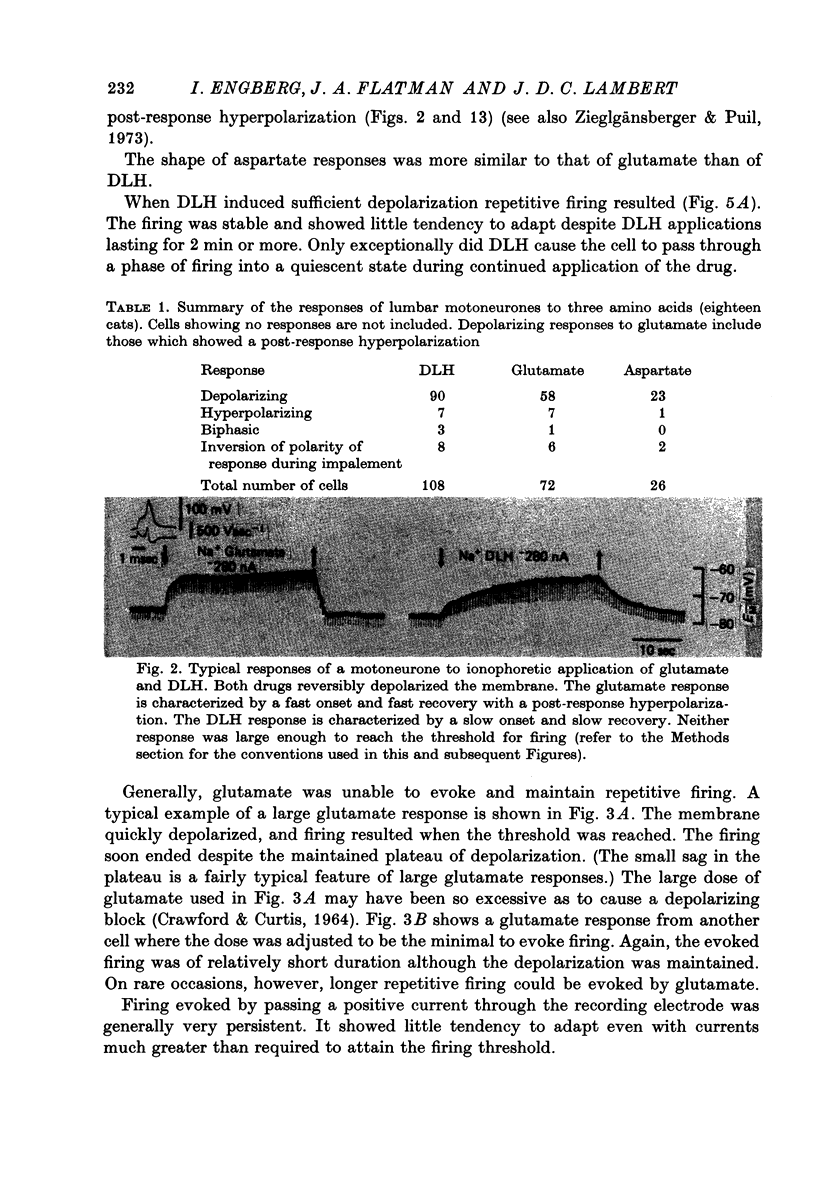
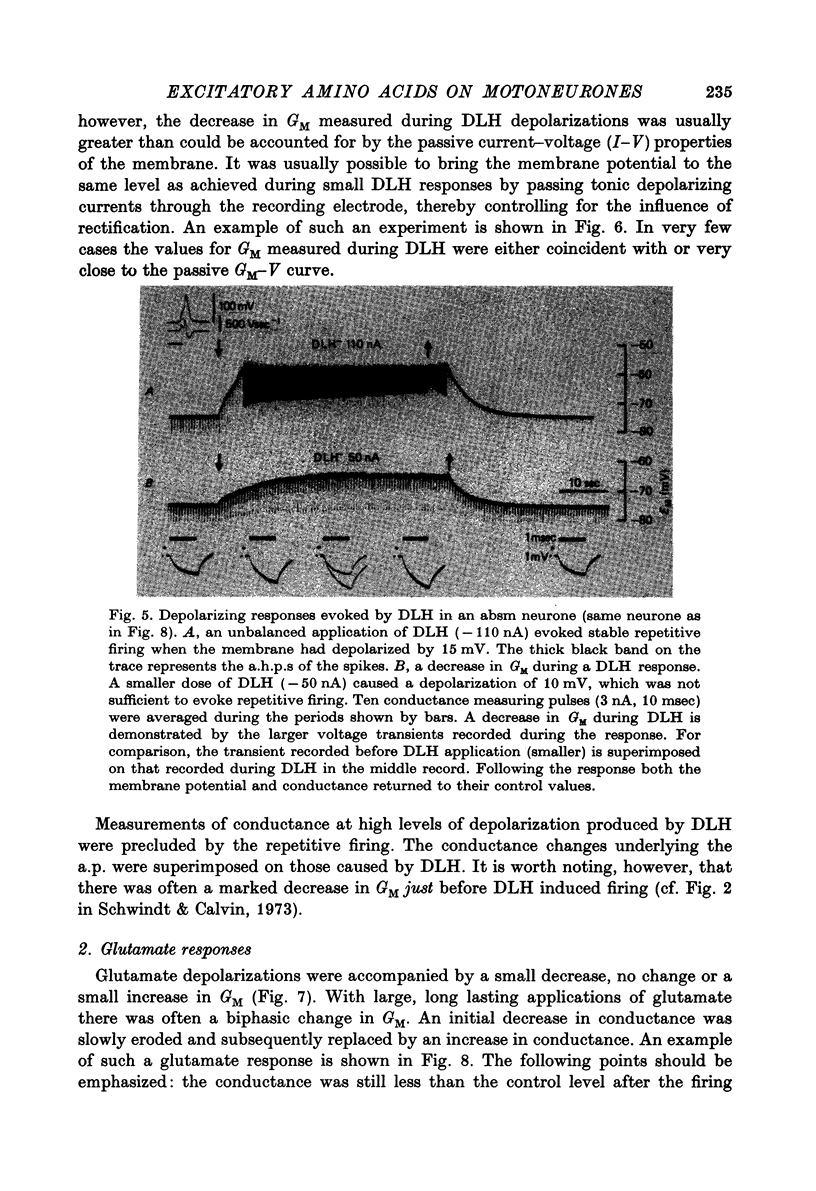
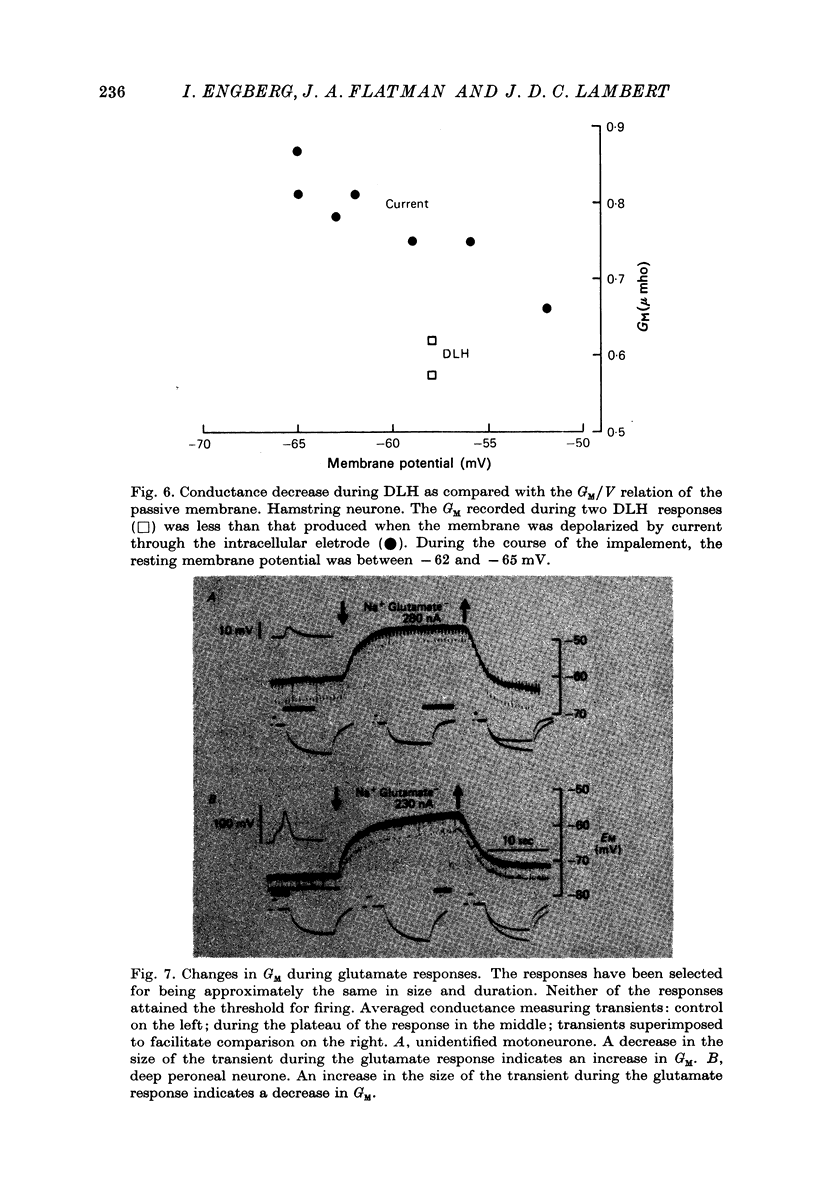
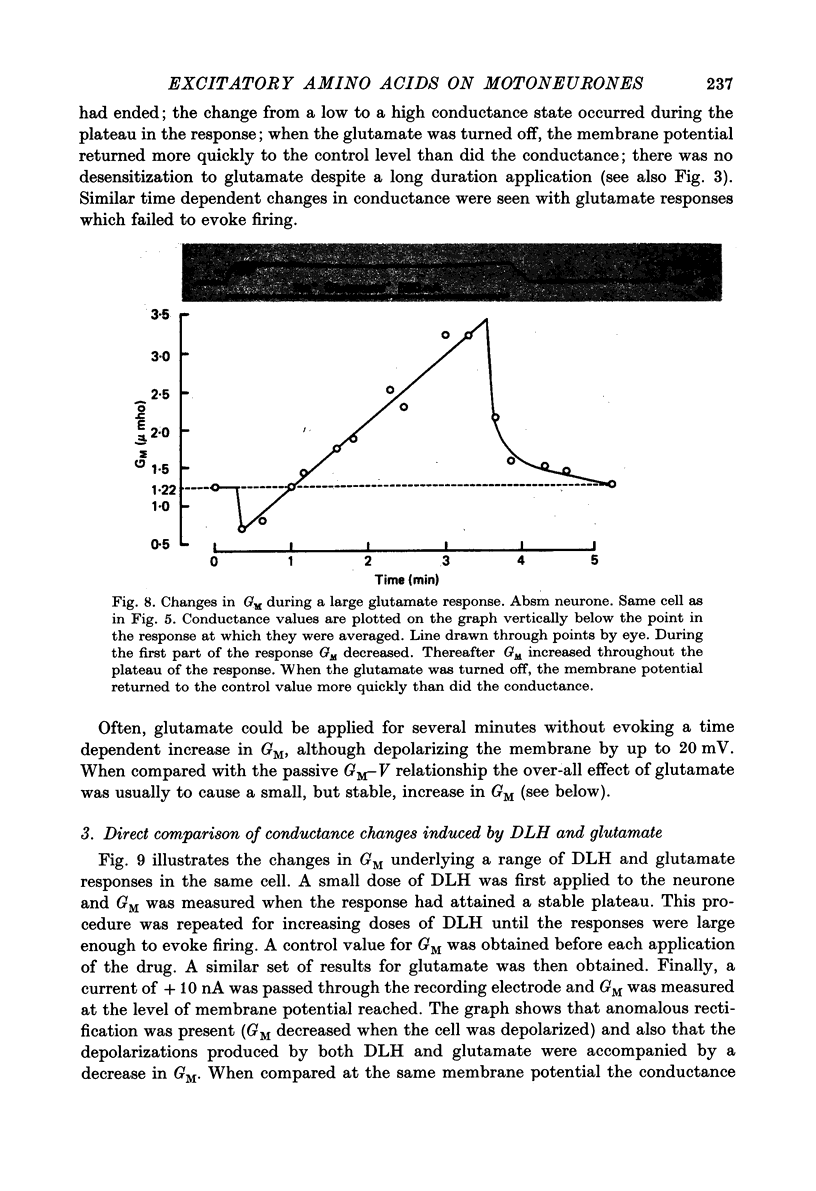
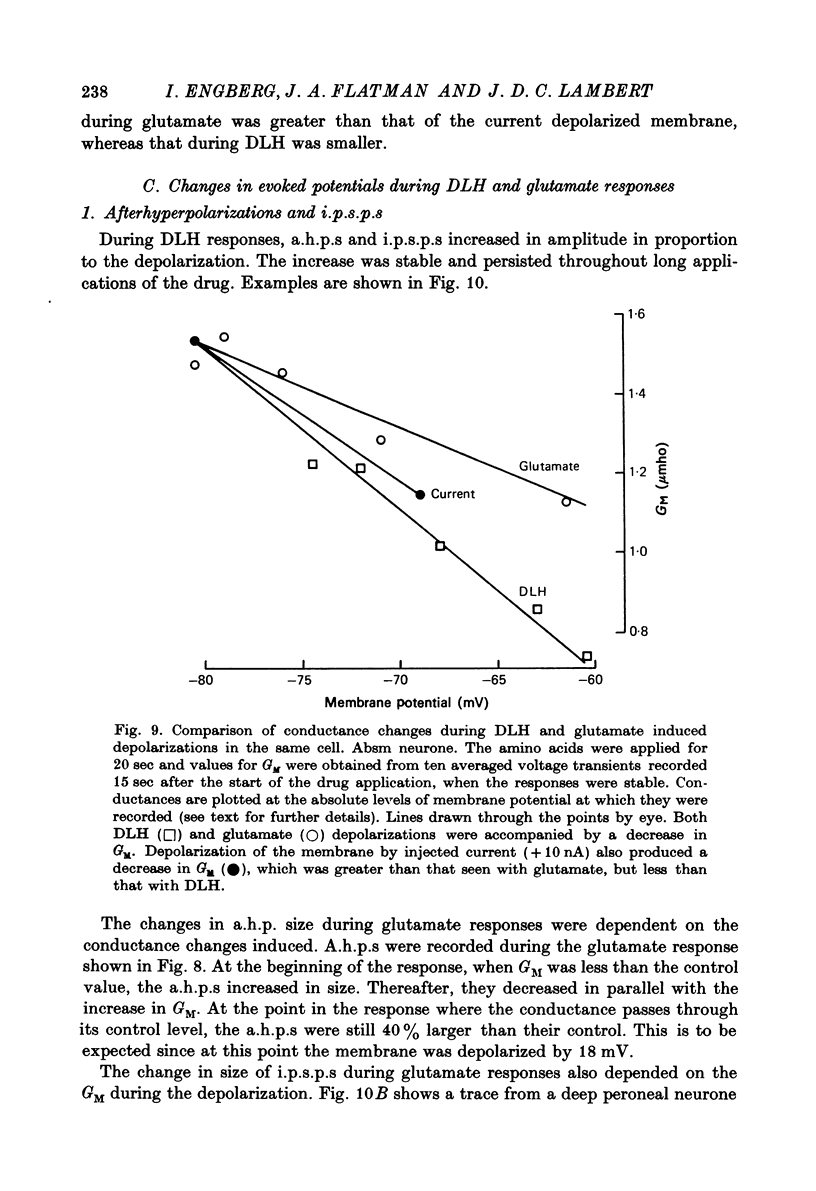
1. Combined recording or ionophoretic electrodes of the concentric type were used to investigate the depolarizing responses of DL-homocysteate (DLH) and L-glutamate in cat lumbar motoneurones. 2. Typically, DLH responses were slow both in onset and recovery, while glutamate responses were fast in onset and recovery and were frequently accompanied by a post-response hyperpolarization. 3. DLH responses (smaller than those necessary to evoke firing) were accompanied by a stable decrease in GM. This decrease was usually more than could be accounted for by anomalous rectification of the membrane. 4. Small glutamate responses were accompanied by either a small decrease, no change or a small increase in GM. There was a biphasic change in GM during large responses: GM decreased during the rising phase and early part of the response plateau and thereafter increased as the depolarization was maintained. It is proposed that the high conductance state during glutamate application (but not the depolarization itself) is a manifestation of glutamate uptake. 5. Firing evoked by DLH was stable during very long applications of the drug. Firing evoked by glutamate was usually of short duration, despite the maintained depolarization. 6. No reversal potential for the DLH responses could be demonstrated, but the responses decreased in size both with hyperpolarization and depolarization of the membrane. A 'null point' of the response in the negative direction was found to be approximately -95 mV. 7. DLH resonses were insensitive to changes in the internal Cl concentration. When the external K concentration was increased by K+ ionophoresis, the DLH responses became smaller. It is concluded that the DLH response is probably mediated via a decrease in K+ conductance and that the availability of this conductance channel is potential dependent. 8. Changes in the sizes of evoked potentials (e.p.s.p.s, i.p.s.p.s and a.h.p.s) with DLH and glutamate responses were investigated. The size of each of these evoked potentials was inversely related to GM during the responses; thus they all showed stable increases during DLH responses. E.p.s.p.s recorded during DLH were of longer half-width and time-to-peak than the control, but there was no change in the maximum slope (V.sec-1). When e.p.s.p.s decreased in size with glutamate the time-to-peak remained constant. 