Abstract
The interactions of Neisseria meningitidis with cells of the leptomeninges are pivotal events in the progression of bacterial leptomeningitis. An in vitro model based on the culture of human meningioma cells was used to investigate the role of the leptomeninges in the inflammatory response. Following challenge with meningococci, meningioma cells secreted specifically the proinflammatory cytokine interleukin-6 (IL-6), the CXC chemokine IL-8, the CC chemokines monocyte chemoattractant protein 1 (MCP-1) and regulated-upon-activation, normal-T-cell expressed and secreted protein (RANTES), and the cytokine growth factor granulocyte-macrophage colony-stimulating factor (GM-CSF). A temporal pattern of cytokine production was observed, with early secretion of IL-6, IL-8, and MCP-1 followed by later increases in RANTES and GM-CSF levels. IL-6 was induced equally by the interactions of piliated and nonpiliated meningococci, whereas lipopolysaccharide (LPS) had a minimal effect, suggesting that other, possibly secreted, bacterial components were responsible. Induction of IL-8 and MCP-1 also did not require adherence of bacteria to meningeal cells, but LPS was implicated. In contrast, efficient stimulation of RANTES by intact meningococci required pilus-mediated adherence, which served to deliver increased local concentrations of LPS onto the surface of meningeal cells. Secretion of GM-CSF was induced by pilus-mediated interactions but did not involve LPS. In addition, capsule expression had a specific inhibitory effect on GM-CSF secretion, which was not observed with IL-6, IL-8, MCP-1, or RANTES. Thus, the data demonstrate that cells of the leptomeninges are not inert but are active participants in the innate host response during leptomeningitis and that there is a complex relationship between expression of meningococcal components and cytokine induction.
Meningitis, which is an inflammation of the meninges that surround the brain within the skull and the spinal cord within the spinal canal (56), is the most common infection of the central nervous system. In humans, the meninges comprise the pachymenix or dura mater (hard mother) and the leptomeninges, which consist of the arachnoid mater and pia mater together with the trabeculae that traverse the cerebrospinal fluid (CSF)-filled subarachnoid space (SAS) (2, 9, 56). Classical bacterial meningitis is predominantly a leptomeningitis, with little or no involvement of the dura mater or the underlying brain. The bacterial etiology of leptomeningitis is broad, and a wide variety of bacteria that gain access to the SAS can initiate an inflammatory response. Susceptibility to the various causative agents is age dependent, and a distinct group of species affect neonates compared to the species that affect subjects over 1 month old (13). After the neonatal period, the most common bacterial agents that cause pyogenic leptomeningitis are Haemophilus influenzae, Streptococcus pneumoniae, and Neisseria meningitidis (meningococci). In affluent countries, vaccines developed against H. influenzae have virtually eliminated the disease (35); in addition, improved vaccines reduce the burden of disease(s) caused by S. pneumoniae (41). However, leptomeningitis and septicemia caused by meningococci will continue to present serious health problems worldwide.
Meningococci initially colonize the nasopharyngeal mucosa, and the most likely route by which the bacteria enter the blood from the pharynx is via penetration of the epithelium and drainage to regional lymph nodes before they enter the blood. The exact routes and mechanisms by which meningococci enter the CSF are poorly understood, although increasing levels of bacteremia closely correlate with invasion of the CSF, which makes the hematogenous route of spread to the meninges the most probable route. Meningococci are likely to enter the CSF-filled spaces of the SAS via the blood-CSF barrier rather than the blood-brain barrier, which is less relevant in meningitis since the bacteria do not enter the brain parenchyma. It is probable that veins in the SAS, and not the choroid plexi (45), are the primary routes of entry for bacteria into the CSF, since meningococci are unable to penetrate the tight junctions of the choroidal epithelium (36) and ventriculitis is only a late complication of bacterial infection. Due to the absence of opsonophagocytic host defense in the CSF (58), uncontrolled proliferation of meningococci is followed by an inflammatory response, centered on the leptomeninges and the SAS, which is characterized by the liberation of proinflammatory cytokines and chemoattractant cytokines (chemokines) (16, 55, 54).
It is likely that the interactions of meningococci with cells of the leptomeninges are pivotal events in the progression of meningitis. In vivo animal models are inappropriate for studying these interactions, due to significant anatomical differences between the meninges in humans and animals. In addition, in vitro models based on primary cultures of cells from the leptomeninges are not possible (9). Recently, we have established an in vitro model based on culture of cells from benign tumors (meningiomas) of the human meninges; these cells have essentially the same profile of cell markers as normal leptomeningeal cells and show identical patterns of reactivity with meningococci (17). However, little is known about the role of the leptomeninges in initiating and sustaining the intracranial inflammatory response and the potential contributions of individual meningococcal components to cytokine induction. The components that may be involved include major surface antigens of the meningococci, such as pili (38, 53), lipopolysaccharide (LPS) (49), polysaccharide capsule (14), and outer membrane (OM) proteins, including the porins and the Opa (opacity), Opc, and iron-binding proteins (48). A recent study in which microarray technology was used revealed the early expression of several cytokine genes, including those encoding tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-8, in meningioma cells following challenge with whole meningococci; however, the difference in gene expression may not have reflected biological significance, and the secretion of bioactive cytokines was not investigated (57). In the present study, we extended the meningioma cell model to investigate (i) the nature of the proinflammatory cytokines and chemokines induced at the levels of mRNA expression and protein secretion and (ii) the effects of specific meningococcal components on the production of these molecules.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
N. meningitidis strain MC58 (B:15:P1.7,16b; Cap+ Pil+ Opa+ Opc+ LPS+) was isolated from an outbreak of meningococcal infections which occurred in Stroud, Gloucestershire, United Kingdom, in the mid 1980s (33), and variants that differed in the expression of pili were obtained by colony selection by using a stereomicroscope (17). Strain C311, a pilE insertion-deletion mutant of strain C311 (53), and a variant of strain MC58 lacking capsule were obtained from M. Virji, University of Bristol. All strains were grown on proteose-peptone agar at 37°C for 18 h in an atmosphere containing 5% (vol/vol) CO2.
Preparation of OM, LPS-depleted OM, and LPS.
OM of N. meningitidis strain MC58 were prepared by extraction of whole cells with lithium acetate as described by Tinsley and Heckels (47), and LPS-depleted OM were produced by extraction of the OM with 1% (wt/vol) sodium deoxycholate in 1 mM Tris-HCl buffer (pH 8.5) containing 10 mM EDTA (4). LPS was purified from strain MC58 by extraction with hot phenol as described previously (25).
Isolation and culture of human meningothelial meningioma cells.
Culture of meningioma cells of the meningothelial histological subtype was carried out as described previously (17). Fresh meningioma tissue was obtained from surgically removed tumors, blood clots and tissue debris were removed, and the tissue was cut into small pieces (1 mm3) and digested with 0.125% (wt/vol) trypsin-0.02% (wt/vol) EDTA in Hanks balanced salt solution (17). Following digestion, the cells were resuspended in culture medium (Dulbecco's modified Eagle's medium [DMEM] [Gibco] supplemented with 10% [vol/vol] fetal calf serum [FCS], Glutamax [stabilized l-glutamine], 100 IU of penicillin ml−1, 100 IU of streptomycin ml−1, and 10 μg of gentamicin ml−1) and cultured in tissue culture flasks that had previously been coated with collagen (type I from rat tail; 5 μg/cm2; Sigma). Cells were grown in a humid environment with 5% CO2 (vol/vol) at 37°C, and fresh medium was added every 3 days. The cultures were expanded and passaged up to eight times with no obvious signs of senescence, and they were stored frozen at −135°C in culture medium containing 10% (vol/vol) dimethyl sulfoxide (17). The cell cultures were characterized by immunohistochemical staining by using antibodies against the specific cellular markers of desmosomal desmoplakin, epithelial membrane antigen, vimentin, and cytokeratin as described previously (17). In addition, the absence of staining in the monolayers after incubation with antibody to CD68 (Dako) confirmed that macrophages were not present in the cultures.
