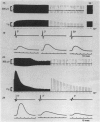
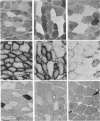
Abstract
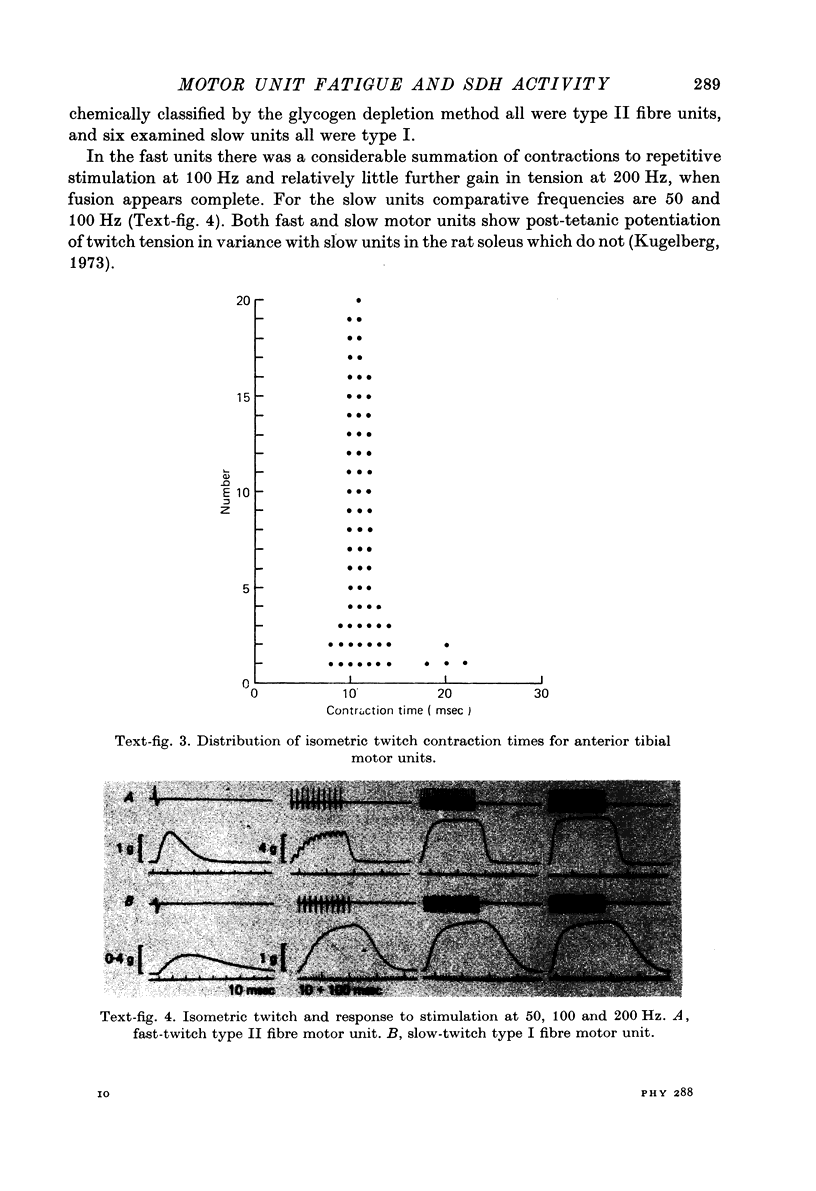
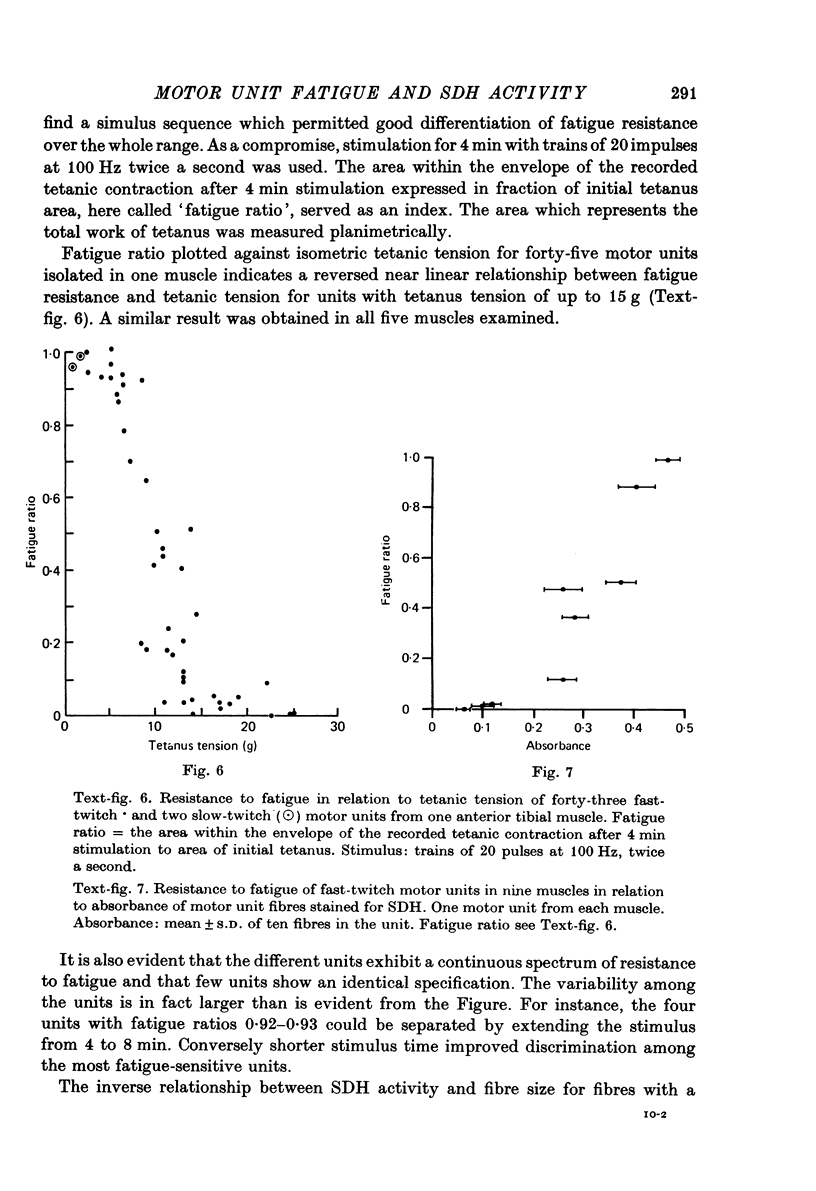
1. The fatigue in rat anterior tibial (a.t.) motor units was studied and related to microphotometric determinations of succinate dehydrogenase (SDH) activity of the motor unit muscle fibres. 2. Anterior tibial contains fast-twitch type II fibre units with an average contraction time of 11 msec and about 5% slow-twitch type I fibre units with an average contraction time of 20 msec. 3. In type II fibres stained for SDH, absorbance varied continuously from 0.046 to 0.569 and inversely to fibre size, except for the largest fibres. 4. Resistance to fatigue of fast motor units to 100 Hz intermittent stimulation varied continuously within a wide range in near linear relations to absorbance for SDH of unit fibres and inversely to tetanic tension, except for motor units with the largest fibres and the largest tetanic tension. 5. Neither resistance to fatigue nor SDH activity lent itself to any categorization of motor units or fibres into well demarcated functional or histochemical types, since both parameters varied continuously in the unit and fibre population of the muscle. 6. The direct relation between resistance to fatigue of fast-twitch motor units and SDH activity of unit fibres appeared valid for fatigue resistance of: (a) neuromuscular transmission, tested with 100 Hz intermittent stimulation which gave concomitant failure of electrical and mechanical response, (b) excitation--contraction coupling, demonstrated by post-stimulatory depression of twitch tension with preserved maximum tetanus tension and action potential, and (c) contractile mechanism; excitation--contraction coupling?, tested with low frequency stimulation which gave decline of twitch and maximum tetanus tension with preserved action potential. 7. It is suggested that the endurance of each link in the chain of events leading to contraction, including neuromuscular junction and the excitation--contraction coupling system, is under aerobic conditions matched to the contractile capacity of the fibre expressed by its oxidative enzyme activity.