9. Acidic amino acids have been implicated as natural excitatory transmitters. The consequence of our results for the mechanism of excitatory transmission is therefore discussed.
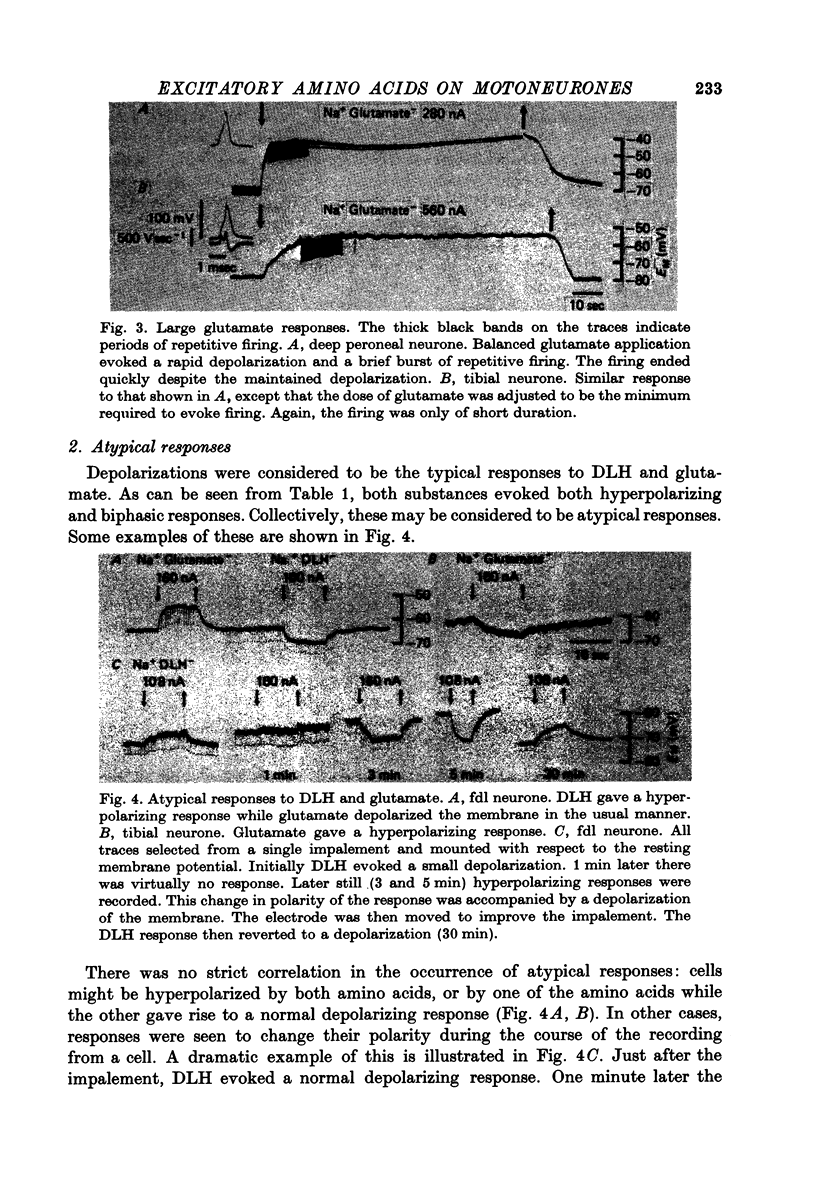
Full text
PDF






















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
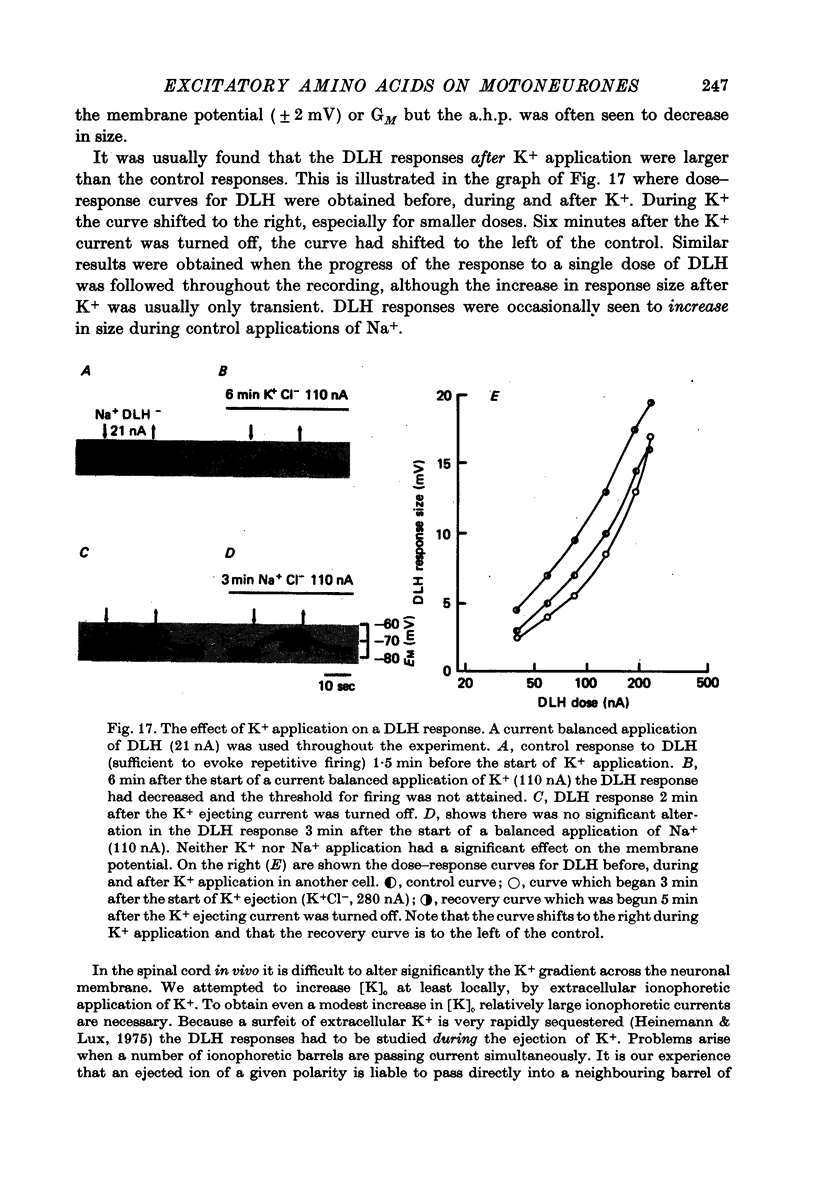
- Altmann H., ten Bruggencate G., Pickelmann P., Steinberg R. Effects of glutamate, aspartate, and two-presumed antagonists on feline rubrospinal neurones. Pflugers Arch. 1976 Aug 24;364(3):249–255. doi: 10.1007/BF00581763. [DOI] [PubMed] [Google Scholar]
- Balcar V. J., Johnston G. A. Glutamate uptake by brain slices and its relation to the depolarization of neurones by acidic amino acids. J Neurobiol. 1972;3(4):295–301. doi: 10.1002/neu.480030403. [DOI] [PubMed] [Google Scholar]
- Balcar V. J., Johnston G. A. High affinity uptake of transmitters: studies on the uptake of L-aspartate, GABA, L-glutamate and glycine in cat spinal cord. J Neurochem. 1973 Feb;20(2):529–539. doi: 10.1111/j.1471-4159.1973.tb12152.x. [DOI] [PubMed] [Google Scholar]
- Balcar V. J., Johnston G. A. The structural specificity of the high affinity uptake of L-glutamate and L-aspartate by rat brain slices. J Neurochem. 1972 Nov;19(11):2657–2666. doi: 10.1111/j.1471-4159.1972.tb01325.x. [DOI] [PubMed] [Google Scholar]
- Barrett J. N., Crill W. E. Influence of dendritic location and membrane properties on the effectiveness of synapses on cat motoneurones. J Physiol. 1974 Jun;239(2):325–345. doi: 10.1113/jphysiol.1974.sp010571. [DOI] [PMC free article] [PubMed] [Google Scholar]
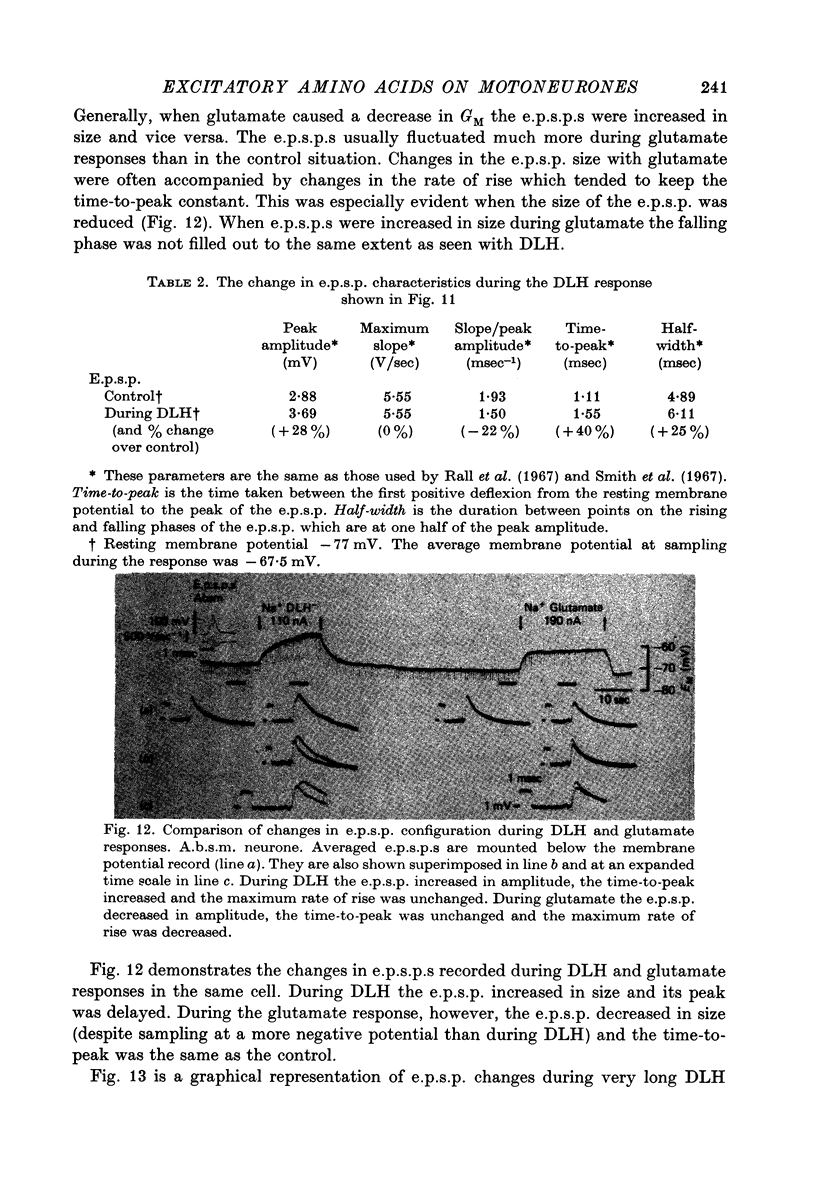
- Bernardi G., Zieglgansberger W., Herz A., Puil E. A. Intracellular studies on the action of L-glutamic acid on spinal neurones of the cat. Brain Res. 1972 Apr 28;39(2):523–525. doi: 10.1016/0006-8993(72)90457-x. [DOI] [PubMed] [Google Scholar]
- Blankenship J. E. Action of tetrodotoxin on spinal motoneurons of the cat. J Neurophysiol. 1968 Mar;31(2):186–194. doi: 10.1152/jn.1968.31.2.186. [DOI] [PubMed] [Google Scholar]
