Abstract
One requirement for the pathogenesis of Yersinia pestis, the causative agent of bubonic plague, is the yersiniabactin (Ybt) siderophore-dependent iron transport system that is encoded within a high-pathogenicity island (HPI) within the pgm locus of the Y. pestis chromosome. Nine gene products within the HPI have demonstrated functions in the nonribosomal peptide synthesis (NRPS)/polyketide (PK) synthesis or transport of Ybt. NRPS/PK synthetase or synthase enzymes are generally activated by phosphopantetheinylation. However, no products with similarities to known phosphopantetheinyl (P-pant) transferases were found within the pgm locus. We have identified a gene, ybtD, encoded outside the HPI and pgm locus, that is necessary for function of the Ybt system and has similarities to other P-pant transferases such as EntD of Escherichia coli. A deletion within ybtD yielded a strain (KIM6-2085+) defective in siderophore production. This strain was unable to grow on iron-deficient media at 37°C but could be cross-fed by culture supernatants from Ybt-producing strains of Y. pestis. The promoter region of ybtD was fused to lacZ; β-galactosidase expression from this reporter was not regulated by the iron status of the bacterial cells or by YbtA, a positive regulator of other genes of the ybt system. The ybtD mutant failed to express indicator Ybt proteins (high-molecular-weight protein 1 [HMWP1], HMWP2, and Psn), a pattern similar to those seen with several other ybt biosynthetic mutants. In contrast, cells containing a single amino acid substitution (S2908A) in the terminal thioesterase domain of HMWP2 failed to exhibit any ybt regulatory defects but did not elaborate extracellular Ybt under iron-deficient conditions.
To cause infections nearly all pathogenic bacteria must remove iron, an essential trace nutrient, from host iron- and/or heme-chelating proteins (14, 50, 75). Yersinia pestis, the causative agent of bubonic and pneumonic plague, possesses an ABC hemoprotein transport system (Hmu) that allows it to use a variety of host hemoproteins as a source of iron (40, 73) as well as a Has/hemophore system that may not be functional (62). Analysis of the sequence of CO92 (Y. pestis biotype orientalis) (53) and KIM10+ (Y. pestis biotype mediaevalis) (21) revealed nine potentially functional inorganic iron transport systems; three of these have demonstrated iron acquisition ability. The Yfu system (35) belongs to a family of ABC iron transporters present in Yersinia enterocolitica (Yfu), neisseriae (Fbp), Haemophilus influenzae (Hit), Actinobacillus pleuropneumoniae (Afu), and Serratia marcescens (Sfu) (8). The Y. pestis Yfe system belongs to a family of cation-transporting ABC systems and transports both iron and manganese. This system acquires iron during the later stages of plague (5, 6).
The yersiniabactin (Ybt) iron transport system produces a siderophore composed of phenolate, thiazoline, and thiazolidine rings (16, 22, 55) via a nonribosomal peptide synthesis (NRPS)/polyketide (PK) synthesis scheme. The Ybt siderophore has considerable similarity to the siderophores pyochelin and anguibactin, produced by Pseudomonas aeruginosa and Vibrio anguillarum, respectively (20, 42). The Ybt iron acquisition system is essential for the virulence of Y. pestis during the early stages of infection in mice (5) and appears to be the primary iron acquisition system of plague (56). Ybt biosynthetic, regulatory, and transport genes are encoded within a high-pathogenicity island (HPI) that is present in highly pathogenic isolates of Y. pestis, Yersinia pseudotuberculosis, and Y. enterocolitica, as well as several types of pathogenic Escherichia coli (10, 11, 15, 30, 38, 60, 66). In Y. pestis, the HPI resides within the pgm locus, a 102-kb region of chromosomal DNA subject to high-frequency deletion (11, 12, 27, 30, 37, 48).
Iron from Fe-Ybt is transported into the cell via a TonB-dependent OM receptor (termed Psn in Y. pestis and FyuA in Y. enterocolitica) in conjunction with an ABC transport system encoded by ybtP and ybtQ. Psn also binds the bacteriocin pesticin (24-26, 39, 45, 61). Both YbtP and YbtQ are necessary for use of iron from Ybt and resemble inner membrane permeases fused to an ATP-binding domain. No periplasmic-binding protein has been identified for the Ybt system (9, 24, 30). YbtA is a transcriptional regulator of the AraC family that activates transcription of ybt biosynthetic and transport operons and represses transcription from its own promoter (23).
Ybt biogenesis uses a mixed NRPS/PK synthesis mechanism that assembles the siderophore from salicylate, a linker group derived from malonyl coenzyme A, three molecules of cysteine, and three methyl groups donated by S-adenosylmethionine (30). The requirement of six gene products (high-molecular-weight protein 1 [HMWP1], HMWP2, YbtE, YbtS, YbtT, and YbtU) for in vivo Ybt synthesis has been clearly demonstrated. YbtS likely participates in salicylate biosynthesis (4, 30). YbtE adenylates salicylate and transfers this activated compound to HMWP2 (31). HMWP2, encoded by irp2, possesses NRPS domains involved in the initial cyclization and condensation reactions involving salicylate and two cysteine molecules (30, 31, 36, 71). YbtU reduces the middle thiazoline ring to a thizolidine structure (51). HMWP1, encoded by irp1, contains PK/fatty acid synthase and modified NRPS domains that add the branched isobutyryl-alcohol linker and the last thiazoline moiety. YbtT contains a thioesterase (TE) domain (4, 30) and may be involved in removing aberrant structures from the enzymatic complex, while the terminal TE domain of HMWP1 is hypothesized to remove the completed siderophore from the enzyme complex (33).
NRPS/PK synthetase or synthase enzymes are generally activated by phosphopantetheinylation. Phosphopantetheinyl (P-pant) transferases transfer the 4′-phosphopantetheine moiety of coenzyme A to a specific site on the NRPS and PK enzymes. Activated acyl groups or amino acids are subsequently added to these tethers in preparation for the assembly of the compounds. Phosphopantetheinylation of a peptidyl carrier protein domain of HMWP1 (PCP3) has been demonstrated in vitro using E. coli EntD (30). No gene encoding an apparent P-pant transferase is present within the HPI or the pgm locus. Here we report on genes involved in the first and last steps of Ybt biogenesis—activation of the NRPS/PK synthetase complex by phosphopantetheinylation and cleavage of the completed siderophore by the terminal TE domain of HMWP1.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
All relevant characteristics of strains used in this study are presented in Table 1. All the Y. pestis strains used in this study were derived from KIM6+, an avirulent strain that possesses all of the known Y. pestis virulence determinants except for pCD1, a 70.5-kb plasmid encoding the low-calcium response (Lcr) regulon. (27, 68) The Lcr virulence regulon is unrelated to the Pgm+ phenotype and has no demonstrable role in iron metabolism (57, 59).
TABLE 1.