Challenge of human meningioma cells with bacteria.
Human meningioma cells from passages 3 to 8 were grown to confluence (approximately 5 × 104 cells) on collagen-coated 24-well tissue culture plates in culture medium with antibiotics. Prior to challenge, the cells were growth arrested for 48 h without antibiotics in DMEM-Glutamax containing 0.1% (vol/vol) decomplemented FCS. Challenge experiments were based on the procedures of Hardy et al. and Virji et al. (17, 52). The medium was removed, and the monolayers were washed with phosphate-buffered saline (PBS) containing 0.1% (vol/vol) decomplemented FCS and then incubated with concentrations of meningococci (1.0 ml per monolayer) ranging from low (approximately 2.5 × 102 CFU ml−1) to intermediate (approximately 2.5 × 104 and 2.5 × 106 CFU ml−1) to high (approximately 2.5 × 108 CFU ml−1), each prepared in DMEM-Glutamax containing 0.1% (vol/vol) decomplemented FCS. During the experiments, samples of each bacterial variant were removed from triplicate wells at intervals for up to 48 h. Supernatant samples were removed and stored at −20°C for the cytokine immunoassay. In order to quantify total bacterial CFU associated with the meningioma cells, washed monolayers were lysed by incubation with 1% (wt/vol) saponin in PBS containing 0.1% (vol/vol) decomplemented FCS for 15 min, and organisms were quantified by viable counting (17). The relative associations of different bacterial variants were evaluated by using one-way analysis of variance to compare the levels of significance between mean values; a P value of <0.05 was considered significant (17).
Incubation of human meningioma cells with OM, LPS-depleted OM, and pure LPS.
Human meningioma cells were grown and growth arrested as described above, and they were incubated with various concentrations of OM (0.01 to 100 μg/monolayer), LPS-depleted OM (0.1 to 100 μg/monolayer), and pure LPS (0.001 to 1.0 μg/monolayer). During the experiments, samples of each test concentration were removed from triplicate wells at intervals for up to 48 h, and supernatant samples were stored at −20°C for cytokine immunoassays.
Viability of human meningioma cells.
The viability of human meningioma cells following different treatments was assessed with the LIVE/DEAD reduced biohazard viability-cytotoxicity assay from Molecular Probes, used according to the manufacturer's instructions. All meningioma cell monolayers were examined with a Leica model TCS 4D confocal microscope (Leitz).
RNA isolation and reverse transcriptase PCR.
Total RNA was isolated from triplicate wells (in 24-well culture plates) by using Trizol reagent (Gibco BRL) according to the manufacturer's instructions, and 1 μg was reverse transcribed into single-stranded cDNA in a reaction mixture (40 μl) containing oligo(dT) (200 ng), Superscript RNase H− reverse transcriptase (200 U; Gibco BRL), each deoxynucleoside triphosphate (Promega) at a concentration of 0.5 mM in the presence of RNase inhibitor (32 U; Promega), 0.01 M dithiothreitol (Gibco BRL), and 0.1 mg of acetylated bovine serum albumin (Promega). Control cDNA preparations (without reverse transcriptase) were also prepared in the absence of the Superscript enzyme. Aliquots of the cDNA preparations were made and stored at −20°C. Amplification of cytokine and chemokine cDNA was carried out with the primer pairs (20 to 30 pmol/μl) described in Table 1 (22) in the presence of Taq DNA polymerase enzyme (1 U; Promega), each deoxynucleoside triphosphate (Promega) at a concentration of 0.01 M, and various concentrations of MgCl2. The temperature profile for the amplification reaction consisted of 30 cycles of denaturation at 94°C for 15 s, followed by annealing for 15 s at the temperatures described in Table 1 and extension at 72°C for 5.5 min. As positive controls for the cytokine reactions, cDNA prepared from concanavalin A-stimulated peripheral blood mononuclear cells and from the HT-29, HUT78, and CASKI cell lines, all of which constitutively express several of the factors, were used. PCR products were visualized after agarose gel electrophoresis in the presence of VistraGreen (1/10,000, vol/vol; Amersham) by scanning chemifluorescent signals with a Storm 860 imager (Molecular Dynamics). Semiquantification of data was performed with ImageQuant software, and cytokine and chemokine signals were standardized by calculation of signal ratios to the glyceraldehyde-3-phosphate dehydrogenase housekeeping gene, which was assayed in every cDNA preparation.
TABLE 1.
Oligonucleotide primers, conditions, and product sizes for cytokine and chemokine reverse transcriptase PCR
| Factor | Direction | Sequence | Annealing temp (°C) | MgCl2 concn (mM) | Product size (bp) |
|---|---|---|---|---|---|
| IL-1αa | Sense | 5′-GTCTCTGAATCAGAAATCCTTCTATC-3′ | 55 | 1.0 | 420 |
| Antisense | 5′-CATGTCAAATTTCACTGCTTCATCC-3′ | ||||
| IL-1βa | Sense | 5′-AAACAGATGAAGTGCTCCTTCCAGG-3′ | 55 | 1.0 | 388 |
| Antisense | 5′-TGGAGAACACCACTTGTTGCTCCA-3′ | ||||
| IL-6a | Sense | 5′-ATGAACTCCTTCTCCACAAGCGC-3′ | 50 | 1.0 | 628 |
| Antisense | 5′-GAAGAGCCCTCAGGCTGGACTG-3′ | ||||
| IL-8a | Sense | 5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ | 50 | 1.0 | 289 |
| Antisense | 5′-TCTCAGCCCTCTTCAAAAACTTCTC-3′ | ||||
| IL-10 | Sense | 5′-GCTCTGTTGCCTGGTCCTCCTGACT-3′ | 65 | 1.5 | 289 |
| Antisense | 5′-GATGCCCCAAGCTGAGAACCAAGAC-3′ | ||||
| IL-12a | Sense | 5′-ATGTCGTAGAATTGGATTGGTATCCG-3′ | 50 | 2.0 | 358 |
| Antisense | 5′-GTACTGATTGTCGTCAGCCACCAGC-3′ | ||||
| GM-CSF | Sense | 5′-CAGCACGCAGCCCTGGGAGCATGTG-3′ | 55 | 1.5 | 253 |
| Antisense | 5′-CCGGGGTTGGAGGGCAGTGCTGCTT-3′ | ||||
| MCP-1a | Sense | 5′-TCTGTGCCTGCTGCTCATAGC-3′ | 50 | 1.0 | 510 |
| Antisense | 5′-GGGTAGAACTGTGGTTCAAGAGG-3′ | ||||
| MIP-1α | Sense | 5′-CAGGTCTCCACTGCTGCC-3′ | 50 | 3.0 | 252 |
| Antisense | 5′-CACTCAGCTCCAGGTCACT-3′ | ||||
| MIP-1β | Sense | 5′-AATACCATGAAGCTCTGCCTG-3′ | 63 | 1.5 | 441 |
| Antisense | 5′-TGACACCTAATACAATAACACGGC-3′ | ||||
| RANTES | Sense | 5′-ATGAAGGTCTCCGCGGCACGCCTCGCTGTC-3′ | 45 | 1.5 | 252 |
| Antisense | 5′-CTAGCTCATCTCCAAAGAGTTGAT-3′ | ||||
| TGFβ | Sense | 5′-GCCGACTACTACGCCAAGGAGGTCA-3′ | 63 | 1.5 | 251 |
| Antisense | 5′-AGCAACAATTCCTGGCGATACCTCA-3′ | ||||
| TNFα | Sense | 5′-GCGGTGCTTGTTCCTGAGCCTCTTC-3′ | 63 | 1.5 | 284 |
| Antisense | 5′-CAATGGCGTGGAGCTGAGAGATAACCA-3′ | ||||
| GAPDHb | Sense | 5′-GGGAAGGTGAAGGTCGGAGT-3′ | 60 | 2.0 | 229 |
| Antisense | 5′-TGGAAGATGGTGATGGGATTTC-3′ |
Primer sequences derived from the study of Jung et al. (22).
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Measurement of cytokine and chemokine protein production.