Full text
PDF




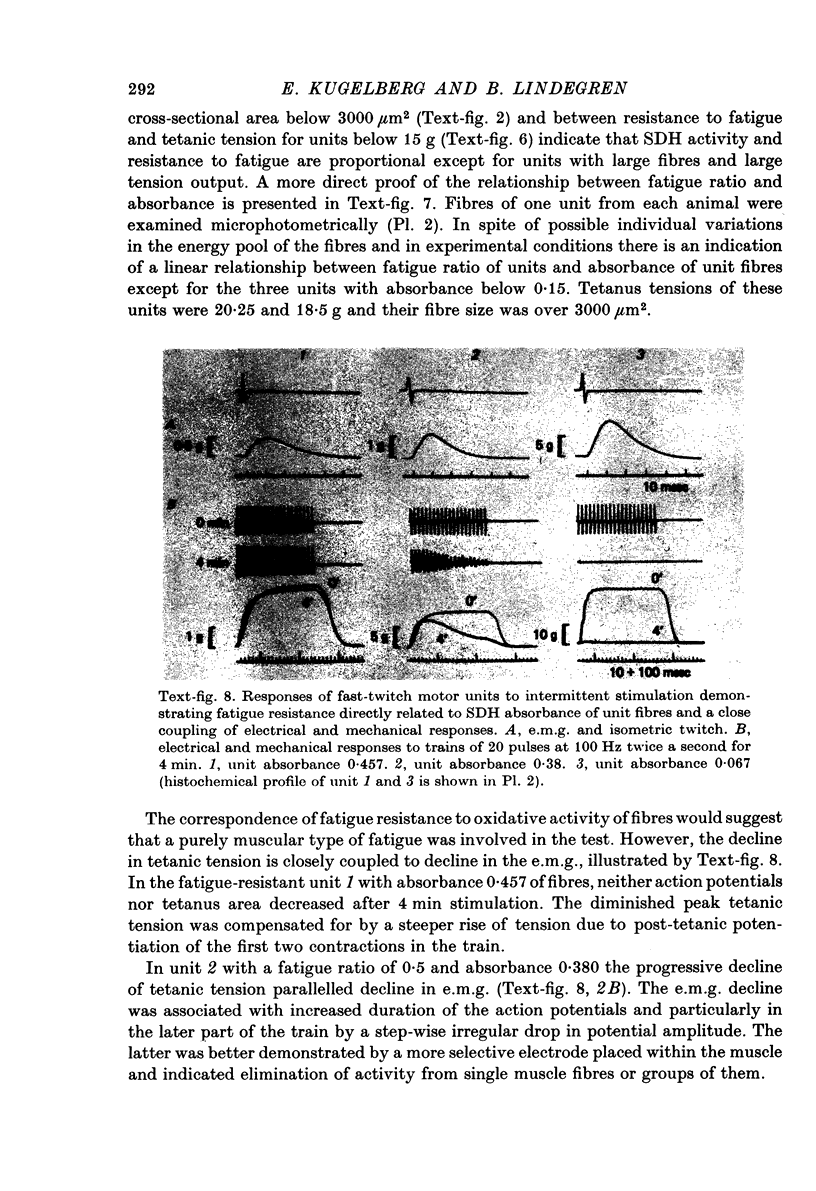







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
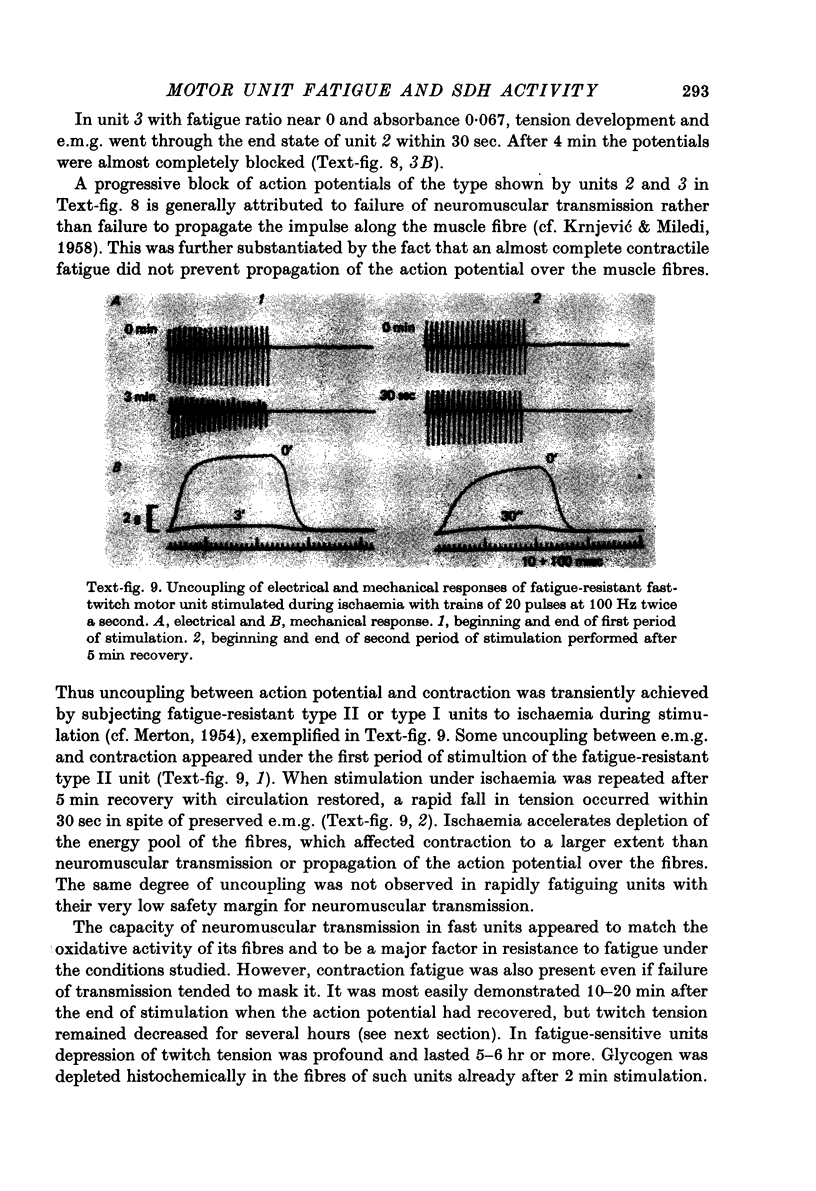
- BIGLAND B., LIPPOLD O. C. Motor unit activity in the voluntary contraction of human muscle. J Physiol. 1954 Aug 27;125(2):322–335. doi: 10.1113/jphysiol.1954.sp005161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKS V. B., THIES R. E. Reduction of quantum content during neuromuscular transmission. J Physiol. 1962 Jul;162:298–310. doi: 10.1113/jphysiol.1962.sp006934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
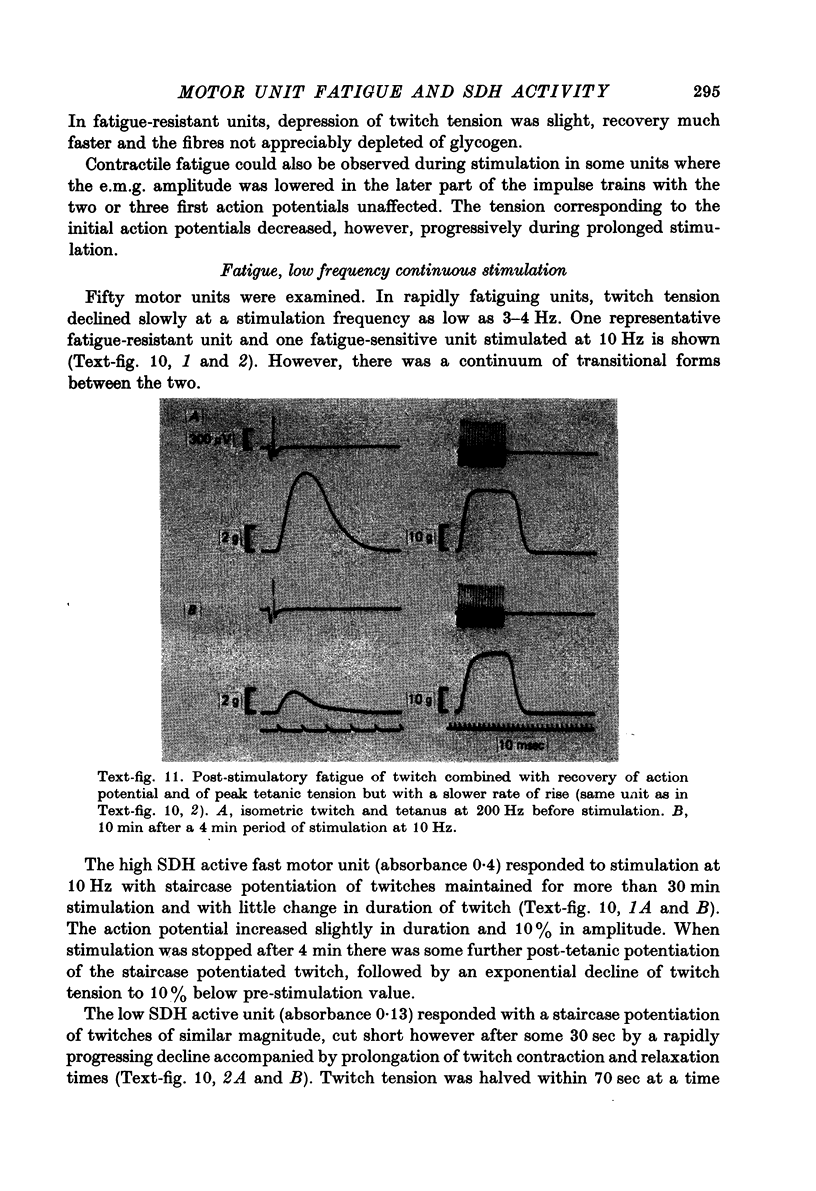
- Close R. Properties of motor units in fast and slow skeletal muscles of the rat. J Physiol. 1967 Nov;193(1):45–55. doi: 10.1113/jphysiol.1967.sp008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eadie M. J., Tyrer J. H., Kukums J. R., Hooper W. D. Aspects of tetrazolium salt reduction relevant to quantitative histochemistry. Histochemie. 1970;21(2):170–180. doi: 10.1007/BF00306184. [DOI] [PubMed] [Google Scholar]
- Edström L., Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink G. Succinic dehydrogenase content of individual muscle fibers at different ages and stages of growth. Life Sci. 1969 Aug 15;8(16):791–808. doi: 10.1016/0024-3205(69)90097-6. [DOI] [PubMed] [Google Scholar]
- Goldspink G., Waterson S. E. The effect of growth and inanition on the total amount of nitroblue tetrazolium deposited in individual muscle fibres of fast and slow rat skeletal muscle. Acta Histochem. 1971;40(1):16–22. [PubMed] [Google Scholar]