Bacterial strains and plasmids used in this studya
| Strain or plasmid | Relevant characteristic(s) | Reference or Source |
|---|---|---|
| E. coli | ||
| DH5α | Cloning host | 2 |
| DH5α (λpir) | Strain for propagating plasmids with R6K origins, derived from DH5α | S. C. Straley |
| Y. pestis | ||
| KIM6+ | Pgm+ (Hms+ Ybt+) Lcr− | 25 |
| KIM6 | Pgm− (Δpgm − Hms− Ybt−) Lcr− | 25 |
| KIM6-2046.1 | Hms+ Ybt− (irp2::kan2046.1) Lcr− Kmr | 25 |
| KIM6-2055 | Hms+ Ybt− (ybtA::kan2055) Lcr− Kmr | 23 |
| KIM6-2085+ | Hms+ Ybt− (ΔybtD2085) Lcr− | This study |
| KIM6-2086 | Hms+ Ybt− (irp1-2086) Lcr− | This study |
| KIM6-2086.1+ | Pgm+ (Hms+ Ybt+) Lcr−; derived from KIM6-2086 by replacement of the irp1-2086 mutation with irp1+ | This study |
| Plasmids | ||
| pACYC184 | 4.2-kb cloning vector; Cmr Tcr | 17 |
| pEU730 | 15.2-kb low-copy-number vector with promoterless lacZ; Spcr Smr | 28 |
| pEUYbtP | 15.4-kb low-copy-number ybtP::lacZ reporter | 24 |
| plasmid, Spcr; iron-, Fur-, and YbtA-regulated expression of β-galactosidase | ||
| pKNG101 | 6.8-kb suicide vector; sacB+, R6K origin; Sucs Smr | 44 |
| pWSK29 | 5.4-kb low-copy-number cloning vector; Apr | 74 |
| pET22b-HMWP1-TEmut | 9.49-kb NcoI/XhoI fragment of irp1, with altered bp, in pET22b; Apr, 15.0 kb, irp1-2086 (HMWP1-S2908A) | Z. Suo and C. T. Walsh |
| pEUYbtD1 | 15.4-kb low-copy-number ybtD::lacZ reporter plasmid; 182-bp AscI/KpnI fragment containing ybtD promoter region cloned into AscI/KpnI sites of pEU730; Spcr Smr; fusion of ybtD 167-bp promoter to lacZ | This study |
| pEUYbtD2 | 15.6-kb low-copy-number ybtD::lacZ reporter plasmid; 355-bp AscI/KpnI fragment containing ybtD promoter region cloned into AscI/KpnI sites of pEU730; Spcr Smr; fusion of ybtD 342-bp promoter to lacZ | This study |
| pIrp1TE2 | 1.37-kb PvuII/XhoI fragment from pET22b-HMWP1-TEmut cloned in SmaI/SalI sites of suicide vector pKNG101; Sucs Smr, 8.2 kb, irp1-2086 (HMWP1-S2908A) | This study |
| pKNGIRP1 | 1,703-bp PCR product from pPSN3 ligated into the | This study |
| BamHI/XbaI site of pKNG101; sacB+, R6K origin; Sucs Smr, 8.5 kb; irp1-TE+ | ||
| pPSN3 | 9,994-bp SalI fragment encompassing the irp1-ybtE region in pBLG2; Apr; 15.8 kb | 24 |
| pYBTD1 | 4.44-kb XhoI/BglII fragment from Y. pestis KIM6+ genomic DNA cloned into XhoI/BamHI sites of pWSK29; Apr; 9.8 kb; ybtD+ | This study |
| pYBTD2 | 774-bp EcoRV/AsuII fragment within ybtD removed from pYBTD1; Apr; 9.0 kb; ΔybtD | This study |
| pYBTD3 | 3.7-kb XbaI/ApaI fragment from pYBTD2 cloned into XbaI/ApaI sites of suicide vector pKNG101; Suc Smr; ΔybtD | This study |
| pYBTD4 | 0.93-kb BamHI/XbaI PCR product of ybtD and its putative promoter region ligated into Bam HI/XbaI sites of pACYC184; Cmr; 10.5 kb; ybtD+ | This study |
Y. pestis strains with a plus sign possess an intact 102-kb pgm locus containing the genes for hemin storage (hms) and the Ybt system. All other Y. pestis strains contain a mutation within the pgm locus due to either a deletion or insertion of an antibiotic resistance cassette. Strains synthesizing the siderophore Ybt are designated Ybt+, while those affected in Ybt production are Ybt−. Lcr− indicates the absence of the low-calcium-response virulence plasmid pCD1. Abbreviations: Apr, Kmr, Spcr, Smr, Tcr, and Cmr, resistance to ampicillin, kanamycin, spectinomycin, streptomycin, tetracycline, and chloramphenicol, respectively.
All strains were stored at −20°C in phosphate-buffered glycerol. Y. pestis cells were grown routinely at 30°C on Congo red (72) from glycerol stocks and then grown in heart infusion broth (Difco Laboratories) or on tryptose-blood agar base (Difco). For iron-deficient growth, Y. pestis cells were grown in the chemically defined medium PMH or PMH2 (33) which had been extracted prior to use with Chelex 100 resin (Bio-Rad Laboratories) (70). Growth of the cultures was monitored by determining the optical density at 620 nm with a Genesys5 spectrophotometer (Spectronic Instruments, Inc.). Residual iron not removed from deferrated PMH or PMH2 by the resin can be precipitated by the addition of 0.5 mM NaCO3, 0.01 mM MnCl2, and 4.0 mM CaCl2 (PMH-S and PMH2-S) or chelated by supplementation with 2,2′-dipyridyl (PMH-DIP) at a concentration of 100 μM. PMH-S, PMH2-S, and PMH-DIP plates were solidified with 1% agarose. PMH-S, PMH2-S, and/or PMH-DIP plates were subsequently used in cross-feeding experiments or to determine the growth characteristics of the ybt mutants at 37°C as previously described (25). For iron-replete growth, Y. pestis strains were cultivated in PMH2 supplemented with 10 μM FeCl3.
All glassware used for iron-restricted studies was soaked overnight in chromic-sulfuric acid (46.3 g of K2Cr2O7 per liter of 11.25 M sulfuric acid) or ScotClean (OWL Scientific, Inc) to remove contaminating iron and copiously rinsed in deionized water. E. coli cells were grown on Luria broth. Where appropriate, ampicillin (100 μg/ml), spectinomycin (100 μg/ml), tetracycline (6.25 μg/ml), streptomycin (50 μg/ml), kanamycin (50 μg/ml), or chloramphenicol (30 μg/ml) was added to cultures.
Plasmids, sequencing, and recombinant DNA techniques.
All the plasmids used in this study are listed in Table 1. Plasmids were purified from overnight cultures by alkaline lysis (7) and further purified when necessary by polyethylene glycol precipitation (41). Standard cloning and recombinant DNA methods (63) were used to construct the various plasmids in Table 1. A standard CaCl2 procedure was used to introduce plasmids into E. coli (63). Y. pestis cells were transformed by electroporation as previously described (25). Plasmid DNA and PCR products were sequenced by either Retrogen, Inc., or in our laboratory. Sequencing reactions in our laboratory were performed via the dideoxynucleotide chain termination method (64) using 35S-dATP (Amersham/USB), Sequenase (version 2.0; Amersham/USB), and 7-deaza-dGTP (Boehringer Mannheim Biochemicals). Samples were electrophoresed through a 6% polyacrylamide gel containing 8.3 M urea (Sigma) cast in Tris-borate-EDTA buffer (63). Dried gels were exposed at room temperature to Kodak Biomax MR film. Synthetic oligonucleotide primers were purchased from Integrated DNA Technologies.
Nucleotide sequence accession number.
The ybtD sequence may be found using GenBank accession number AE009952, which contains the entire Yersinia pestis KIM10+ genome sequence (21). BLAST searches of the Yersinia pestis KIM10+ genome may be performed at the Web site (http://magpie.genome.wisc.edu/cgi-bin/Authenticate.cgi/uwgp blast.html) for the Genome Center of Wisconsin. KIM10+ is a derivative of KIM6+ lacking plasmid pPCP1 (58)
Generating ybtD and irp2TE mutant strains.
All Y. pestis mutant strains were generated by homologous recombination using mutated DNA fragments cloned into suicide vectors carrying the sacB gene and an R6K origin of replication. For construction of a ΔybtD strain (KIM6-2085+), a 4.44-kb XhoI/BglII fragment of Y. pestis KIM6+ genomic DNA was cloned into XhoI/BamHI sites of pWSK29. Isolates containing recombinant plasmids were screened by PCR using Taq polymerase with primers ENTD1 (5′-GCCAAGTGTGATTTTGAGGTGA-3′) and ENTD2 (5′-ACGCACGTTGGTTATTATGGCT-3′). Reaction mixtures contained 0.2 mM deoxynucleoside triphosphates and 0.2 μM primers. Reactions consisted of 4 min at 94°C followed by 25 cycles of 20s s at 94°C, 30 s at 55°C, and 30 s at 72°C and a single cycle at 72°C for 7 min. One clone containing the desired insert was designated pYBTD1. A deletion encompassing most of the ybtD gene was made by removal of a 774-bp EcoRV/AsuII fragment from pYBTD1 to yield pYBTD2 (see Fig. 2). A 3.7-kb XbaI/ApaI fragment from pYBTD2 was cloned into the XbaI/ApaI sites of the suicide vector pKNG101 creating pYBTD3. The recombinant suicide plasmid was introduced into Y. pestis KIM6+ by electroporation. Y. pestis isolates with the plasmid recombined into the chromosome were selected on tryptose-blood agar base plates containing 50 μg of streptomycin/ml. As previously described, cells grown overnight without antibiotics were used to select sucrose-resistant isolates that had completed allelic exchange (5). Isolates containing the chromosomal ΔybtD mutation were identified by PCR using Taq polymerase with primers ENTD1 (see above) and ENTD3 (5′-CGATTGGCTAGAGAAAGCAGGA-3′). Reaction mixtures contained 0.2 mM deoxynucleoside triphosphates and 0.2 μM primers. Reactions consisted of 3 min at 94°C followed by 25 cycles of 15s s at 94°C, 30 s at 55°C, and 90 s at 72°C and a single cycle at 72°C for 7 min. One isolate, strain KIM6-2085+ (Table 1), was selected for further characterization.