The levels of cytokine and chemokine proteins produced after bacterial challenge of meningioma cells were quantified by a sandwich immunoassay by using matched pairs of specific antibodies. The wells of 96-well FluoroNunc Maxisorp immunoassay plates were coated, by incubation at room temperature for 16 h, with 100-μl solutions (2 to 4 μg ml−1) of the capture monoclonal antibodies in sodium carbonate buffer (pH 9.6). The antibodies used were directed against the proinflammatory cytokine mediators IL-1α, IL-1β, IL-6, and TNF-α (all from R&D Systems); the anti-inflammatory cytokines IL-10 and transforming growth factor β (TGF-β) (R&D); the growth factor granulocyte-macrophage colony stimulating factor (GM-CSF) (Biosource International); the C-C chemokine family members monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and regulated-upon-activation, normal-T-cell expressed and secreted protein (RANTES) (R&D); and the C-X-C family chemokine IL-8 (R&D). After the wells were washed with 25 mM Tris-phosphate buffer (pH 8.0) containing 100 mM NaCl and 0.05% (vol/vol) Tween 20, they were blocked by incubation with PBS containing 1% (wt/vol) bovine serum albumin and 5% (wt/vol) sucrose for 1 h at 37°C. Samples of culture medium were diluted with proprietary assay buffer (Delfia; Wallac) and added to immunoassay wells. Matched biotinylated detector antibodies (0.5 to 20 ng ml−1) were added to the wells and incubated for 2 h at 37°C. For measurement of bound biotin-labeled antibodies, a time-resolved fluorimetry system (Delfia; Wallac) was used (5). Europium (Eu)-labeled streptavidin (100 ng ml−1) was added to each well, and following 1 h of incubation, any bound label was detected by the addition of Delfia enhancement solution and subsequent measurement of emitted fluorescence with a 1234 Delfia fluorimeter (Wallac). The concentration of each cytokine or chemokine was determined by comparison with standard solutions of the corresponding purified recombinant protein (Peprotech) treated similarly. A two-sample t test was used to compare the mean levels of cytokine secretion following certain treatments, and a P value of <0.05 was considered significant.
RESULTS
Profile of cytokine and chemokine production by human meningioma cells challenged with N. meningitidis.
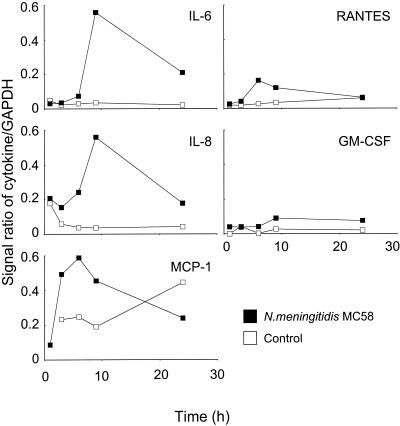
Human meningioma cells of the meningothelial subtype are thought to resemble normal leptomeningeal cells closely. In order to investigate the role of cells of the leptomeninges in the inflammatory response, meningothelial meningioma cell lines were established from three different patients. Meningeal cell lines were characterized by phase-contrast microscopy and immunocytochemical analysis with antibodies directed against specific cellular markers and by the similar quantitative patterns of adherence of meningococci in challenge experiments over time (17). The N. meningitidis that is normally recovered from infected CSF is both capsulated (Cap+) and piliated (Pil+) (6, 44). The meningioma cell lines were challenged with a high dose (approximately 2.5 × 108 CFU per monolayer) of Cap+ Pil+ N. meningitidis strain MC58, and reverse transcriptase PCR was used to screen for up-regulation of a panel of proinflammatory cytokines and chemokines. Compared to the results obtained with control uninfected cells, challenge with meningococci induced significant up-regulation of mRNA for the proinflammatory cytokine IL-6 and the chemokines IL-8 and MCP-1 and lower levels of the chemokine RANTES and the cytokine growth factor GM-CSF (Fig. 1). In general, maximum expression of mRNA was observed between 6 and 9 h after challenge, and expression declined by 24 h. In contrast, little or no significant mRNA for IL-1α, IL-1β, TNF-α, MIP-1α, MIP-1β, TGF-β, IL-10, or IL-12 was detected in cells challenged with meningococci.
FIG. 1.
Cytokine and chemokine mRNA expression in human meningioma cells challenged with 2.5 × 108 CFU of N. meningitidis parent strain MC58 (Cap+ Pil+ Opa+ Opc+ LPS+). The data are median values for five independent experiments performed with three different meningothelial meningioma cell lines. GAPDH, glyceraldehyde-3-phosphate dehygrogenase.
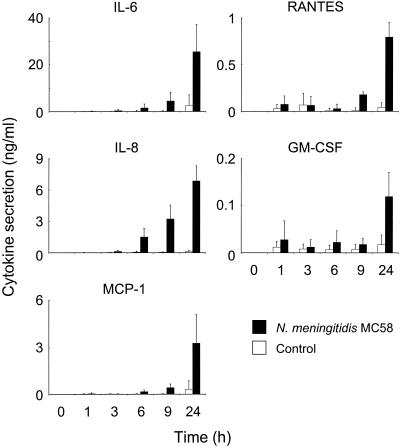
The accumulation of individual cytokine and chemokine proteins was also measured at each of the time points during the experiments. There was a positive correlation between up-regulation of mRNA expression and secretion of protein, which continued to increase after the decline in mRNA and reached the maximum level 24 h after challenge (Fig. 2). Challenge with meningococci induced high levels of IL-6 (25 ± 12 ng/ml), which were approximately 10-fold greater than the levels observed with control cells. Smaller amounts of IL-8 (7 ± 1 ng/ml) and MCP-1 (3 ±2 ng/ml) were secreted, but these amounts were approximately 58- and 10-fold greater, respectively, than the amounts in the control cells (Fig. 2). In addition, although the levels of RANTES (1 ± 0.2 ng/ml) and GM-CSF (0.2 ± 0.05 ng/ml) were the lowest levels detected, they were still approximately 18- and 7-fold higher, respectively, than the control levels (Fig. 2). As expected, in the absence of mRNA message, no protein secretion was observed for IL-1α, IL-1β, TNF-α, MIP-1α, MIP-1β, TGF-β, IL-10, or IL-12 cytokine.
FIG. 2.
Cytokine and chemokine protein secretion by human meningioma cells challenged with 2.5 × 108 CFU of N. meningitidis parent strain MC58 (Cap+ Pil+ Opa+ Opc+ LPS+). The data are mean cytokine levels, and the error bars indicate the standard errors of the means of five independent experiments performed with three different meningothelial meningioma cell lines.
The reproducibility of the assays was demonstrated by the similar results obtained in five independent experiments performed with the three different meningothelial meningioma cell lines. Therefore, subsequent experiments were carried out with one representative cell line. In addition, the viability of meningioma cell monolayers challenged with the high dose of meningococci was investigated. Even after 48 h of bacterial challenge, the confluent monolayers were intact (still containing approximately 5 × 104 cells), and cell viability was >99% (data not shown). Thus, meningioma cells in vitro were resistant to killing by meningococci, even after prolonged contact with a high concentration of the bacteria.
Role of pili in modulating cytokine production by human meningioma cells.
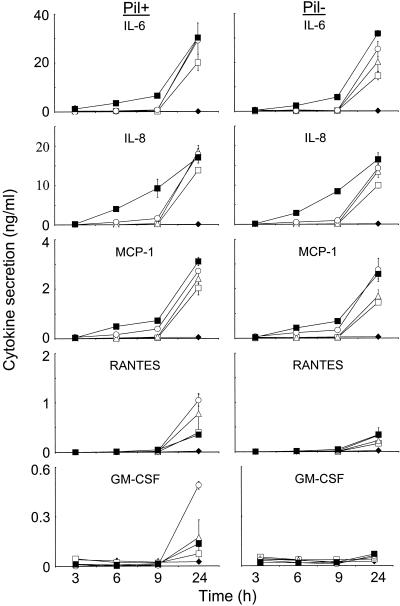
The role of pili in modulating cytokine and chemokine production by meningioma cells was determined by investigating the dose- and time-dependent kinetics of protein secretion induced by piliated (Pil+) and nonpiliated (Pil−) variants of capsulated (Cap+) strain MC58. Meningothelial meningioma cells were challenged with various concentrations of meningococci in order to mimic the events that may occur in the SAS when initial low levels of entering bacteria are followed by unchecked proliferation. Regardless of the concentration of the bacterial inoculum, Pil+ meningococci always showed significantly greater adherence (P < 0.05) to meningioma cells than the corresponding Pil− variant showed, in accordance with previous observations (17). In addition, the cell monolayers remained viable and intact throughout the experiments following challenge with the various concentrations of either Pil+ or Pil− meningococci.