- Grabowski W., Lobsiger E. A., Lüttgau H. C. The effect of repetitive stimulation at low frequencies upon the electrical and mechanical activity of single muscle fibres. Pflugers Arch. 1972;334(3):222–239. doi: 10.1007/BF00626225. [DOI] [PubMed] [Google Scholar]
- Hammarberg C., Kellerth J. O. Studies of some twitch and fatigue properties of different motor unit types in the ankle muscles of the adult cat. Acta Physiol Scand. 1975 Nov;95(3):231–242. doi: 10.1111/j.1748-1716.1975.tb10047.x. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Failure of neuromuscular propagation in rats. J Physiol. 1958 Mar 11;140(3):440–461. [PMC free article] [PubMed] [Google Scholar]
- Kugelberg E. Adaptive transformation of rat soleus motor units during growth. J Neurol Sci. 1976 Mar;27(3):269–289. doi: 10.1016/0022-510x(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Edström L. Differential histochemical effects of muscle contractions on phosphorylase and glycogen in various types of fibres: relation to fatigue. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):415–423. doi: 10.1136/jnnp.31.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugelberg E. Histochemical composition, contraction speed and fatiguability of rat soleus motor units. J Neurol Sci. 1973 Oct;20(2):177–198. doi: 10.1016/0022-510x(73)90029-4. [DOI] [PubMed] [Google Scholar]