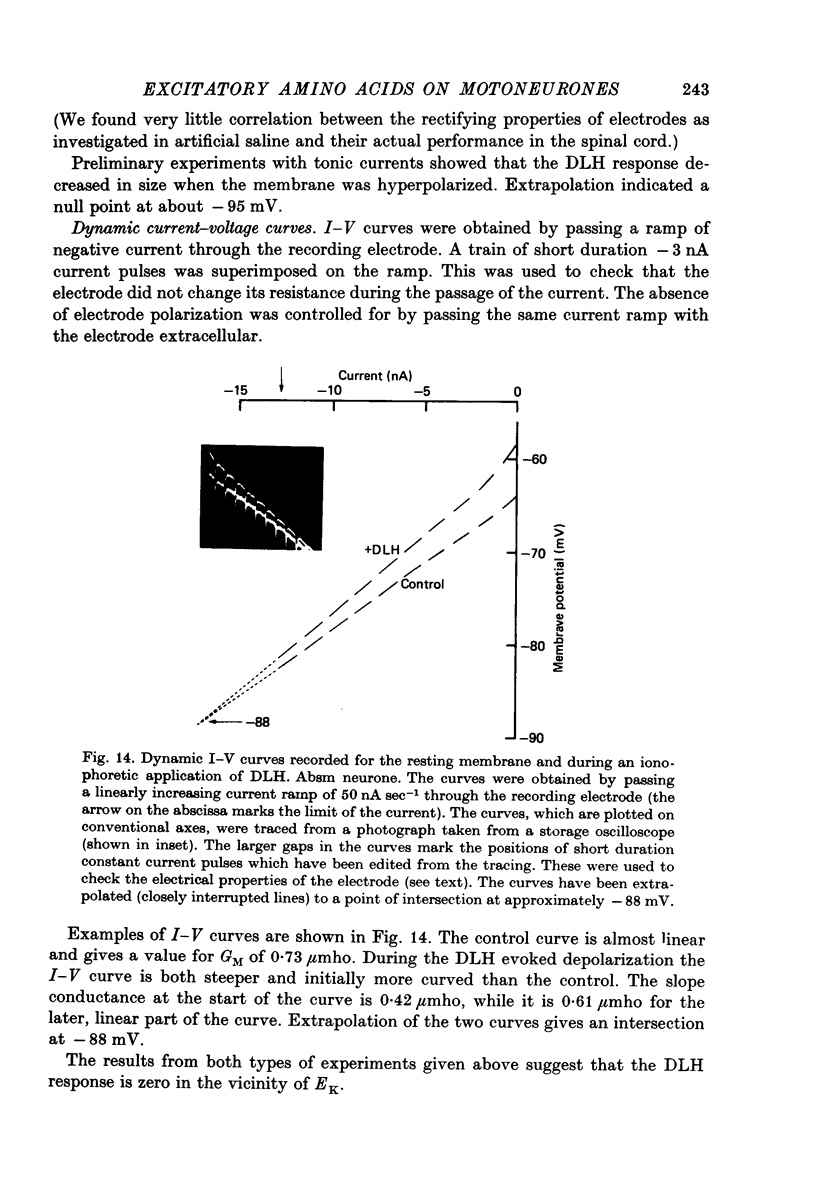
- Brown J. E., Muller K. J., Murray G. Reversal potential for an electrophysiological event generated by conductance changes: mathematical analysis. Science. 1971 Oct 15;174(4006):318–318. doi: 10.1126/science.174.4006.318. [DOI] [PubMed] [Google Scholar]
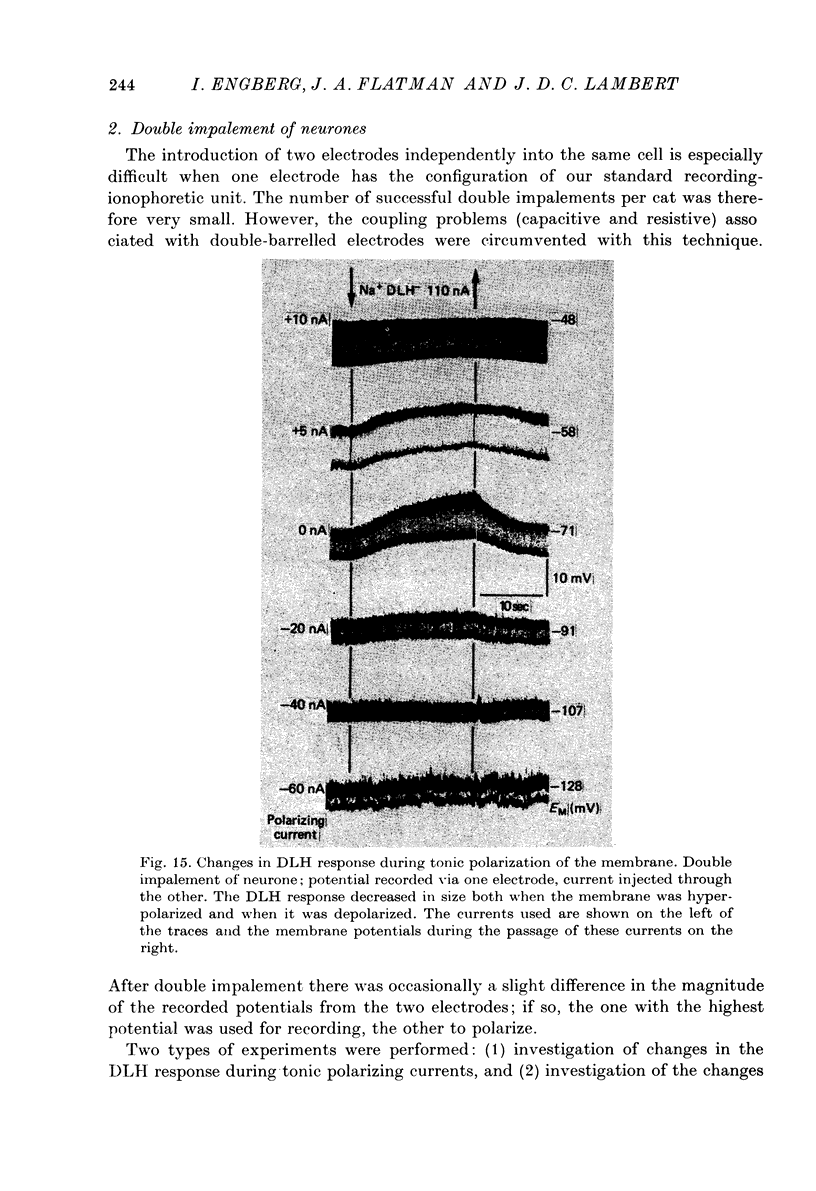
- Burke R. E. Composite nature of the monosynaptic excitatory postsynaptic potential. J Neurophysiol. 1967 Sep;30(5):1114–1137. doi: 10.1152/jn.1967.30.5.1114. [DOI] [PubMed] [Google Scholar]
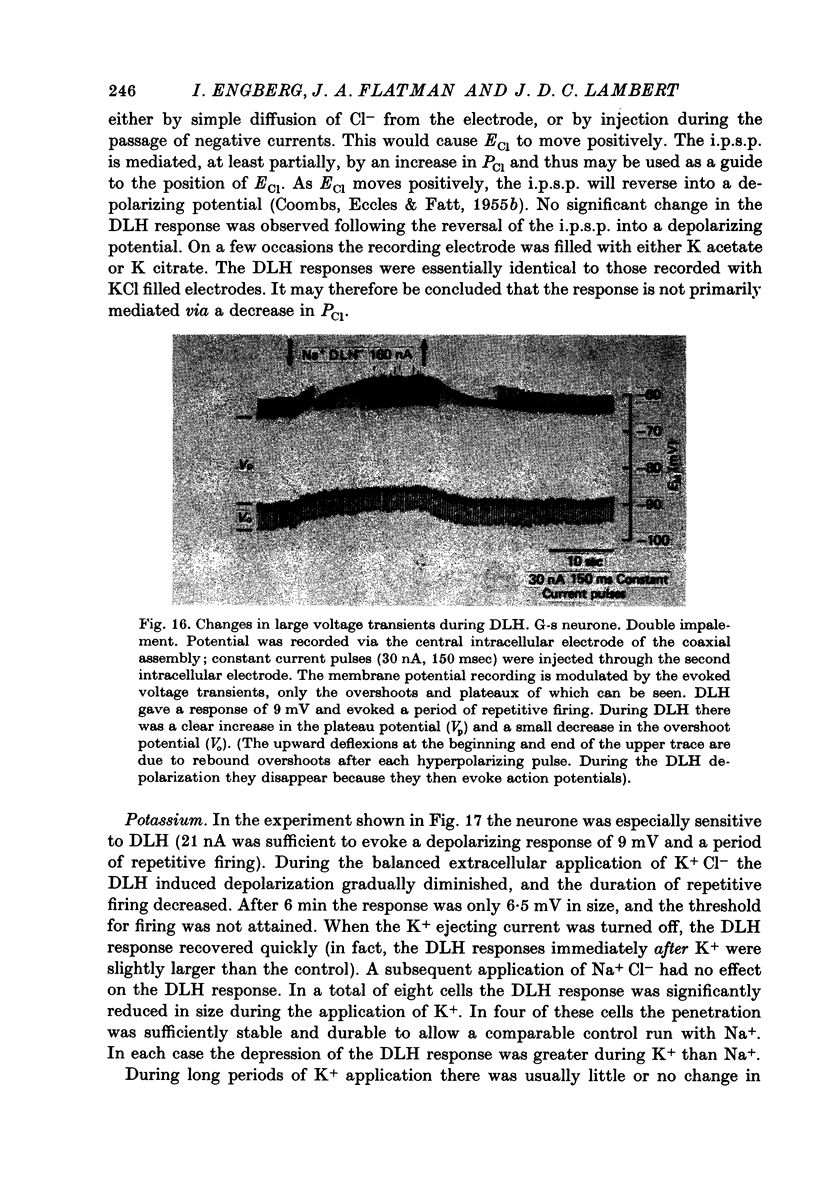
- COOMBS J. S., ECCLES J. C., FATT P. The electrical properties of the motoneurone membrane. J Physiol. 1955 Nov 28;130(2):291–325. doi: 10.1113/jphysiol.1955.sp005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The specific ionic conductances and the ionic movements across the motoneuronal membrane that produce the inhibitory post-synaptic potential. J Physiol. 1955 Nov 28;130(2):326–374. doi: 10.1113/jphysiol.1955.sp005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD J. M., CURTIS D. R. THE EXCITATION AND DEPRESSION OF MAMMALIAN CORTICAL NEURONES BY AMINO ACIDS. Br J Pharmacol Chemother. 1964 Oct;23:313–329. doi: 10.1111/j.1476-5381.1964.tb01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., PERRIN D. D., WATKINS J. C. The excitation of spinal neurones by the ionophoretic application of agents which chelate calcium. J Neurochem. 1960 Aug;6:1–20. doi: 10.1111/j.1471-4159.1960.tb13443.x. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., PHILLIS J. W., WATKINS J. C. The chemical excitation of spinal neurones by certain acidic amino acids. J Physiol. 1960 Mar;150:656–682. doi: 10.1113/jphysiol.1960.sp006410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., WATKINS J. C. Acidic amino acids with strong excitatory actions on mammalian neurones. J Physiol. 1963 Apr;166:1–14. doi: 10.1113/jphysiol.1963.sp007087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin W. H. Dendritic synapses and reversal potentials: theoretical implications of the view from the soma. Exp Neurol. 1969 Jun;24(2):248–264. doi: 10.1016/0014-4886(69)90018-1. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Watkins J. C. Stereoselective uptake of L-homocysteate by rat brain slices [proceedings]. Br J Pharmacol. 