The dose-dependent kinetics of cytokine and chemokine protein secretion by meningioma cells following challenge with Pil+ and Pil− meningococci are shown in Fig. 3. The highest concentration of bacteria induced early increases in the IL-6 and IL-8 levels, which at 6 and 9 h were approximately 10-fold higher than those induced by the other challenge concentrations. A significant time lag was observed with the intermediate and lower challenge concentrations, with secretion occurring between 9 and 24 h after challenge. However, by 24 h the levels of cytokine accumulation induced by the various concentrations of bacteria were essentially similar (P > 0.05). Significantly, there was no difference in the kinetics and quantitative production of both IL-6 and IL-8 from cells challenged with Pil+ meningococci and cells challenged with Pil− meningococci (P > 0.05) (Fig. 3). The highest concentration of bacteria induced only an approximately twofold increase in MCP-1 at the early time points compared with the level induced by the intermediate dose (2.5 × 106 CFU) but more-than-10-fold increases compared with the levels induced by the lower challenge concentrations. However, as was observed with IL-6 and IL-8, the levels of MCP-1 accumulation at 24 h were comparable (P > 0.05), regardless of the concentration of the bacterial inoculum and the expression of pili (Fig. 3).
FIG. 3.
Role of pili in modulating cytokine production by human meningioma cells. Meningothelial meningioma cells were challenged with the following concentrations of piliated (Pil+) and nonpiliated (Pil−) strain MC58: 2.5 × 102 CFU per monolayer □, 2.5 × 104 CFU per monolayer ▵, 2.5 × 106 CFU per monolayer ○, and 2.5 × 108 CFU per monolayer ▪. Control wells contained culture medium alone ♦. The data are the mean levels of cytokine and chemokine secretion in triplicate wells, and the error bars indicate the standard deviations.
Different dose-dependent kinetics of secretion were observed for RANTES and GM-CSF. A time lag in secretion of RANTES was observed with all of the challenge concentrations of Pil+ and Pil− meningococci, with significant secretion at levels above control levels occurring between 9 and 24 h. Increasing the concentration of the Pil+ variant from 2.5 × 102 to 2 × 106 CFU per monolayer resulted in an approximately two- to threefold increase in RANTES secretion. In addition, at these concentrations Pil+ meningococci induced levels of RANTES that were two to fourfold higher than the levels induced by equivalent concentrations of the Pil− variant. However, an unexpected pattern was observed following challenge with the highest concentration of Pil+ meningococci: this concentration did not stimulate RANTES production at the early time points, and at 24 h the level of secretion was significantly lower (P = 0.006) than the level of secretion induced by the intermediate concentrations of Pil+ meningococci and was similar to the level of secretion obtained with the lowest concentration (Fig. 3). Furthermore, although Pil− meningococci increased RANTES secretion compared to the secretion induced by the control, there were no significant differences in the levels of secretion induced by the various concentrations of Pil− inoculum (P > 0.05).
A time lag in secretion of GM-CSF was also observed with all of the challenge concentrations of Pil+ meningococci, and significant secretion occurred between 9 and 24 h (Fig. 3). In contrast to the RANTES data, challenge with 2 × 106 CFU of the Pil+ variant per monolayer induced approximately three- to sevenfold increases in GM-CSF levels compared with the levels induced by the other concentrations, all of which induced similar levels of secretion. Regardless of the concentration of the bacterial inoculum, Pil− meningococci did not induce secretion of GM-CSF from meningioma cells. Challenge experiments with a variant of N. meningitidis strain C311, which contains an insertion-deletion in pilE and produces no pili, confirmed that Pil− meningococci were unable to induce GM-CSF secretion from meningioma cells (data not shown).
We also investigated whether the reductions in RANTES and GM-CSF levels observed with the highest concentration of Pil+ meningococci were due to an ability of the Pil+ variant, but not the Pil− variant, to adsorb these cytokines from the supernatants. Each variant (>108 CFU) was incubated in the presence of 1 ng of purified RANTES or GM-CSF cytokine per ml for up to 24 h. There were no significant differences (P > 0.05) in the concentrations of each cytokine in supernatant samples from wells containing Pil+ meningococci, wells containing Pil− meningococci, and wells containing no bacteria (data not shown). Therefore, neither Pil+ nor Pil− meningococci adsorbed either RANTES or GM-CSF cytokines from the supernatants.
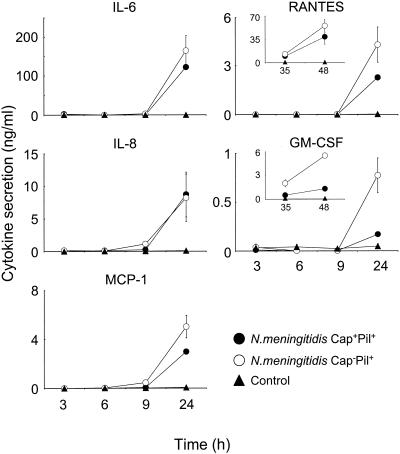
Effect of capsule expression on modulation of cytokine production by human meningioma cells.
Previous studies have shown that Cap− Pil+ meningococci associate more intimately with the surface of meningioma cells than the corresponding Cap+ Pil+ variant does (17). In addition, the intimate association was linked to an apparent increase in the activity of the host cell membrane. The role of capsule expression in modulating the induction of cytokine protein secretion was determined by challenging meningioma cells with the Cap+ Pil+ and Cap− Pil+ variants. Similar kinetics and quantitative secretion of IL-6, IL-8, and MCP-1 were observed with the two variants (Fig. 4). Challenge with Cap− Pil+ meningococci induced a slightly higher level of RANTES secretion at 24 h, but the level was only marginally higher (P = 0.063) than the level observed with the Cap+ Pil+ variant (Fig. 4). In contrast, Cap− meningococci induced fivefold-higher production of GM-CSF than the Cap+ variant induced at 24 h (P = 0.033). In order to confirm whether the increases were statistically significant and to determine whether RANTES and GM-CSF secretion occurred later than secretion of the other cytokines, supernatant samples were also collected at intervals up to 48 h after challenge. Figure 4 (inset) clearly shows that RANTES production increased with time, and the data confirmed that there was no significant difference between the levels of secretion induced by the Cap− Pil+ and Cap+ Pil+ variants (P = 0.135). In contrast, the Cap− Pil+ variant did induce significantly (P = 0.003) greater amounts of GM-CSF than the Cap+ Pil+ variant, and the amounts were still increasing at 48 h after challenge (Fig. 4, inset).
FIG. 4.
Effect of capsule expression on cytokine production by human meningioma cells. Meningothelial meningioma cells were challenged with approximately 2.5 × 104 CFU of capsulated (Cap+) and noncapsulated (Cap−) variants of Pil+ strain MC58 meningococci per monolayer. Control wells contained culture medium alone. The data are the mean concentrations of cytokine and chemokine protein secreted from triplicate wells, and the error bars indicate the standard deviations.
As was observed in all other challenge experiments with viable meningococci, meningioma cell monolayers were still viable and completely intact after 48 h of challenge with either a Cap+ or Cap−variant (data not shown).
Effect of OM and LPS on modulation of cytokine production by human meningioma cells.