- MASHIMA H., MATSUMURA M., NAKAYAMA Y. On the coupling relation between action potential and mechanical response during repetitive stimulation in frog sartorius muscle. Jpn J Physiol. 1962 Jun 15;12:324–336. doi: 10.2170/jjphysiol.12.324. [DOI] [PubMed] [Google Scholar]
- MERTON P. A. Voluntary strength and fatigue. J Physiol. 1954 Mar 29;123(3):553–564. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte J., Pette D. Microphotometric determination of enzyme activity in single cells in cryostat section. II. Succinate dehydrogenase, lactate dehydrogenase and triosephosphate dehydrogenase activities in red, intermediate and white fibers of soleus and rectus femoris muscles of rat. J Histochem Cytochem. 1972 Aug;20(8):577–582. doi: 10.1177/20.8.577. [DOI] [PubMed] [Google Scholar]
- Olson C. B., Swett C. P., Jr Effect of prior activity on properties of different types of motor units. J Neurophysiol. 1971 Jan;34(1):1–16. doi: 10.1152/jn.1971.34.1.1. [DOI] [PubMed] [Google Scholar]
- Pullen A. H. The distribution and relative sizes of three histochemical fibre types in the rat tibialis anterior muscle. J Anat. 1977 Feb;123(Pt 1):1–19. [PMC free article] [PubMed] [Google Scholar]
- STEIN J. M., PADYKULA H. A. Histochemical classification of individual skeletal muscle fibers of the rat. Am J Anat. 1962 Mar;110:103–123. doi: 10.1002/aja.1001100203. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H., Kamieniecka Z. Histochemical fiber typing and staining intensity in cat and rat muscles. J Histochem Cytochem. 1975 Jun;23(6):395–401. doi: 10.1177/23.6.125297. [DOI] [PubMed] [Google Scholar]