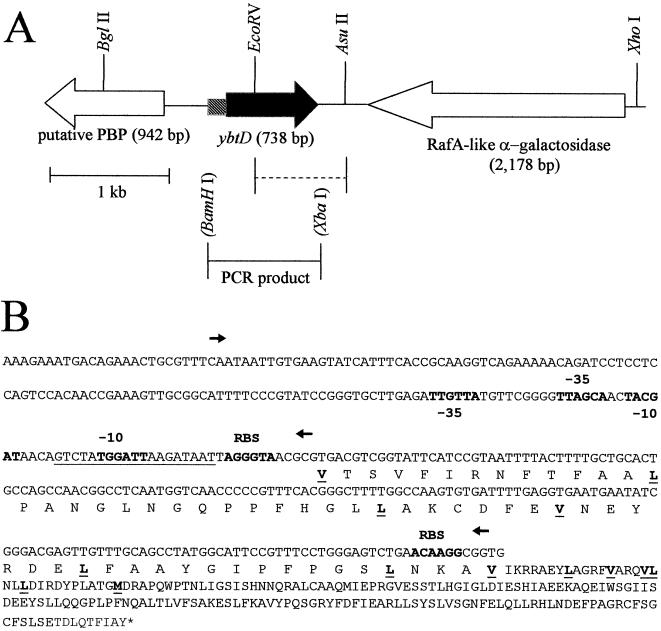
FIG. 2.
Genetic organization of the Y. pestis ybtD region showing restriction sites used. (A) Dashed line indicates the region deleted in the ΔybtD mutant. The PCR product used in complementation studies is also indicated. The BamHI and XbaI sites in parentheses are artificial restriction sites introduced by PCR. (B) Putative −10 and −35 regions, potential ribosomal binding sites (RBS), and a region with similarity to a Fur binding site (underlined nucleotides), as well as the potential protein start sites, are indicated (underlined and in boldface type). Arrows show the two promoter regions tested in expression studies.
For trans complementation of the ΔybtD mutation, a 0.93-kb PCR product was amplified from pYBTD1 using ProofStart Taq polymerase (Qiagen) with primers ENTDC-1 (5′-CGCGGATCCTCCTCCAGTCCACAACC-3′) and ENTDC-2 (5′-GCTCTAGACTTCTTTCATATTCAGCCC-3′). Reaction mixtures contained 0.2 mM deoxynucleoside triphosphates and 0.2 μM primers. Reactions consisted of 3 min at 94°C followed by 25 cycles of 20 s at 94°C, 30 s at 55°C, and 90 s at 72°C and a single cycle at 72°C for 7 min. Following digestion with BamHI and XbaI, the product was ligated into the BamHI/XbaI of pACYC184 generating pYBTD4.
pET22b-HMWP1-TEmut (Table 1) was used to construct an HMWP1-TE− mutant (KIM6-2086). This plasmid contains a 9,491-bp NcoI/XhoI fragment from irp1 in which a single base pair replacement results in the substitution of alanine for serine in the TE domain of HMWP1 at residue 2908. A 1,372-bp PvuII/XhoI fragment of pET22b-HMWP1-TEmut was subcloned into the SmaI/SalI sites of the suicide plasmid pKNG101, generating pIrp1TE1. The mutation was introduced into Y. pestis KIM6+ by allelic exchange as described previously (5). Individual colonies were analyzed for the ability to grow at 37°C in iron-depleted PMH-DIP. One of the isolates that was unable to grow after 24 to 48 h of incubation was analyzed for incorporation of the irp1 mutation. The irp1-TE region of this putative mutant was amplified by PCR using primers TE-PCR1 (5′-CTGTTCAGCCATTCGACG-3′) and TE-PCR2 (5′-AGATGCGCGATGTTGTCG-3′). Reactions consisted of 5 min at 94°C followed by 35 cycles for 30 s at 94°C, 30 s at 50°C, and 30 s at 72°C and a single cycle at 72°C for 7 min. The predicted 595-bp product was purified using a G100-120 Sephadex column. The mutation was confirmed by sequencing the PCR product using primers TE-Seq1 (5′-GTATGTCGGGTGCATCCG-3′) and TE-Seq2 (5′-CTCGCCTTTGGCGTACAG-3′). For complementation of the irp1-2086 mutation, this chromosomal mutation was replaced by wild-type irp1 sequences. A 1,703-bp PCR product from the cloned irp1 gene (spanning the TE regions) in pPSN3 was amplified using primers YTE-XbaI-1 (5′-GCTCTAGAGACGGAGCGAAACAGCGTATTCC-3′) and TE-BamHI-2 (5′-CGGGATCCGGATGCTCCTGAATGACGTGTACG-3′). Reactions consisted of 4 min at 94°C followed by 30 cycles for 20 s at 94°C, 30 s at 67°C, and 120 s at 72°C and a single cycle at 72°C for 7 min. After digestion with BamHI and XbaI, the PCR fragment was ligated into the BamHI/XbaI sites of pKNG101, generating pKNGIRP1. The wild-type sequence was introduced into Y. pestis KIM6-2086 by allelic exchange as described previously (5). Allelic exchange was confirmed by sequencing a PCR product from the irp1-TE region.
Protein analyses.
To label cellular proteins, whole cells of Y. pestis strains were grown through two passages in PMH2, with or without 10 μM FeCl3, for a total of approximately six generations and labeled with 35S-amino acids (DuPont NEN Research Products) for 1 h as previously described (26). To analyze the effect of Ybt on protein synthesis, Ybt, in the form of KIM6+ culture supernatant, was added at the same time as 35S-amino acids to cells acclimated to iron starvation. An equivalent number of counts was electrophoresed on 9% polyacrylamide gels containing sodium dodecyl sulfate (SDS). Dried gels were exposed to Kodak BioMax MR film at room temperature.
Ybt bioassay.
Culture supernatants were obtained from Y. pestis cells inoculated into deferrated PMH2 and grown for a total of six to nine generations at 37°C as previously described (25). Cells were pelleted by centrifugation, and the supernatant was filtered through a 0.2-μm-pore-size filter. For growth responses, PMH-S, PMH2-S, and/or PMH-DIP plates were overlayered with 0.04 optical density (at 620 nm) units of KIM6-2046.1 (irp2::kan2046.1) cells grown in deferrated PMH2 and 25 μl of filtered supernatants from iron-deficient cultures was added to wells in the plates.
The ΔybtD and irp1-2086 mutants were also tested for their ability to promote the growth of KIM6-2046.1 at 37°C by streaking the mutants adjacent to KIM6-2046.1 on PMH-S, PMH2-S, and/or PMH-DIP plates. Prior to streaking, the mutants were adapted to iron-deficient growth conditions as described above. Y. pestis strains that do not produce Ybt are unable to grow on PMH-S, PMH2-S, or PMH-DIP at 37°C but can be cross-fed by Ybt-producing strains (33).
Generating ybtD promoter fusions with lacZ.
Two PCR products were amplified from pYBTD1 using ProofStart Taq polymerase, digested with KpnI and AscI, and ligated into the AscI/KpnI sites of pEU730. The forward primer for both constructs was EntD.pF (5′-AGGCGCGCCAATAATTGTGAAGTATCATTTCA-3′). For the 167-bp insert (pEUYbtD1) and the 342-bp insert (pEUYbtD2), primers EntD.pR-1 (5′-GGGGTACCGCGTTACCCTAATTATCTTAATC-3′) and EntD.pR-2 (5′-GGGGTACCGCCTTGTTCAGACTCCCAG-3′) were used, respectively. Reactions for both products consisted of 3 min at 94°C, followed by 25 cycles of 20 s at 94°C, 30 s at 55°C, and 30 s at 72°C and a single cycle at 72°C for 7 min. The cloned promoter regions were sequenced to confirm that no PCR errors had been introduced.
β-Galactosidase assays.
Lysates were prepared from cells carrying the ybtP::lacZ or ybtD::lacZ reporter plasmid. The cells were grown in PMH2 in the presence or absence of iron through two transfers for a total of approximately six generations, as previously described (69). β-Galactosidase activities were measured spectrophotometrically with a Genesys5 spectrophotometer (Spectronic Instruments, Inc.) following cleavage of ONPG (4-nitro-phenyl-β-d-galactopyranoside). Activities are expressed in Miller units (52).
RESULTS
Mutation of ybtD causes a loss of Ybt siderophore production.