OM and purified LPS induced secretion of IL-6, IL-8, MCP-1, and RANTES (Table 2). There were no significant differences in the levels of IL-6 secretion induced by the various concentrations of either OM or LPS, and the levels were approximately 20- to 40-fold lower than the levels observed with viable bacteria. The levels of IL-8 secretion induced by either OM or purified LPS were also similar within the range of concentrations tested but were only approximately two- to threefold lower than the levels induced by viable bacteria. In contrast, the various concentrations of OM, purified LPS, and viable meningococci all induced similar amounts of MCP-1 (Table 2). Although increased concentrations of OM resulted in increased levels of RANTES secretion, these levels were approximately 5- to 40-fold lower the levels induced by viable bacteria; however, purified LPS induced levels of RANTES similar to the levels induced by viable bacteria. In contrast to the other cytokines and chemokines, neither OM nor purified LPS induced significant levels of GM-CSF compared with the levels induced by viable bacteria (Table 2).
TABLE 2.
Effects of OM and purified LPS on cytokine and chemokine secretion by human meningioma cellsa
| Challenge | Doseb | Cytokine levels (ng/ml)b
|
||||
|---|---|---|---|---|---|---|
| IL-6 | IL-8 | MCP-1 | RANTES | GM-CSF | ||
| N. meningitidis MC58 | 2.5 × 104 -2.5 × 106 | 30c | 18c | 2.4-2.7c | 0.8-1.5c | 0.2-0.5c |
| OM (MC58) | 1.0 | 1.0 ± 0.2d | 6.3 ± 0.6 | 3.8 ± 0.6 | 0.3 ± 0.08 | NDe |
| 0.1 | 1.4 ± 0.1 | 6.3 ± 0.6 | 4.6 ± 1.3 | 0.06 ± 0.03 | ND | |
| 0.01 | 0.7 ± 0.2 | 6.1 ± 1.7 | 2.9 ± 0.9 | 0.02 ± 0.02 | ND | |
| LPS (MC58) | 1.0 | 1.5 ± 0.2 | 6.8 ± 0.6 | 2.2 ± 0.1 | 2.1 ± 0.5 | 0.09 ± 0.02 |
| 0.1 | 1.3 ± 0.3 | 6.4 ± 1.5 | 2.3 ± 0.1 | 2.5 ± 0.6 | 0.04 ± 0.02 | |
| 0.01 | 1.3 ± 0.3 | 8.5 ± 2.6 | 2.3 ± 0.2 | 2.2 ± 0.4 | 0.07 ± 0.07 | |
| 0.001 | 0.5 ± 0.02 | 5.7 ± 0.3 | 2.2 ± 0.2 | 0.97 ± 0.2 | 0.02 ± 0.002 | |
| Control (medium only) | 0.03 | 0.19 | 0.11 | 0.02 | 0.02 | |
Data for OM and purified LPS are compared to the levels of cytokine secretion induced by the intermediate concentrations of Pil+ bacteria used to challenge meningioma cells (see Fig. 3); at these concentrations the relative amounts of LPS in all the preparations were similar (18, 30).
Doses are given in micrograms per milliliter, except for the N. meningitidis MC58 dose, which is given in CFU per milliliter; cytokine levels measure protein accumulation at 24 h.
Level of protein secreted by cells challenged with viable bacteria.
The data are means ± standard deviations based on triplicate wells.
ND, not detected.
To investigate further the contribution of LPS to the induction of cytokine and chemokine secretion, meningioma cells were incubated with OM from strain MC58, which had been depleted of LPS (Table 3). At a concentration of 0.1 μg of protein per ml, the levels of IL-6, IL-8, MCP-1, and RANTES induced by LPS-depleted OM were reduced by 80 to 100% compared with the values observed for cells incubated with native OM (Table 3). However, when higher concentrations of LPS-depleted OM were used (10 to 100 μg/monolayer), increased production of IL-6, IL-8, MCP-1, and RANTES was observed, which in many cases was similar to that observed with native OM (Table 3); this was probably due to the stimulatory effect of increasing levels of residual LPS in the LPS-depleted OM preparation. Again, small amounts of GM-CSF were observed with both OM and LPS-depleted OM preparations (Table 3).
TABLE 3.
Effects of OM and LPS-depleted OM preparations on cytokine production by human meningioma cells
| Prepn | Concn (μg/ml) | Cytokine levels (ng/ml)a
|
||||
|---|---|---|---|---|---|---|
| IL-6 | IL-8 | MCP-1 | RANTES | GM-CSF | ||
| MC58 OM | 100 | 3.0 ± 0.1 | 5.9 ± 1.0 | 2.5 ± 0.1 | 0.3 ± 0.02 | 0.14 ± 0.01 |
| 10 | 2.1 ± 0.07 | 6.4 ± 0.8 | 2.4 ± 0.4 | 0.7 ± 0.07 | 0.13 ± 0.01 | |
| 0.1 | 0.5 ± 0.07 | 5.7 ± 1.1 | 2.1 ± 0.2 | 0.3 ± 0.09 | 0.05 ± 0.02 | |
| MC58 LPS-depleted OM | 100 | 1.5 ± 0.5 | 6.3 ± 1.3 | 2.3 ± 0.13 | 1.0 ± 0.1 | 0.04 ± 0.01 |
| 10 | 0.4 ± 0.04 | 5.0 ± 0.4 | 2.0 ± 0.08 | 0.3 ± 0.1 | 0.04 ± 0 | |
| 0.1 | 0.02 ± 0.02 | 0.2 ± 0.04 | 0.4 ± 0.01 | NDb | 0.01 ± 0.01 | |
| Control (medium only) | ND | 0.07 ± 0 | 0.176 ± 0.05 | ND | 0.02 ± 0.02 | |
Protein accumulation at 24 h. The data are means ± standard deviations based on triplicate wells.
ND, not detected.
During these experiments, the meningioma cell monolayers were also examined by microscopy and were found to be intact, demonstrating that incubation with OM or LPS had no significant effect on cell viability (data not shown).
DISCUSSION
In this study, meningioma cells challenged with N. meningitidis were found to secrete a specific subset of proinflammatory and chemoattractant cytokines, and several surface components of the bacteria were also identified as factors that contribute to activation of this inflammatory response. The major proinflammatory cytokines that have been detected in the CSF of patients with bacterial meningitis are IL-6, IL-1β, and TNF-α (27, 32, 50, 51, 55), and elevated levels of these cytokines are found early during the intracranial inflammatory response. Following challenge with N. meningitidis, meningioma cells produce large amounts of IL-6, suggesting that the leptomeninges is likely to be a major producer of this cytokine. In contrast, meningioma cells did not secrete IL-1β and TNF-α, which implies that other cells in the SAS are sources of these two cytokines. Production of IL-6 by meningioma cells was induced equally by the interactions of Pil+ and Pil− meningococci, despite the clear association between pilus expression and increased bacterial adhesion to meningioma cells. In addition, OM and purified LPS were poor stimulators of IL-6 production compared with viable bacteria, suggesting that LPS had a minimal effect on IL-6 secretion by meningioma cells and that other, possibly secreted, bacterial components were responsible. Although these modulins remain to be identified, recent studies have shown that purified immunoglobulin A1 protease and peptidoglycan fragments, both of which are secreted by pathogenic neisseriae (7, 29), up-regulate IL-6 production by peripheral blood mononuclear cells.
In the present study, meningioma cells challenged with N. meningitidis secreted large amounts of the CXC chemokine IL-8 and the CC chemokines MCP-1 and RANTES, as well as the cytokine growth factor GM-CSF. This is consistent with the presence of elevated levels of IL-8, MCP-1, and RANTES observed in the CSF of patients with bacterial meningitis (24, 28, 31, 42, 43), implying that the leptomeninges plays a critical role in the recruitment of inflammatory cells into the SAS. In addition, other chemokines (namely, growth-related oncogene alpha, MIP-1α, MIP-1β, and the GM-CSF-related molecules granulocyte colony-stimulating factor and macrophage colony-stimulating factor) have also been observed in patients with leptomeningitis (11, 21, 40, 42, 43). However, meningioma cells did not secrete these chemokines and growth factors, which implicates other cells in the SAS in their production during the inflammatory response.