1976 Jul;57(3):433P–434P. [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A., Teb ecis A. K., Watkins J. C. Excitation of mammalian central neurones by acidic amino acids. Brain Res. 1972 Jun 22;41(2):283–301. doi: 10.1016/0006-8993(72)90503-3. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Johnston G. A. The inactivation of extracellularly administered amino acids in the feline spinal cord. Exp Brain Res. 1970 Jun 25;10(5):447–462. doi: 10.1007/BF00234262. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Johnston G. A. Amino acid transmitters in the mammalian central nervous system. Ergeb Physiol. 1974;69(0):97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Johnston G. A., Game C. J., McCulloch R. M. Antagonism of neuronal excitation by 1-hydroxy-3-aminopyrrolidone-2. Brain Res. 1973 Jan 30;49(2):467–470. doi: 10.1016/0006-8993(73)90444-7. [DOI] [PubMed] [Google Scholar]
- Diamond J., Huxley A. F. The activation and distribution of GABA and L-glutamate receptors on goldfish Mauthner neurones: an analysis of dendritic remote inhibition. J Physiol. 1968 Feb;194(3):669–723. doi: 10.1113/jphysiol.1968.sp008432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan A. W. The differential sensitivity to L-glutamate and L-aspartate of spinal interneurones and Renshaw cells. Exp Brain Res. 1974 Mar 29;19(5):522–528. doi: 10.1007/BF00236115. [DOI] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. Non-quantal fluctuations and transmission failures in charge transfer at Ia synapses on spinal motoneurones. J Physiol. 1976 Aug;259(3):689–704. doi: 10.1113/jphysiol.1976.sp011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. Statistical fluctuations in charge transfer at Ia synapses on spinal motoneurones. J Physiol. 1976 Aug;259(3):665–688. doi: 10.1113/jphysiol.1976.sp011488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. The effect of polarizing currents on unitary Ia excitatory post-synaptic potentials evoked in spinal motoneurones. J Physiol. 1976 Aug;259(3):705–723. doi: 10.1113/jphysiol.1976.sp011490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I., Marshall K. C. Mechanism of noradrenaline hyperpolarization in spinal cord motoneurones of the cat. Acta Physiol Scand. 1971 Sep;83(1):142–144. doi: 10.1111/j.1748-1716.1971.tb05061.x. [DOI] [PubMed] [Google Scholar]