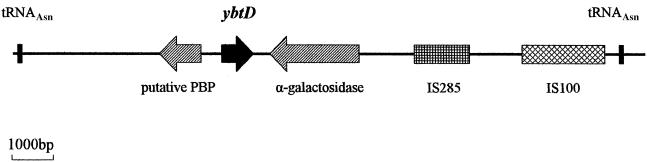
The initiating step in assembling the siderophore on the NRPS/PKS complex is phosphopantetheinylation of carrier sites on HMWP1 and HMWP2. However, a P-pant transferase is not encoded within the HPI of the yersiniae. A BLAST search of the two Y. pestis genomes using the amino acid sequence of E. coli EntD identified two strong P-pant transferase candidates. One gene product was highly similar to E. coli ACPS (acyl carrier protein synthase; 77% identity and 91.3% similarity over the 126 amino acids of both proteins) and is likely the essential P-pant transferase for fatty acid synthesis (46). The other gene was designated ybtD and lies between two tRNAAsn approximately 14.2 kb apart (Fig. 1). The ybtD open reading frame has multiple potential start sites encoding proteins ranging from 27.5 to 17.5 kDa (246 to 156 amino acids; Fig. 2). YbtD shows high similarities to Photorhabdus luminescens NgrA and Vibrio cholerae VibB—both involved in siderophore biosynthesis (18, 76). Figure 3 shows an alignment of YbtD, using the first potential start site, with NgrA, VibB, and EntD, the E. coli P-pant transferase required for enterobactin biosynthesis (1, 19, 32). In addition to containing the two conserved domains found in P-pant transferases, (46) these proteins also display strong homology at the N terminus (residues 34 to 112 of YbtD in Fig. 3).
FIG. 1.
Region of Y. pestis KIM10+ genome containing ybtD. The genes encoding two asparaginyl tRNAs, a putative periplasmic binding protein (PBP) for a C4-dicarboxylate ABC transporter, a RafA-like α-galactosidase, and YbtD are indicated as well as IS285 and IS100 elements. Arrows indicate the direction of transcription of selected genes.
FIG. 3.
Amino acid sequence alignment of YbtD from Y. pestis, NgrA from P. luminescens, VibD from V. cholerae, and EntD from E. coli. Residues with identity to YbtD are in white with a solid black background. Conservative and semiconservative amino acid substitutions are shaded. The consensus line shows identical residues in all four proteins (uppercase letters) and identical residues in two or more proteins (lowercase letters). The identity and similarity of YbtD to each of these proteins are 34 and 65.3% (NgrA), 31.3 and 60.2% (VibD), and 27.2 and 58.5% (EntD). The residues below the consensus line indicate conserved (lowercase) and highly conserved (uppercase) amino acids within the proposed P-pant transferase domain derived from comparison of 22 P-pant transferases (46).
A ybtD deletion was constructed and crossed into Y. pestis KIM6+ using allelic exchange (see Materials and Methods). PMH-S, PMH2-S, and/or PMH-DIP were used to examine the effect of the mutation on the ability of this strain to grow at 37°C under iron-chelated conditions. KIM6-2085+ (ΔybtD2085) did not grow at 37°C on the iron-chelated media (Table 2), indicating that the mutated strain lost the ability to either synthesize and secrete or utilize Ybt. Supernatants from iron-deficient cultures of KIM6-2085+ were unable to stimulate the growth of a Y. pestis strain (KIM6-2046.1) defective in Ybt synthesis (Table 2). However, culture supernatants from KIM6+, a Ybt-producing strain of Y. pestis, restored growth of KIM6-2046.1 cells under these conditions. In addition, KIM6+ was able to cross-feed KIM6-2085+ as well as KIM6-2046.1 cells (Table 2). This suggests that the YbtD− mutant is defective in synthesis of the Ybt siderophore but is still able to use it.
TABLE 2.
Growth of Y. pestis KIM6+ and ybt mutants on PMH-S, PMH2-S, and/or PMH-DIP
| Strain | Relevant characteristic | Growth on PMH-DIP, PMH-S, and/or PMH2-Sa | Growth stimulation of KIM6-2046.1 on PMH-DIP, PMH-S, and/or PMH2-Sb | Growth stimulation by KIM6+ on PMH-DIP, PMH-S, and/or PMH2-Sc |
|---|---|---|---|---|
| KIM6+ | ybt+ | + | + | NDd |
| KIM6-2046.1 | irp2::kan2046.1 | − | − | + |
| KIM6-2085+ | ΔybtD20285 | − | − | + |
| KIM6-2085(pYBTD4)+ | ΔybtD20285/ybtD+ | + | + | ND |
| KIM6-2086 | irp2-2086 | − | − | + |
| KIM6-2086.1+ | ybt+ | + | + | ND |
The presence or absence of growth on PMH-S, PMH2-S, and PMH-DIP plates at 37°C is denoted as + or −, respectively.
Each strain was tested for its ability to promote the growth of KIM6-2046.1 (irp2::kan2046.1) at 37°C on PMH-S, PMH2-S, and PMH-DIP plates, either by streaking adjacent to KIM6-2046.1 or by spent culture supernatants.
Strains were tested for their ability to use exogenous Ybt siderophore at 37°C by streaking adjacent to KIM6+ on PMH-S, PMH2-S, and/or PMH-DIP plates or by spent culture supernatants.
ND, not determined.
For complementation analyses, a PCR product encompassing the ybtD gene was cloned into pACYC184 and designated pYBTD4. The recombinant plasmid restored the ability of KIM6-2085+ cells to grow on iron-chelated plates at 37°C. In addition, culture supernatant from the complemented strain was able to promote the growth of KIM6-2046.1 (irp2::kan2046.1) on PMH-DIP plates (Table 2).
The ΔybtD2085 mutation results in decreased expression of ybt operons.
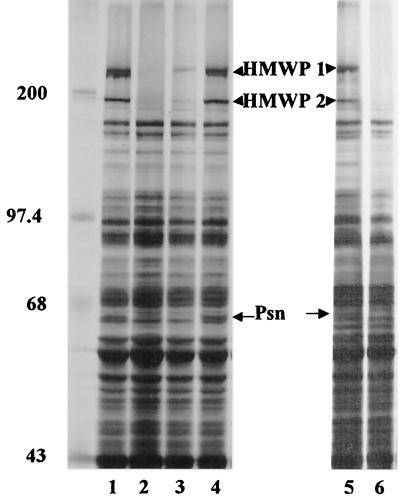
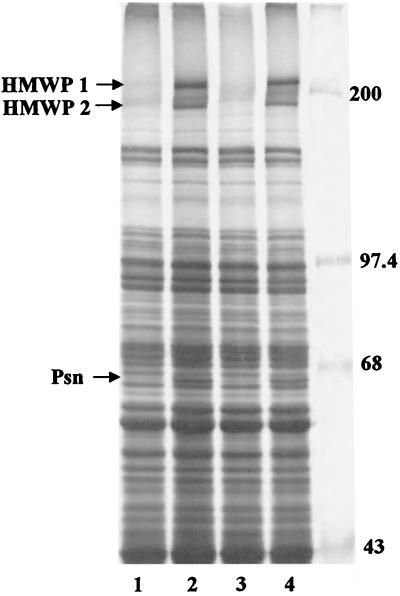
Previously we showed that most mutations (e.g., ΔybtE, ΔybtU, Δirp2, and irp1::kan) which result in the loss of siderophore production lower the expression of other Ybt biosynthetic genes (HMWP1, HMWP2, and YbtE) as well as the Ybt receptor (Psn) (4, 23, 33). However, two mutations in genes encoding Ybt biosynthetic enzymes (ΔybtT and ΔybtS) did not affect the expression of these indicator proteins (33). To determine the effect, if any, of the ΔybtD2085 mutation on the expression of these indicator proteins, total 35S-labeled proteins synthesized by cells grown under iron-sufficient and iron-deficient conditions were analyzed by SDS-polyacrylamide gel electrophoreis (PAGE). The protein expression pattern of the YbtD− mutant was similar to that of the ΔybtE, ΔybtU, Δirp2, and irp1::kan mutants that are defective in siderophore biosynthesis. In the absence of iron, the level of expression of HMWP1, HMWP2, and Psn proteins was greatly reduced in KIM6-2085+ (ΔybtD2085) cells (Fig. 4, lane 3) compared to that in the parental strain KIM6+ (Fig. 4, lane 1). In this experiment, YbtE was not detected due to inadequate separation of the polypeptides in this size range. The reduced level of Psn expressed in the YbtD− mutant was similar to that observed in KIM6-2046.1 (Fig. 4, lane 2); HMWP1 and HMWP2 are not detected in KIM6-2046.1 cells because of the irp2::kan2046.1 mutation and its polar effects. Complementation of the YbtD− mutant with pYBTD4, encoding ybtD, restored expression of HMWP1, HMWP2, and Psn (Fig. 4, lane 4). We have previously shown that addition of purified Ybt or supernatant containing Ybt to Y. pestis Ybt biosynthetic mutants restores expression of HMWP1, HMWP2, YbtE, and Psn (4, 23, 33, 55). Likewise wild-type levels of HMWP1, HMWP2, and Psn were expressed by iron-deficient cultures of the YbtD− cells labeled in the presence of supernatant from KIM6+ (expressing Ybt siderophore) but not with supernatant from the Ybt-biosynthetic mutant KIM6-2046.1 (Fig. 4, lanes 5 and 6).