During the course of leptomeningitis, the large numbers of meningococci found in the CSF and associated with the leptomeninges release high levels of LPS, probably in the form of OM vesicles (44). Furthermore, high concentrations of intracranial LPS have been associated with increased chemokine and cytokine production, disease severity, and a poor neurological outcome for the patient (3, 55). In the present study, a temporal pattern of chemokine production by meningioma cells was observed following challenge with meningococci, with early secretion of IL-8 and MCP-1 followed by later increases in RANTES levels. Both IL-8 and MCP-1 were induced equally by the interactions of Pil+ and Pil− meningococci, despite differences in the adhesion of these variants to meningioma cells. The abilities of OM and purified LPS preparations to induce similar levels of MCP-1 secretion from meningioma cells, coupled with the significant reduction observed with LPS-depleted OM, suggested that LPS was an important stimulus for production of this chemokine. In addition, LPS was also sufficient for IL-8 secretion, although the levels induced by OM and purified LPS were less than those observed with viable bacteria, suggesting that other bacterial components contribute to production of this chemokine. These observations indicate that induction of MCP-1 and IL-8 secretion may be due to release of LPS and other components from meningococci in free suspension in the CSF and is not dependent on adherence of bacteria to the leptomeninges. In contrast, whereas secretion of RANTES was also induced by Pil+ meningococci, OM, and pure LPS, Pil− meningococci induced secretion of lower levels of this chemokine than the levels induced by the Pil+ variant. In addition, reduction of LPS from OM also resulted in lower levels of RANTES production. Therefore, it is likely that efficient stimulation of RANTES production by intact meningococci requires pilus-mediated adherence, which may deliver increased local concentrations of LPS and other components to the surface of meningeal cells. This is in accord with observations made with type 1 pili and fimbriae of Escherichia coli, which have been reported to augment cytokine responses by delivering LPS-dependent activation signals to epithelial cells (19, 39).
The present data indicate that cells of the leptomeninges play a pivotal role in the innate immune response to meningococcal infection in the SAS. Significantly, the response to LPS is characterized by the production of chemokines rather than proinflammatory cytokines. This was further demonstrated by the fact that purified LPS or LPS-containing OM did not induce significant production of the cytokine growth factor GM-CSF. Secretion of this cytokine, which was observed to occur later in the inflammatory response, was dependent on pilus-mediated adhesion and was also influenced by expression of capsule by the bacteria. A direct association between adhesion to host cells, mediated by pili and fimbriae, and the production of cytokines has been reported for other bacteria. Pilus-mediated adherence of Neisseria gonorrhoeae has been shown to up-regulate the secretion by epithelial cells of GM-CSF, as well as IL-8 and TNF-α (5, 34). In addition, the specific interaction of P-fimbriae from E. coli has been reported to increase the production of IL-6 by epithelial cells (20), and this activation was independent of the LPS-CD14 signaling pathway (10). In the present study, despite the ability of Pil+ meningococci to induce GM-CSF secretion, the expression of capsule had an inhibitory effect. This effect of capsule was specific to GM-CSF, since the expression did not modulate the production of IL-6, IL-8, MCP-1, or RANTES. In addition, capsulated and noncapsulated meningococci adhered similarly to meningioma cells, confirming previous observations that capsule expression does not modulate pilus-mediated adherence to these cells (17). Therefore, it is likely that increased GM-CSF production was the result of stimulation by OM components that are more exposed in the absence of capsule. Since N. meningitidis isolated from CSF is invariably capsulated, these data demonstrate that in addition to its primary function of conferring resistance to opsonophagocytosis in the blood, the meningococcal capsule also acts as a virulence factor in the SAS by inhibiting the production of GM-CSF. One consequence of this effect may be to reduce the phagocytic activities of macrophages during the later stages of leptomeningeal inflammation.
An unexpected finding of this study was that high concentrations (>108 CFU) of Pil+ meningococci resulted in reduced secretion of both RANTES and GM-CSF by meningioma cells. This was not due to an ability of Pil+ meningococci to adsorb these cytokines from the supernatant. At least two hypotheses can be presented to account for these observations, and they are not mutually exclusive. First, autoagglutination of Pil+ meningococci may have competed with the ligand-receptor interaction on the meningioma cell surface, and this effect may have been exacerbated by autolysis of dead bacteria following nutrient stress. Second, it is also possible that a negative regulatory mechanism was induced by the other cytokines secreted during the response.
The data obtained in this study are consistent with the limited information available regarding the sequence of events that occur in patients during the course of leptomeningitis. Following invasion, uncontrolled proliferation of meningococci is observed in the SAS of patients, and our study suggests that the interactions of bacterial components with cells of the leptomeninges induce early secretion of IL-6, IL-8, and MCP-1, followed by later secretion of RANTES and GM-CSF. This secretion is consistent with the elevated levels of these cytokines found in the CSF of patients and their role in effecting multiple biological and physiological changes in the SAS. The secretion of IL-8 by meningeal cells observed in the present study is also consistent with the role of IL-8 in the in vivo chemotaxis of inflammatory cells in the CSF. A direct correlation between IL-8 and chemotaxis has been demonstrated by the ability of antibodies to IL-8 to reduce the in vitro chemotaxis of polymorphonuclear leukocytes (PMNL) mediated by a sample of CSF from a patient with meningococcal meningitis (42). In patients, the trafficking of PMNL into the SAS that is observed during the early phase of infection is believed to be stimulated by up-regulation of cell adhesion molecules on the endothelium of blood vessels by IL-8, as well as by RANTES, MIP-1α, and MIP-1β (1, 8). In addition, it is also likely that the direct binding of IL-8 to selective receptors (IL-8RA) on human PMNL also mediates chemotaxis. In the later stages of the disease, the pattern of cell infiltration into the CSF of patients is observed to gradually change to mononuclear cells (24). It is likely that monocyte accumulation in the SAS is effected by MCP-1, produced in part by the leptomeninges, and the coordinated chemotactic activity of macrophage colony-stimulating factor and MIP-1α, which are likely to be secreted by other cells, as suggested by a study of children with meningitis (21). In addition, the later production of GM-CSF by meningeal cells would be consistent with the role of GM-CSF as a maturation factor for monocytes (12) entering the SAS of patients during this reconstitution phase.
During the course of leptomeningitis, significant cell and tissue injury is observed in the SAS, and this is likely to result in part from the secretion of proinflammatory cytokines and chemokines by meningeal cells. The secretion of high levels of IL-6, which is known to contribute to leukocytosis and induction of the fever response in patients, is consistent with a major role for this cytokine in intracranial inflammation (15, 37). It is likely that PMNL and mononuclear cells recruited into the SAS aggravate the inflammatory response with further secretion of proinflammatory mediators (46) and that activation of these cells contributes to tissue injury through the release of cytotoxic mediators (23, 26). In addition, the proinflammatory nature of the response of the leptomeninges to meningococci would be further exacerbated by the absence of meningeal cell secretion of the anti-inflammatory cytokines IL-10 and TGF-β following bacterial challenge, as observed in this study. Taken together, these biological effects have a significant influence on the neuropathology of leptomeningitis and the eventual prognosis for the patient, which may include permanent neurological sequelae and death.
In summary, cells of the leptomeninges are not inert but are active participants in the inflammatory response, and they play a major role in innate host defense within the SAS during bacterial leptomeningitis. Furthermore, the complex relationship between expression of meningococcal ligands and induction of cytokine release suggests several targets at the molecular level of the bacterium and the host cell for potential anti-inflammatory therapies.
Acknowledgments
This work was supported by grants from the Meningitis Trust and the University of Southampton Strategic Development Fund. M. I. Fowler is the recipient of an MRC studentship.