- Freeman A. R. Polyfunctional role of glutamic acid in excitatory synaptic transmission. Prog Neurobiol. 1976;3(2):137–153. doi: 10.1016/0301-0082(76)90012-5. [DOI] [PubMed] [Google Scholar]
- Geller H. M., Woodward D. J. Responses of cultured cerebellar neurons to iontophoretically applied amino acids. Brain Res. 1974 Jul 5;74(1):67–80. doi: 10.1016/0006-8993(74)90112-7. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M., Paupardin-Tritsch D. Ionic mechanisms and receptor properties underlying the responses of molluscan neurones to 5-hydroxytryptamine. J Physiol. 1974 Dec;243(2):427–456. doi: 10.1113/jphysiol.1974.sp010761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L. Electrical changes in the membrane in junctional transmission. Biochim Biophys Acta. 1973 Nov 28;300(3):289–317. doi: 10.1016/0304-4157(73)90007-5. [DOI] [PubMed] [Google Scholar]
- Graham L. T., Jr, Shank R. P., Werman R., Aprison M. H. Distribution of some synaptic transmitter suspects in cat spinal cord: glutamic acid, aspartic acid, gamma-aminobutyric acid, glycine and glutamine. J Neurochem. 1967 Apr;14(4):465–472. doi: 10.1111/j.1471-4159.1967.tb09545.x. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschlag R., Weinreich D. Glutamic acid and primary afferent transmission. Adv Biochem Psychopharmacol. 1972;6:165–180. [PubMed] [Google Scholar]
- Heinemann U., Lux H. D. Undershoots following stimulus-induced rises of extracellular potassium concentration in cerebral cortex of cat. Brain Res. 1975 Jul 25;93(1):63–76. doi: 10.1016/0006-8993(75)90286-3. [DOI] [PubMed] [Google Scholar]
- Henn F. A., Hamberger A. Glial cell function: uptake of transmitter substances. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2686–2690. doi: 10.1073/pnas.68.11.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hösli E., Hösli L. Uptake of L-glutamate and L-aspartate in neurones and glial cells of cultured human and rat spinal cord. Experientia. 1976 Feb 15;32(2):219–222. doi: 10.1007/BF01937776. [DOI] [PubMed] [Google Scholar]
- Hösli L., Andrès P. F., Hösli E. Ionic mechanisms underlying the depolarization of L-glutamate on rat and human spinal neurones in tissue culture. Experientia. 1973 Oct 15;29(10):1244–1247. doi: 10.1007/BF01935098. [DOI] [PubMed] [Google Scholar]