FIG. 4.
SDS-PAGE analysis of whole-cell proteins from Y. pestis strains grown in iron-deficient PMH2. Cultures from Y. pestis KIM6+ (lane 1), KIM6-2046.1 (irp2::kan2046.1) (lane 2), KIM6-2085+ (ΔybtD2085) (lane 3), and KIM6-2085(pYBTD4)+ (ΔybtD2085/ybtD+) (lane 4) were incubated with 35S-labeled amino acids for 1 h. To demonstrate the effect of exogenous siderophore on expression of proteins by KIM6-2085+ cells, KIM6+ culture supernatant containing Ybt siderophore (lane 5) or KIM6-2046.1 culture supernatant (lane 6) was added 1:1 at the same time as 35S-labeled amino acids. Total cellular proteins were separated on a 9% polyacrylamide gel and visualized by autoradiography. Sizes of molecular mass markers (in kilodaltons) are indicated. Arrows point to the iron-regulated proteins HMWP1 (240 kDa), HMWP2 (190 kDa), and Psn (68 kDa).
We used pEUYbtP, a ybtP promoter fusion to lacZ, to test the effect of the ybtD2085 mutation on gene transcription. Previous studies showed that the ybtP promoter is regulated by Fur, iron, YbtA, and the Ybt siderophore (23, 24, 33). Because our Y. pestis strains are phenotypically β-galactosidase negative, any β-galactosidase activity is due to the presence of the reporter plasmid (33, 70). The β-galactosidase activities of cells bearing pEUYbtP and grown in deferrated PMH2 in the presence or absence of added iron, are presented in Table 3. As expected, expression of lacZ from the ybtP promoter was iron regulated in KIM6+, which contains all the genes needed for Ybt synthesis and utilization; there was an 18.4-fold repression of β-galactosidase activity in cells grown in the presence of surplus iron compared to those cultured under iron-deficient conditions. Expression of the ybtP::lacZ reporter in the YbtD− mutant, KIM6-2085+, was still somewhat iron regulated (14-fold repression); however, compared to KIM6+, the overall expression was greatly reduced (a 9.8-fold reduction in iron-starved cultures [Table 3]). Similar results were observed with the ybtP::lacZ reporter in KIM6-2046.1, an HMWP2− mutant unable to synthesize Ybt (Table 3). As in previous studies (24), lacZ expression from pEUYbtP in a Δpgm mutant was even lower, likely due to the absence of the YbtA transcriptional activator (Table 3). These studies suggest that loss of expression of HMWP1, HMWP2, and Psn in the YbtD− mutant is the result of decreased transcription from the relevant ybt promoters.
TABLE 3.
β-Galactosidase activities of Y. pestis strains containing either a ybtP::lacZ or a ybtD::lacZ reporter plasmida
| Reporter | Strain | Mean β-galactosidase activityb (Miller units) ± SD of cells grown:
|
Ratio (growth without Fe/growth with Fe) | Ratio (WT/Mutant) for cells grown:
|
||
|---|---|---|---|---|---|---|
| Without iron | With iron | Without iron | With iron | |||
| pEUYbtP (ybtP::lacZ) | KIM6+ (ybt+) | 29,929 ± 1,582 | 1,626 ± 249 | 18.4 | NDc | ND |
| KIM6 (Δpgm [ybt]) | 656 ± 142 | 216 ± 26 | 3.0 | 45.6 | 7.5 | |
| KIM6-2046.1 (irp2::kan2046.1) | 4,351 ± 1,705 | 292 ± 20 | 14.9 | 6.9 | 5.6 | |
| KIM6-2085+ (ΔybtD2085) | 3,044 ± 249 | 218 ± 17 | 14.0 | 9.8 | 7.5 | |
| KIM6-2086 (irp1-2086) | 34,004 ± 4,963 | 2,058 ± 580 | 16.5 | 0.88 | 0.79 | |
| pEUYbtD1 (ybtD::lacZ; 167-bp promoter region) | KIM6+ (ybt+) | 4,252 ± 1,415 | 4,674 ± 1,236 | 0.91 | ND | ND |
| KIM6-2055 (ybtA::kan2055) | 4,811 ± 982 | ND | ND | 0.88 | ND | |
| pEUYbtD2 (ybtD::lacZ; 342-bp promoter region) | KIM6+ (ybt+) | 2,637 ± 446 | 2,072 ± 307 | 1.3 | ND | ND |
Cells were harvested during exponential growth at 37°C after approximately six generations in PMH2 containing either no added iron or 10 μM FeCl3.
Enzyme activities are expressed in Miller units (52). The values ± standard deviations represent an average of three to four individual reactions from two or more independent cultures.
ND, not determined or not applicable.
Transcription of ybtD is not affected by YbtA or iron status of the cell.
The promoters of other ybt genes encoding siderophore biosynthetic and transport functions are repressed by iron through the action of Fur and are activated by YbtA and the siderophore (4, 23, 24, 69). Due to the multiple possible protein start sites and the two potential −10 and −35 regions (Fig. 2B), we constructed two transcriptional reporters to examine expression from the ybtD promoter. Both constructs start with the same upstream site (left-pointing arrow in Fig. 2B) and include both −10 and −35 regions and the potential Fur binding site; the 167-bp promoter fragment (pEUYbtD1) ends at the first potential valine start site, while the longer 342-bp construct (pEUYbtD2) ends at the third potential valine start (two right-pointing arrows in Fig. 2B) to ensure that all potential transcriptional regulatory elements were included. These regions were cloned into the lacZ transcriptional fusion vector, pEU730 (28), which contains an RNase III site upstream of lacZ (29). Processing of the message at this site removes any sequences which might alter message stability or affect translational efficiency (47). The level of β-galactosidase activity from Y. pestis KIM6(pEUYbtD2)+ cells grown under iron-sufficient or iron-deficient conditions was ∼2-fold lower than that determined in Y. pestis KIM6(pEUYbtD1)+ cells (Table 3). Since the amino acid similarities between YbtD and other P-pant transferases start upstream of the third potential valine start, we used pEUYbtD1 to further characterize the ybtD promoter region. The ybtD::lacZ reporter failed to show any repression under iron-sufficient growth conditions (Table 3). In addition, the level of β-galactosidase activity was similar in YbtA+ and YbtA− strains of Y. pestis bearing pEUYbtD1 (Table 3). Thus, the ybtD promoter does not appear to be regulated by iron or YbtA.
A single amino acid substitution in the TE domain of HMWP1 causes loss of siderophore production.
HMWP1 contains an internal TE domain which is hypothesized to be involved in the final step of siderophore biogenesis: release of the siderophore from the biosynthetic machinery. Previously we showed that YbtT, which contains a TE domain, is required for siderophore synthesis in Y. pestis (33) and likely serves an editing function to release aberrant intermediates on carrier sites of HMWP1 and HMWP2. To determine if the internal TE domain in HMWP1 is required for Ybt synthesis, we constructed a mutation that results in the substitution of an alanine for the serine at residue 2908 within the catalytic TE domain of HMWP1 (Fig. 5). Mutational analysis of several TEs showed that the conserved serine residue in the G(Y/W/H)SXG motif is a required catalytic nucleophile (67). Allelic exchange was used to introduce this mutation into KIM6+ generating strain KIM6-2086. KIM6-2086 cells were unable to grow on PMH2-S or PMH-DIP plates at 37°C unless supplied with culture supernatant from KIM6+ cells containing the Ybt siderophore. Growth on PMH2-S plates at 37°C was also restored when the chromosomal mutation was replaced by the wild-type sequence (strain KIM6-2086.1+, Table 2). Finally, culture supernatant from iron-starved KIM6-2086 cells did not allow the growth of the Ybt-biosynthetic mutant, KIM6-2046.1, on PMH2-S plates (Table 2). These results indicate that the mutation in the TE domain of irp1 caused a loss of siderophore production and/or secretion.
FIG. 5.
Conserved TE domains of YbtT and HMWP2. The TE consensus sequence is described in reference 67. The serine residue in HMWP1 that was changed to an alanine in KIM6-2086 is underlined.
The irp1-2086 mutation does not affect the expression of ybt operons.