Editor: J. D. Clements
REFERENCES
- 1.Adams, D. H., and A. R. Lloyd. 1997. Chemokines: leucocyte recruitment and activation of cytokines. Lancet 349:490-495. [DOI] [PubMed] [Google Scholar]
- 2.Alcolado, R., R. O. Weller, E. P. Parrish, and D. Garrod. 1988. The cranial arachnoid and pia mater in man: anatomical and ultrastructural observations. Neuropathol. Appl. Neurobiol. 14:1-17. [DOI] [PubMed] [Google Scholar]
- 3.Brandtzaeg, P., R. Ovstebo, and P. Kierulf. 1992. Compartmentalization of lipopolysaccharide production correlates with clinical presentation in meningococcal disease. J. Infect. Dis. 166:650-652. [DOI] [PubMed] [Google Scholar]
- 4.Christodoulides, M., J. L. Brooks, E. Rattue, and J. E. Heckels. 1998. Immunisation with recombinant class 1 outer membrane protein from Neisseria meningitidis: influence of liposomes and adjuvants on antibody avidity, recognition of native protein and the induction of a bactericidal immune response against meningococci. Microbiology 144:3027-3037. [DOI] [PubMed] [Google Scholar]
- 5.Christodoulides, M., J. S. Everson, B. Liu, P. R. Lambden, P. J. Watt, E. J. Thomas, and J. E. Heckels. 2000. Interaction of primary human endometrial cells with Neisseria gonorrhoeae expressing green fluorescent protein. Mol. Microbiol. 35:32-43. [DOI] [PubMed] [Google Scholar]
- 6.Devoe, I. W., and J. E. Gilchrist. 1975. Pili on meningococci from primary cultures of nasopharyngeal carriers and cerebrospinal fluid of patients with acute disease. J. Exp. Med. 141:297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dokter, W. H. A., A. J. Dijkstra, S. B. Koopmans, B. K. Stulp, W. Keck, M. R. Halie, and E. Vellenga. 1994. G(ANH)MTETRA, a natural bacterial cell wall breakdown product, induces interleukin-1-beta and interleukin-6 expression in human monocytes—a study of the molecular mechanisms involved in inflammatory cytokine expression. J. Biol. Chem. 269:4201-4206. [PubMed] [Google Scholar]
- 8.Fassbender, K., U. Schminke, S. Ries, A. Ragoschke, U. Kischka, M. Fatar, and M. Hennerici. 1997. Endothelial-derived adhesion molecules in bacterial meningitis: association to cytokine release and intrathecal leukocyte-recruitment. J. Neuroimmunol. 74:130-134. [DOI] [PubMed] [Google Scholar]
- 9.Feurer, D. J., and R. O. Weller. 1991. Barrier functions of the leptomeninges: a study of normal meninges and meningiomas in tissue culture. Neuropathol. Appl. Neurobiol. 17:391-405. [DOI] [PubMed] [Google Scholar]
- 10.Frendeus, B., C. Wachtler, M. Hedlund, H. Fischer, P. Samuelsson, M. Svensson, and C. Svanborg. 2001. Escherichia coli P fimbriae utilize the Toll-like receptor 4 pathway for cell activation. Mol. Microbiol. 40:37-51. [DOI] [PubMed] [Google Scholar]
- 11.Gallo, P., S. Pagni, B. Giometto, M. G. Piccinno, F. Bozza, V. Argentiero, and B. Tavolato. 1990. Macrophage-colony stimulating factor (M-CSF) in the cerebrospinal fluid. J. Neuroimmunol. 29:105-112. [DOI] [PubMed] [Google Scholar]
- 12.Geissler, K., M. Harrington, C. Srivastava, T. Leemhuis, G. Tricot, and H. E. Broxmeyer. 1989. Effects of recombinant human colony stimulating factors (CSF) (granulocyte macrophage CSF, granulocyte CSF, and CSF-1) on human monocyte macrophage differentiation. J. Immunol. 143:140-146. [PubMed] [Google Scholar]
- 13.Gray, F., and P. Nordmann. 1997. Bacterial infections, p. 113-156. In D. I.Graham and P. L. Lantos (ed.), Greenfields neuropathology: infections. Arnold, London, United Kingdom.
- 14.Griffiss, J. M. 1995. Mechanisms of host immunity, p. 35-70. In K. A. V. Cartwright (ed.), Meningococcal disease. John Wiley & Sons, Chichester, United Kingdom.
- 15.Gruol, D. L., and T. E. Nelson. 1997. Physiological and pathological roles of interleukin-6 in the central nervous system. Mol. Neurobiol. 15:307-339. [DOI] [PubMed] [Google Scholar]
- 16.Halstensen, A., M. Ceska, P. Brandtzaeg, H. Redl, A. Naess, and A. Waage. 1993. Interleukin-8 in serum and cerebrospinal fluid from patients with meningococcal disease. J. Infect. Dis. 167:471-475. [DOI] [PubMed] [Google Scholar]
- 17.Hardy, S. J., M. Christodoulides, R. O. Weller, and J. E. Heckels. 2000. Interactions of Neisseria meningitidis with cells of the human meninges. Mol. Microbiol. 36:817-829. [DOI] [PubMed] [Google Scholar]
- 18.Heckels, J. E. 1977. The surface properties of Neisseria gonorrhoeae: isolation of the major components of the outer membrane. J. Gen. Microbiol. 99:333-341. [DOI] [PubMed] [Google Scholar]
- 19.Hedlund, M., B. Frendeus, C. Wachtler, L. Hang, H. Fischer, and C. Svanborg. 2001. Type 1 fimbriae deliver an LPS- and TLR4-dependent activation signal to CD14-negative cells. Mol. Microbiol. 39:542-552. [DOI] [PubMed] [Google Scholar]
- 20.Hedlund, M., M. Svensson, A. Nilson, R. D. Duan, and C. Svanborg. 1996. Role of the ceramide-signaling pathway in cytokine responses to P-fimbriated Escherichia coli. J. Exp. Med. 183:1037-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inaba, Y., A. Ishiguro, and T. Shimbo. 1997. The production of macrophage inflammatory protein-1 alpha in the cerebrospinal fluid at the initial stage of meningitis in children. Pediatr. Res. 42:788-793. [DOI] [PubMed] [Google Scholar]
- 22.Jung, H. C., L. Eckmann, S. K. Yang, A. Panja, J. Fierer, E. Morzycka Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koedel, U., and H. W. Pfister. 1999. Oxidative stress in bacterial meningitis. Brain Pathol. 9:57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lahrtz, F., L. Piali, K. S. Spanaus, J. Seebach, and A. Fontana. 1998. Chemokines and chemotaxis of leukocytes in infectious meningitis. J. Neuroimmunol. 85:33-43. [DOI] [PubMed] [Google Scholar]
- 25.Lambden, P. R., and J. E. Heckels. 1982. Synthesis of immunogenic oligosaccharide-protein conjugates from the lipopolysaccharide of Neisseria gonorrhoeae p9. J. Immunol. Methods 48:233-240. [DOI] [PubMed] [Google Scholar]
- 26.Leib, S. L., and M. G. Tauber. 1999. Pathogenesis of bacterial meningitis. Infect. Dis. Clin. N. Am. 13:527-548. [DOI] [PubMed] [Google Scholar]
- 27.Leist, T. P., K. Frei, S. Kamhansen, R. M. Zinkernagel, and A. Fontana. 1988. Tumor necrosis factor-alpha in cerebrospinal fluid during bacterial, but not viral, meningitis—evaluation in murine model infections and in patients. J. Exp. Med. 167:1743-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Cortes, L. F., M. Cruz-Ruiz, J. Gomez-Mateos, P. Viciana-Fernandez, F. J. Martinez-Marcos, and J. Pachon. 1995. Interleukin-8 in cerebrospinal fluid from patients with meningitis of different etiologies—its possible role as neutrophil chemotactic factor. J. Infect. Dis. 172:581-584. [DOI] [PubMed] [Google Scholar]