- Ito M., Oshima T. Electrical behaviour of the motoneurone membrane during intracellularly applied current steps. J Physiol. 1965 Oct;180(3):607–635. doi: 10.1113/jphysiol.1965.sp007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Miller S., Porter R., Redman S. J. The time course of minimal excitory post-synaptic potentials evoked in spinal motoneurones by group Ia afferent fibres. J Physiol. 1971 Jun;215(2):353–380. doi: 10.1113/jphysiol.1971.sp009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J. An electrical description of the motoneurone, and its application to the analysis of synaptic potentials. J Physiol. 1971 Jun;215(2):321–352. doi: 10.1113/jphysiol.1971.sp009473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Aprison M. H. The distribution of glutamic acid, a transmitter candidate, and other amino acids in the dorsal sensory neuron of the cat. Brain Res. 1970 Dec 1;24(2):285–292. doi: 10.1016/0006-8993(70)90107-1. [DOI] [PubMed] [Google Scholar]
- Johnson J. L. Glutamic acid as a synaptic transmitter in the nervous system. A review. Brain Res. 1972 Feb 11;37(1):1–19. doi: 10.1016/0006-8993(72)90343-5. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., PHILLIS J. W. Iontophoretic studies of neurones in the mammalian cerebral cortex. J Physiol. 1963 Feb;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S., Otsuka M. The effects of substance P and other peptides on spinal neurons of the frog. Brain Res. 1974 Jan 18;65(3):397–410. doi: 10.1016/0006-8993(74)90231-5. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Morris M. E. An excitatory action of substance P on cuneate neurones. Can J Physiol Pharmacol. 1974 Jun;52(3):736–744. doi: 10.1139/y74-094. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Pumain R., Renaud L. The mechanism of excitation by acetylcholine in the cerebral cortex. J Physiol. 1971 May;215(1):247–268. doi: 10.1113/jphysiol.1971.sp009467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Schwartz S. Some properties of unresponsive cells in the cerebral cortex. Exp Brain Res. 1967;3(4):306–319. doi: 10.1007/BF00237557. [DOI] [PubMed] [Google Scholar]
- Logan W. J., Snyder S. H. High affinity uptake systems for glycine, glutamic and aspaspartic acids in synaptosomes of rat central nervous tissues. Brain Res. 1972 Jul 20;42(2):413–431. doi: 10.1016/0006-8993(72)90540-9. [DOI] [PubMed] [Google Scholar]