To determine whether expression of Ybt proteins was affected by the irp1-2086 mutation, total 35S-labeled proteins synthesized by cells grown under iron-deficient conditions were analyzed by SDS-PAGE. The protein expression pattern of the HMWP1-TE mutant (KIM6-2086) was similar to that of Ybt+ strain KIM6+; i.e., HMWP1, HMPW2, and Psn were highly expressed in the mutant (Fig. 6). In addition, the β-galactosidase activity of KIM6-2086 cells bearing pEUYbtP and grown in deferrated PMH2 was repressed by iron (16.5-fold repression). The levels of lacZ expression were similar to that observed with KIM6+ which produces and uses the Ybt siderophore (Table 3). Thus, the irp1-2086 mutation did not affect the expression of ybt genes.
FIG. 6.
SDS-PAGE analysis of whole-cell proteins from Y. pestis strains grown in iron-sufficient and iron-deficient PMH2. Cultures from Y. pestis KIM6+ (lanes 1 and 2), KIM6-2046.1 (irp2::kan2046.1) (lane 3), and KIM6-2086 (irp1-2086) (lane 4) were incubated with 35S-labeled amino acids for 1 h. Total cellular proteins were separated on a 9% polyacrylamide gel and visualized by autoradiography. Cell extracts from iron-deficient cultures (lanes 2 to 4) or iron-sufficient cultures (lane 1) are shown. Sizes of molecular mass markers (in kilodaltons) are indicated. Arrows point to the iron-regulated proteins HMWP1 (240 kDa), HMWP2 (190 kDa), and Psn (68 kDa).
DISCUSSION
PK synthases, fatty acid synthases, and nonribosomal peptide synthetases all require posttranslational modification of the acyl, aryl, and/or peptidyl carrier protein domains for catalytic activation, the first step in the biogenesis of their products. The last step in this process is release of the product from the enzyme complex by a TE. In this study we have examined the roles of YbtD, a putative P-pant transferase for the Ybt synthetase complex, and the TE domain of HMWP1, which likely releases the completed molecule, in siderophore production.
P-pant transferases activate the enzyme complex by catalyzing transfer of P-pant moieties from coenzyme A molecules to the carrier domains of PK synthases, fatty acid synthases, and nonribosomal peptide synthetases (46). Ybt siderophore is synthesized via a mixed NRPS/PK synthetase mechanism. HMWP1 contains one acyl and one peptidyl carrier domain, while HMWP2 possesses an aryl and two peptidyl carrier domains (30). While there are some differences among the HPIs of the pathogenic yersiniae, all encode an essentially identical and interchangeable siderophore biosynthesis and transport system and none of the pathogenicity islands possess a gene encoding the essential P-pant transferase activity (12, 24, 25, 30, 54, 55, 60).
The HPI of Y. pseudotuberculosis can insert at any one of three different tRNAAsn genes; only one HPI insertion site has been identified in three different strains of Y. pestis (10, 30, 38, 53). The region of KIM10+ chromosomal DNA that contains ybtD is flanked by two tRNAAsn genes (Fig. 1). Sequences adjacent to the Y. pseudotuberculosis and Y. enterocolitica HPIs (3, 10, 38, 60) are homologous to the sequences flanking these tRNA genes. Thus, it is possible that in some strains of Y. pseudotuberculosis and Y. enterocolitica the HPI is located close to the region containing ybtD. In KIM10+, the two tRNAAsn in the vicinity of ybtD are approximately 14.2 kb apart (Fig. 1). However, in CO92, these same two tRNA genes are separated by >179 kb. (53) This difference between KIM10+ and CO92 probably results from an inversion involving the IS285 element (Fig. 1).
We have demonstrated that ybtD is essential for normal production of the Ybt siderophore. Although the authentic start site for YbtD remains to be determined, this protein contains two conserved P-pant transferase domains (Fig. 3), suggesting that it is required to activate the carrier domains of HMWP1 and HMWP2. Bioassays using the ΔybtD mutant indicate that it is defective in Ybt siderophore production (Table 2). Our bioassay detects Ybt in iron-deficient culture supernatants diluted 1:16 (data not shown). Thus, if any Ybt siderophore is present in culture supernatants of the YbtD− mutant it is present at <6% of wild-type levels.
Surprisingly, transcription from the ybtD promoter is not regulated by the iron status of the cell or by YbtA (Table 3). This suggests that ybtD may have been recently converted for use in the Ybt system or that this P-pant transferase is used to activate more than one system. The Y. pestis KIM10+ (21) and CO92 genomes (53) both contain two NRPS systems in addition to Ybt that would require activation by a P-pant transferase. One system encodes enzymes with homologies to Bordetella siderophore biosynthetic enzymes as well as an OM receptor and an ABC transporter related to similar components in other iron transport systems (34, 43, 56). The second putative NRPS system contains open reading frames showing similarities to Yersinia HMWP1 and HMWP2 proteins and to YbtP, a fused function permease/ATP hydrolase ABC transporter component. To be functional, both these systems would require activation by a P-pant transferase, possibly YbtD. It is unlikely that the Y. pestis ACPS would work since in E. coli, the ACPS P-pant transferase activity is specific for fatty acid biosynthesis (46). However, it is unknown whether either of these putative NRPS systems is functional.
The Ybt system, like many bacterial NRPS and/or PK synthase systems, possesses a C-terminal TE domain as part of the NRPS/PK synthetase enzyme (HMWP1) in addition to a separate gene encoding an external TE (YbtT). We have now shown that both YbtT (33) and the HMWP1-TE domain (this study) are required for normal levels of Ybt siderophore production. Thus, these putative TEs do not perform redundant functions but are apparently required in separate aspects of Ybt biogenesis. It has previously been proposed that an internal TE domain likely releases the completed molecule from the enzyme complex while an external TE may serve an editing function by removing aberrant structures on mischarged NRPSs caused by nonspecific thioesterification (13, 49, 65).
In Y. pestis, we proposed that Ybt (or the Ybt-Fe complex) functions as a signal molecule in concert with the AraC-type regulator YbtA to activate transcription of other genes in the Ybt system and repress transcription of ybtA. Thus, YbtA− mutants showed reduced β-galactosidase activity from psn::lacZ and ybtP::lacZ reporter plasmids but elevated expression from a ybtA::lacZ reporter. An irp2::kan2046.1 mutation also lowered expression of the psn and ybtP promoters but to a lesser extent than the ybtA::kan2055 mutation (23, 24) (Table 3). This suggests that YbtA alone may partially activate promoters controlled by this regulator (23). In addition, strains with large deletions or insertions in irp1, irp2, ybtE, or ybtD genes, encoding products involved in Ybt synthesis, had significantly reduced expression of HMWP1, HMWP2, as well as Psn (25) (Fig. 4 and 6). In contrast, mutations in ybtT or ybtS and now in the TE domain of irp1 eliminate siderophore synthesis without affecting ybt gene expression (33) (Fig. 6). These results bring into question the role of Ybt in regulating expression of the ybt operons. However, it is possible that both the HMWP1-TE− and YbtT− mutants produce amounts of Ybt siderophore sufficient for regulatory activity but below our level of detection (∼6% of wild-type levels) in bioassays. If YbtT serves a proofreading function and removes aberrant structures from the enzyme complex, low levels of authentic Ybt might be produced in vivo in this mutant. Indeed, YbtT is not required for in vitro synthesis of Ybt using purified compounds (51). Alternatively, YbtT− mutants may produce an aberrant compound(s) that can function as an inducer in concert with YbtA yet not be effective in iron transport. The TE domain of HMWP1 likely releases the completed siderophore from the enzyme complex; noncatalytic hydrolysis of the thioester bond could release low levels of Ybt sufficient to fulfill regulatory functions without providing observable growth stimulation. Finally, YbtS is hypothesized to synthesize salicylate, which is activated by YbtE, transferred to the N-terminal aryl carrier protein domain of HMWP2, and initiates Ybt synthesis (30). Albeit at a much lower efficiency, YbtE also adenylates 2,3-dihydroxybenzoate (31). Thus, in the YbtS− mutant, YbtE may activate 2,3-dihydroxybenzoate or another phenolate compound which then initiates synthesis of an aberrant Ybt molecule. The low efficiency of YbtE-catalyzed activation of an alternate phenolate moiety or poor chain elongation from this aberrant structure may lead to low levels of an altered siderophore that interacts with YbtA and allows normal regulation of the Ybt system. Further experiments will be necessary to completely characterize this regulatory system and to determine the nature of the signal molecule in Ybt+ cells as well as in YbtT−, YbtS−, and HMWP1-TE− mutants.
Acknowledgments
This work was supported by Public Health Service grants AI42738 and AI33481 from the National Institutes of Health.
We thank Jennifer Abney for assistance with some of the β-galactosidase assays. We thank Christopher Walsh and Zucai Suo for providing pET22b-HMWP1-TEmut.