- 29.Lorenzen, D. R., F. Dux, U. Wolk, A. Tsirpouchtsidis, G. Haas, and T. F. Meyer. 1999. Immunoglobulin A1 protease, an exoenzyme of pathogenic neisseriae, is a potent inducer of proinflammatory cytokines. J. Exp. Med. 190:1049-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makepeace, B., P. J. Watt, J. E. Heckels, and M. Christodoulides. 2001. Interactions of Neisseria gonorrhoeae with mature human macrophage opacity proteins influence production of proinflammatory cytokines. Infect. Immun. 69:1909-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mastroianni, C. M., L. Lancella, F. Mengoni, M. Lichtner, P. Santopadre, C. D'Agostino, F. Ticca, and V. Vullo. 1998. Chemokine profiles in the cerebrospinal fluid (CSF) during the course of pyogenic and tuberculous meningitis. Clin. Exp. Immunol. 114:210-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuzono, Y., M. Narita, Y. Akutsu, and T. Togashi. 1995. Interleukin-6 in cerebrospinal fluid of patients with central nervous system infections. Acta Paediatr. 84:879-883. [DOI] [PubMed] [Google Scholar]
- 33.McGuinness, B. T., I. N. Clarke, P. R. Lambden, A. K. Barlow, J. T. Poolman, D. M. Jones, and J. E. Heckels. 1991. Point mutation in meningococcal porA gene associated with increased endemic disease. Lancet 337:514-517. [DOI] [PubMed] [Google Scholar]
- 34.Naumann, M., S. Wessler, C. Bartsch, B. Wieland, and T. F. Meyer. 1997. Neisseria gonorrhoeae epithelial cell interaction leads to the activation of the transcription factors nuclear factor kappa b and activator protein 1 and the induction of inflammatory cytokines. J. Exp. Med. 186:247-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peltola, H. 2000. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin. Microbiol. Rev. 13: 302-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pron, B., M. K. Taha, C. Rambaud, J. C. Fournet, N. Pattey, J. P. Monnet, M. Musilek, J. L. Beretti, and X. Nassif. 1997. Interaction of Neisseria meningitidis with the components of the blood-brain barrier correlates with an increased expression of PilC. J. Infect. Dis. 176:1285-1292. [DOI] [PubMed] [Google Scholar]
- 37.Rothwell, N. J. 1994. CNS regulation of thermogenesis. Crit. Rev. Neurobiol. 8:1-10. [PubMed] [Google Scholar]
- 38.Sauer, F. G., M. A. Mulvey, J. D. Schilling, J. J. Martinez, and S. J. Hultgren. 2000. Bacterial pili: molecular mechanisms of pathogenesis. Curr. Opin. Microbiol. 3:65-72. [DOI] [PubMed] [Google Scholar]
- 39.Schilling, J. D., M. A. Mulvey, C. D. Vincent, R. G. Lorenz, and S. J. Hultgren. 2001. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J. Immunol. 166:1148-1155. [DOI] [PubMed] [Google Scholar]
- 40.Shimoda, K., S. Okamura, F. Omori, Y. Mizuno, T. Hara, T. Aoki, K. Ueda, and Y. Niho. 1991. Granulocyte colony-stimulating factor in cerebrospinal fluid from patients with meningitis. Blood 77:2214-2217. [PubMed] [Google Scholar]
- 41.Shinefield, H. R., and S. Black. 2000. Efficacy of pneumococcal conjugate vaccines in large scale field trials. Pediatr. Infect. Dis. J. 19:394-397. [DOI] [PubMed] [Google Scholar]
- 42.Spanaus, K. S., D. Nadal, H. W. Pfister, J. Seebach, U. Widmer, K. Frei, S. Gloor, and A. Fontana. 1997. C-X-C and C-C chemokines are expressed in the cerebrospinal fluid in bacterial meningitis and mediate chemotactic activity on peripheral blood-derived polymorphonuclear and mononuclear cells in vitro. J. Immunol. 158:1956-1964. [PubMed] [Google Scholar]
- 43.Sprenger, H., A. Rosler, P. Tonn, H. J. Braune, G. Huffmann, and D. Gemsa. 1996. Chemokines in the cerebrospinal fluid of patients with meningitis. Clin. Immunol. Immunopathol. 80:155-161. [DOI] [PubMed] [Google Scholar]
- 44.Stephens, D. S., K. M. Edwards, and F. M. G. Morris. 1982. Pili and outer membrane appendages on Neisseria meningitidis in the cerebrospinal fluid of an infant. J. Infect. Dis. 146:568. [DOI] [PubMed] [Google Scholar]
- 45.Strazielle, N., and J. F. Ghersi-Egea. 2000. Choroid plexus in the central nervous system: biology and physiopathology. J. Neuropathol. Exp. Neurol. 59:561-574. [DOI] [PubMed] [Google Scholar]
- 46.Tauber, M. G., and B. Moser. 1999. Cytokines and chemokines in meningeal inflammation: biology and clinical implications. Clin. Infect. Dis. 28:1-12. [DOI] [PubMed] [Google Scholar]
- 47.Tinsley, C. R., and J. E. Heckels. 1986. Variation in the expression of pili and outer membrane protein by Neisseria meningitidis during the course of meningococcal infection. J. Gen. Microbiol. 132:2483-2490. [DOI] [PubMed] [Google Scholar]
- 48.Tzeng, Y. L., and D. S. Stephens. 2000. Epidemiology and pathogenesis of Neisseria meningitidis. Microb. Infect. 2:687-700. [DOI] [PubMed] [Google Scholar]
- 49.van Deuren, M., P. Brandtzaeg, and J. van der Meer. 2000. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin. Microbiol. Rev. 13:144-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Deuren, M., J. van der Venjongekrijg, E. Vannier, R. van Dalen, G. Pesman, A. K. M. Bartelink, C. A. Dinarello, and J. W. M. van der Meer. 1997. The pattern of interleukin-1 beta (IL-1 beta) and its modulating agents IL-1 receptor antagonist and IL-1 soluble receptor type II in acute meningococcal infections. Blood 90:1101-1108. [PubMed] [Google Scholar]
- 51.van Furth, A. M., J. J. Roord, and R. van Furth. 1996. Roles of proinflammatory and anti-inflammatory cytokines in pathophysiology of bacterial meningitis and effect of adjunctive therapy. Infect. Immun. 64:4883-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Virji, M., K. Makepeace, D. J. P. Ferguson, M. Achtman, J. Sarkari, and E. R. Moxon. 1992. Expression of the Opc protein correlates with invasion of epithelial and endothelial cells by Neisseria meningitidis. Mol. Microbiol. 6:2785-2795. [DOI] [PubMed] [Google Scholar]
- 53.Virji, M., J. R. Saunders, G. Sims, K. Makepeace, D. Maskell, and D. J. P. Ferguson. 1993. Pilus-facilitated adherence of Neisseria meningitidis to human epithelial and endothelial cells: modulation of adherence phenotype occurs concurrently with changes in primary amino acid sequence and the glycosylation status of pilin. Mol. Microbiol. 10:1013-1028. [DOI] [PubMed] [Google Scholar]
- 54.Waage, A., A. Halstensen, T. Espevik, and P. Brandtzaeg. 1993. Compartmentalization of TNF and IL-6 in meningitis and septic shock. Med. Inflamm. 2:23-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waage, A., A. Halstensen, and R. Shalaby. 1989. Local production of tumor necrosis factor α, interleukin 1 and interleukin 6 in meningococcal meningitis. J. Exp. Med. 170:1859-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weller, R. O. 1995. Fluid compartments and fluid balance in the central nervous system, p. 1202-1204. In P. L.Williams (ed.), Gray's anatomy. Churchill Livingstone, Edinburgh, United Kingdom.
- 57.Wells, D. B., P. J. Tighe, K. G. Wooldridge, K. Robinson, and D. A. A. A. Aldeen. 2001. Differential gene expression during meningeal-meningococcal interaction: evidence for self-defense and early release of cytokines and chemokines. Infect. Immun. 69:2718-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zwahlen, A., U. E. Nydegger, P. Vaudaux, P. H. Lambert, and F. A. Waldvogel. 1982. Complement-mediated opsonic activity in normal and infected human cerebrospinal fluid early response during bacterial meningitis. J. Infect. Dis. 145:635-646. [DOI] [PubMed] [Google Scholar]