- Lux H. D. Ammonium and chloride extrusion: hyperpolarizing synaptic inhibition in spinal motoneurons. Science. 1971 Aug 6;173(3996):555–557. doi: 10.1126/science.173.3996.555. [DOI] [PubMed] [Google Scholar]
- Lux H. D., Neher E. The equilibration time course of (K + ) 0 in cat cortex. Exp Brain Res. 1973 Apr 30;17(2):190–205. doi: 10.1007/BF00235028. [DOI] [PubMed] [Google Scholar]
- Martin A. R., Wickelgren W. O., Ber1anek R. Effects of iontophoretically applied drugs on spinal interneurons of the lamprey. J Physiol. 1970 May;207(3):653–665. doi: 10.1113/jphysiol.1970.sp009086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan H. Actions of excitatory amino acids and their antagonism. Neuropharmacology. 1974 Jun;13(6):449–454. doi: 10.1016/0028-3908(74)90133-6. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Frank K. Anomalous rectification in cat spinal motoneurons and effect of polarizing currents on excitatory postsynaptic potential. J Neurophysiol. 1967 Sep;30(5):1097–1113. doi: 10.1152/jn.1967.30.5.1097. [DOI] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. Permeability changes produced by L-glutamate at the excitatory post-synaptic membrane of the crayfish muscle. J Physiol. 1976 Mar;255(3):669–685. doi: 10.1113/jphysiol.1976.sp011302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves R. D. Function of muscarinic and nicotinic acetylcholine receptors. Nature. 1976 May 13;261(5556):149–151. doi: 10.1038/261149a0. [DOI] [PubMed] [Google Scholar]
- RALL W. Theory of physiological properties of dendrites. Ann N Y Acad Sci. 1962 Mar 2;96:1071–1092. doi: 10.1111/j.1749-6632.1962.tb54120.x. [DOI] [PubMed] [Google Scholar]
- Rall W., Burke R. E., Smith T. G., Nelson P. G., Frank K. Dendritic location of synapses and possible mechanisms for the monosynaptic EPSP in motoneurons. J Neurophysiol. 1967 Sep;30(5):1169–1193. doi: 10.1152/jn.1967.30.5.1169. [DOI] [PubMed] [Google Scholar]
- Rinzel J., Rall W. Transient response in a dendritic neuron model for current injected at one branch. Biophys J. 1974 Oct;14(10):759–790. doi: 10.1016/S0006-3495(74)85948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt P. C., Calvin W. H. Nature of conductances underlying rhythmic firing in cat spinal motoneurons. J Neurophysiol. 1973 Nov;36(6):955–973. doi: 10.1152/jn.1973.36.6.955. [DOI] [PubMed] [Google Scholar]