Editor: J. T. Barbieri
REFERENCES
- 1.Armstrong, S. K., G. S. Pettis, L. J. Forrester, and M. A. McIntosh. 1989. The Escherichia coli enterobactin biosynthesis gene, entD: nucleotide sequence and membrane localization of its protein product. Mol. Microbiol. 3:757-766. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1987. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 3.Bach, S., C. Buchrieser, M. Prentice, A. Guiyoule, T. Msadek, and E. Carniel. 1999. The high-pathogenicity island of Yersinia enterocolitica Ye8081 undergoes low-frequency deletion but not precise excision, suggesting recent stabilization in the genome. Infect. Immun. 67:5091-5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bearden, S. W., J. D. Fetherston, and R. D. Perry. 1997. Genetic organization of the yersiniabactin biosynthetic region and construction of avirulent mutants in Yersinia pestis. Infect. Immun. 65:1659-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bearden, S. W., and R. D. Perry. 1999. The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol. Microbiol. 32:403-414. [DOI] [PubMed] [Google Scholar]
- 6.Bearden, S. W., T. M. Staggs, and R. D. Perry. 1998. An ABC transporter system of Yersinia pestis allows utilization of chelated iron by Escherichia coli SAB11. J. Bacteriol. 180:1135-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun, V., K. Hantke, and W. Köster. 1998. Bacterial iron transport: mechanisms, genetics, and regulation, p. 67-145. In A. Sigel and H. Sigel (ed.), Metal ions in biological systems, vol. 35. Iron transport and storage in microorganisms, plants, and animals. Marcel Dekker, Inc., New York, N.Y. [PubMed]
- 9.Brem, D., C. Pelludat, A. Rakin, C. A. Jacobi, and J. Heesemann. 2001. Functional analysis of yersiniabactin transport genes of Yersinia enterocolitica. Microbiology 147:1115-1127. [DOI] [PubMed] [Google Scholar]
- 10.Buchrieser, C., R. Brosch, S. Bach, A. Guiyoule, and E. Carniel. 1998. The high-pathogenicity island of Yersinia pseudotuberculosis can be inserted into any of the three chromosomal asn tRNA genes. Mol. Microbiol. 30:965-978. [DOI] [PubMed] [Google Scholar]
- 11.Buchrieser, C., M. Prentice, and E. Carniel. 1998. The 102-kilobase unstable region of Yersinia pestis comprises a high-pathogenicity island linked to a pigmentation segment which undergoes internal rearrangement. J. Bacteriol. 180:2321-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchrieser, C., C. Rusniok, L. Frangeul, E. Couve, A. Billault, F. Kunst, E. Carniel, and P. Glaser. 1999. The 102-kilobase pgm locus of Yersinia pestis: sequence analysis and comparison of selected regions among different Yersinia pestis and Yersinia pseudotuberculosis strains. Infect. Immun. 67:4851-4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler, A. R., N. Bate, and E. Cundliffe. 1999. Impact of thioesterase activity on tylosin biosynthesis in Streptomyces fradiae. Chem. Biol. 6:287-292. [DOI] [PubMed] [Google Scholar]
- 14.Byers, B. R., and E. L. Arceneaux. 1998. Microbial iron transport: iron acquisition by pathogenic microorganisms, p. 37-66. In A. Sigel and H. Sigel (ed.), Metal ions in biological systems, vol. 35. Iron transport and storage in microorganisms, plants, and animals. Marcel Dekker, Inc., New York. [PubMed]
- 15.Carniel, E., I. Guilvout, and M. Prentice. 1996. Characterization of a large chromosomal “high-pathogenicity island” in biotype 1B Yersinia enterocolitica. J. Bacteriol. 178:6743-6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chambers, C. E., D. D. McIntyre, M. Mouck, and P. A. Sokol. 1996. Physical and structural characterization of yersiniophore, a siderophore produced by clinical isolates of Yersinia enterocolitica. BioMetals 9:157-167. [DOI] [PubMed] [Google Scholar]
- 17.Chang, A. C. Y., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciche, T. A., S. B. Bintrim, A. R. Horswill, and J. C. Ensign. 2001. A phosphopantetheinyl transferase homolog is essential for Photorhabdus luminescens to support growth and reproduction of the entomopathogenic nematode Heterorhabditis bacteriophora. J. Bacteriol. 183:3117-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coderre, P. E., and C. F. Earhart. 1989. The entD gene of the Escherichia coli K12 enterobactin gene cluster. J. Gen. Microbiol. 135:3043-3055. [DOI] [PubMed] [Google Scholar]
- 20.Cox, C. D., K. L. Rinehart, Jr., M. L. Moore, and C. J. Cook, Jr. 1981. Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 78:4256-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng, W., V. Burland, G. Plunkett III, A. Boutin, G. F. Mayhew, P. Liss, N. T. Perna, D. J. Rose, B. Mau, S. Zhou, D. C. Schwartz, J. D. Fetherston, L. E. Lindler, R. R. Brubaker, G. V. Plano, S. C. Straley, K. A. McDonough, M. L. Nilles, J. S. Matson, F. R. Blattner, and R. D. Perry. Genome sequence of Yersinia pestis KIM. J. Bacteriol., in press. [DOI] [PMC free article] [PubMed]
- 22.Drechsel, H., H. Stephan, R. Lotz, H. Haag, H. Zähner, K. Hantke, and G. Jung. 1995. Structure elucidation of yersiniabactin, a siderophore from highly virulent Yersinia strains. Liebigs Ann. 1995:1727-1733. [Google Scholar]
- 23.Fetherston, J. D., S. W. Bearden, and R. D. Perry. 1996. YbtA, an AraC-type regulator of the Yersinia pestis pesticin/yersiniabactin receptor. Mol. Microbiol. 22:315-325. [DOI] [PubMed] [Google Scholar]
- 24.Fetherston, J. D., V. J. Bertolino, and R. D. Perry. 1999. YbtP and YbtQ: two ABC transporters required for iron uptake in Yersinia pestis. Mol. Microbiol. 32:289-299. [DOI] [PubMed] [Google Scholar]
- 25.Fetherston, J. D., J. W. Lillard, Jr., and R. D. Perry. 1995. Analysis of the pesticin receptor from Yersinia pestis: role in iron-deficient growth and possible regulation by its siderophore. J. Bacteriol. 177:1824-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fetherston, J. D., and R. D. Perry. 1994. The pigmentation locus of Yersinia pestis KIM6+ is flanked by an insertion sequence and includes the structural genes for pesticin sensitivity and HMWP2. Mol. Microbiol. 13:697-708. [DOI] [PubMed] [Google Scholar]
- 27.Fetherston, J. D., P. Schuetze, and R. D. Perry. 1992. Loss of the pigmentation phenotype in Yersinia pestis is due to the spontaneous deletion of 102 kb of chromosomal DNA which is flanked by a repetitive element. Mol. Microbiol. 6:2693-2704. [DOI] [PubMed] [Google Scholar]
- 28.Froehlich, B., L. Husmann, J. Caron, and J. R. Scott. 1994. Regulation of rns, a positive regulatory factor for pili of enterotoxigenic Escherichia coli. J. Bacteriol. 176:5385-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Froehlich, B. J., and J. R. Scott. 1991. A single-copy promoter-cloning vector for use in Escherichia coli. Gene 108:99-101. [DOI] [PubMed] [Google Scholar]
- 30.Gehring, A. M., E. DeMoll, J. D. Fetherston, I. Mori, G. F. Mayhew, F. R. Blattner, C. T. Walsh, and R. D. Perry. 1998. Iron acquisition in plague: modular logic in enzymatic biogenesis of yersiniabactin by Yersinia pestis. Chem. Biol. 5:573-586. [DOI] [PubMed] [Google Scholar]
- 31.Gehring, A. M., I. Mori, R. D. Perry, and C. T. Walsh. 1998. The nonribosomal peptide synthetase HMWP2 forms a thiazoline ring during biogenesis of yersiniabactin, an iron-chelating virulence factor of Yersinia pestis. Biochemistry 37:11637-11650. [DOI] [PubMed] [Google Scholar]
- 32.Gehring, A. M., I. Mori, and C. T. Walsh. 1998. Reconstitution and characterization of the Escherichia coli enterobactin synthetase from EntB, EntE, and EntF. Biochemistry 37:2648-2659. [DOI] [PubMed] [Google Scholar]
- 33.Geoffroy, V. A., J. D. Fetherston, and R. D. Perry. 2000. Yersinia pestis YbtU and YbtT are involved in synthesis of the siderophore yersiniabactin but have different effects on regulation. Infect. Immun. 68:4452-4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giardina, P. C., L. A. Foster, S. I. Toth, B. A. Roe, and D. W. Dyer. 1997. Analysis of the alcABC operon encoding alcaligin biosynthesis enzymes in Bordetella bronchiseptica. Gene 194:19-24. [DOI] [PubMed] [Google Scholar]