- Smith T. G., Wuerker R. B., Frank K. Membrane impedance changes during synaptic transmission in cat spinal motoneurons. J Neurophysiol. 1967 Sep;30(5):1072–1096. doi: 10.1152/jn.1967.30.5.1072. [DOI] [PubMed] [Google Scholar]
- Sonnhof U. A multi-barrelled coaxial electrode for iontophoresis and intracellular recording with a gold shield of the central pipette for capacitance neutralization. Pflugers Arch. 1973 Jul 31;341(4):351–358. doi: 10.1007/BF01023677. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Blockade by amino acid antagonists of neuronal excitation mediated by the pyramidal tract. J Physiol. 1976 May;257(1):187–198. doi: 10.1113/jphysiol.1976.sp011363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A. T. Mobilization of synaptic membrane-bound calcium by acidic amino acids. J Neurochem. 1975 Jan;24(1):127–134. doi: 10.1111/j.1471-4159.1975.tb07638.x. [DOI] [PubMed] [Google Scholar]
- VAN HARREVELD A. Compounds in brain extracts causing spreading depression of cerebral cortical activity and contraction of crustacean muscle. J Neurochem. 1959 Feb;3(4):300–315. doi: 10.1111/j.1471-4159.1959.tb12636.x. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Votava J. Slow synaptic excitation in sympathetic ganglion cells: evidence for synaptic inactivation of potassium conductance. Science. 1970 Nov 13;170(3959):755–758. doi: 10.1126/science.170.3959.755. [DOI] [PubMed] [Google Scholar]
- Werman R., Carlen P. L. Unusual behavior of the La EPSP in cat spinal motoneurons. Brain Res. 1976 Aug 13;112(2):395–401. doi: 10.1016/0006-8993(76)90294-8. [DOI] [PubMed] [Google Scholar]
- Zieglgänsberger W., Puil E. A. Actions of glutamic acid on spinal neurones. Exp Brain Res. 1973 Mar 29;17(1):35–49. doi: 10.1007/BF00234562. [DOI] [PubMed] [Google Scholar]
- Zieglgänsberger W., Puil E. A. Tetrodotoxin interference of CNS excitation by glutamic acid. Nat New Biol. 1972 Oct 18;239(94):204–205. doi: 10.1038/newbio239204a0. [DOI] [PubMed] [Google Scholar]
- Zieglgänsberger W., Reiter C. A cholinergic mechanism in the spinal cord of cats. Neuropharmacology. 1974 Jun;13(6):519–527. doi: 10.1016/0028-3908(74)90141-5. [DOI] [PubMed] [Google Scholar]