- 35.Gong, S., S. W. Bearden, V. A. Geoffroy, J. D. Fetherston, and R. D. Perry. 2001. Characterization of the Yersinia pestis Yfu ABC iron transport system. Infect. Immun. 67:2829-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guilvout, I., O. Mercereau-Puijalon, S. Bonnefoy, A. P. Pugsley, and E. Carniel. 1993. High-molecular-weight protein 2 of Yersinia enterocolitica is homologous to AngR of Vibrio anguillarum and belongs to a family of proteins involved in nonribosomal peptide synthesis. J. Bacteriol. 175:5488-5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hare, J. M., and K. A. McDonough. 1999. High-frequency RecA-dependent and -independent mechanisms of Congo red binding mutations in Yersinia pestis. J. Bacteriol. 181:4896-4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hare, J. M., A. K. Wagner, and K. A. McDonough. 1999. Independent acquisition and insertion into different chromosomal locations of the same pathogenicity island in Yersinia pestis and Yersinia pseudotuberculosis. Mol. Microbiol. 31:291-303. [DOI] [PubMed] [Google Scholar]
- 39.Heesemann, J., K. Hantke, T. Vocke, E. Saken, A. Rakin, I. Stojiljkovic, and R. Berner. 1993. Virulence of Yersinia enterocolitica is closely associated with siderophore production, expression of an iron-repressible outer membrane polypeptide of 65 000 Da and pesticin sensitivity. Mol. Microbiol. 8:397-408. [DOI] [PubMed] [Google Scholar]
- 40.Hornung, J. M., H. A. Jones, and R. D. Perry. 1996. The hmu locus of Yersinia pestis is essential for utilization of free haemin and haem-protein complexes as iron sources. Mol. Microbiol. 20:725-739. [DOI] [PubMed] [Google Scholar]
- 41.Humphreys, G. O., G. A. Willshaw, and E. S. Anderson. 1975. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim. Biophys. Acta 383:457-463. [DOI] [PubMed] [Google Scholar]
- 42.Jalal, M. A. F., M. B. Hossain, D. van der Helm, J. Sanders-Loehr, L. A. Actis, and J. H. Crosa. 1989. Structure of anguibactin, a unique plasmid-related bacterial siderophore from the fish pathogen Vibrio anguillarum. J. Am. Chem. Soc. 111:292-296. [Google Scholar]
- 43.Kang, H. Y., T. J. Brickman, F. C. Beaumont, and S. K. Armstrong. 1996. Identification and characterization of iron-regulated Bordetella pertussis alcaligin siderophore biosynthesis genes. J. Bacteriol. 178:4877-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaniga, K., I. Delor, and G. R. Cornelis. 1991. A wide-host-range suicide vector for improving reverse genetics in Gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109:137-141. [DOI] [PubMed] [Google Scholar]
- 45.Kutyrev, V. V., A. A. Filippov, O. S. Oparina, and O. A. Protsenko. 1992. Analysis of Yersinia pestis chromosomal determinants Pgm+ and Psts associated with virulence. Microb. Pathog. 12:177-186. [DOI] [PubMed] [Google Scholar]
- 46.Lambalot, R. H., A. M. Gehring, R. S. Flugel, P. Zuber, M. LaCelle, M. A. Marahiel, R. Reid, C. Khosla, and C. T. Walsh. 1996. A new enzyme superfamily: the phosphopantetheinyl transferases. Chem. Biol. 3:923-936. [DOI] [PubMed] [Google Scholar]
- 47.Linn, T., and R. St. Pierre. 1990. Improved vector system for constructing transcriptional fusions that ensures independent translation of lacZ. J. Bacteriol. 172:1077-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lucier, T. S., and R. R. Brubaker. 1992. Determination of genome size, macrorestriction pattern polymorphism, and nonpigmentation-specific deletion in Yersinia pestis by pulsed-field gel electrophoresis. J. Bacteriol. 174:2078-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marahiel, M. A., T. Stachelhaus, and H. D. Mootz. 1997. Modular peptide synthetases involved in nonribosomal peptide synthesis. Chem. Rev. 97:2651-2673. [DOI] [PubMed] [Google Scholar]
- 50.Mietzner, T. A., and S. A. Morse. 1994. The role of iron-binding proteins in the survival of pathogenic bacteria. Annu. Rev. Nutr. 14:471-493. [DOI] [PubMed] [Google Scholar]
- 51.Miller, D. A., L. Luo, N. Hillson, T. A. Keating, and C. T. Walsh. 2002. Yersiniabactin synthetase: a four protein assembly line producing the nonribosomal peptide/polyketide hybrid siderophore of Yersinia pestis. Chem. Biol. 9:333-344. [DOI] [PubMed] [Google Scholar]
- 52.Miller, J. H. 1992. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 53.Parkhill, J., B. W. Wren, N. R. Thomson, R. W. Titball, M. T. Holden, M. B. Prentice, M. Sebaihia, K. D. James, C. Churcher, K. L. Mungall, S. Baker, D. Basham, S. D. Bentley, K. Brooks, A. M. Cerdeno-Tarraga, T. Chillingworth, A. Cronin, R. M. Davies, P. Davis, G. Dougan, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Leather, S. Moule, P. C. Oyston, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413:523-527. [DOI] [PubMed] [Google Scholar]
- 54.Pelludat, C., A. Rakin, C. A. Jacobi, S. Schubert, and J. Heesemann. 1998. The yersiniabactin biosynthetic gene cluster of Yersinia enterocolitica: organization and siderophore-dependent regulation. J. Bacteriol. 180:538-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perry, R. D., P. B. Balbo, H. A. Jones, J. D. Fetherston, and E. DeMoll. 1999. Yersiniabactin from Yersinia pestis: biochemical characterization of the siderophore and its role in iron transport and regulation. Microbiology 145:1181-1190. [DOI] [PubMed] [Google Scholar]
- 56.Perry, R. D., S. W. Bearden, and J. D. Fetherston. 2001. Iron and heme acquisition and storage systems of Yersinia pestis. Recent Res. Dev. Microbiol. 5:13-27. [Google Scholar]
- 57.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis: etiologic agent of plague. Clin. Microbiol. Rev. 10:35-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perry, R. D., M. L. Pendrak, and P. Schuetze. 1990. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J. Bacteriol. 172:5929-5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perry, R. D., S. C. Straley, J. D. Fetherston, D. J. Rose, J. Gregor, and F. R. Blattner. 1998. DNA sequencing and analysis of the low-Ca2+-response plasmid pCD1 of Yersinia pestis KIM5. Infect. Immun. 66:4611-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rakin, A., C. Noelting, S. Schubert, and J. Heesemann. 1999. Common and specific characteristics of the high-pathogenicity island of Yersinia enterocolitica. Infect. Immun. 67:5265-5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rakin, A., E. Saken, D. Harmsen, and J. Heesemann. 1994. The pesticin receptor of Yersinia enterocolitica: a novel virulence factor with dual function. Mol. Microbiol. 13:253-263. [DOI] [PubMed] [Google Scholar]
- 62.Rossi, M.-S., J. D. Fetherston, S. Létoffé, E. Carniel, R. D. Perry, and J.-M. Ghigo. 2001. Identification and characterization of the hemophore-dependent heme acquisition system of Yersinia pestis. Infect. Immun. 69:6707-6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 64.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schneider, A., and M. A. Marahiel. 1998. Genetic evidence for a role of thioesterase domains, integrated in or associated with peptide synthetases, in non-ribosomal peptide biosynthesis in Bacillus subtilis. Arch. Microbiol. 169:404-410. [DOI] [PubMed] [Google Scholar]
- 66.Schubert, S., A. Rakin, H. Karch, E. Carniel, and J. Heesemann. 1998. Prevalence of the “high-pathogenicity island” of Yersinia species among Escherichia coli strains that are pathogenic to humans. Infect. Immun. 66:480-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shaw-Reid, C., N. Kelleher, H. C. Losey, A. M. Gehring, C. Berg, and C. T. Walsh. 1999. Assembly line enzymology by multimodular nonribosomal peptide synthetases: the thioesterase domain of Escherichia coli EntF catalyzes both elongation and cyclolactonization. Chem. Biol. 6:385-400. [DOI] [PubMed] [Google Scholar]
- 68.Sikkema, D. J., and R. R. Brubaker. 1987. Resistance to pesticin, storage of iron, and invasion of HeLa cells by yersiniae. Infect. Immun. 55:572-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Staggs, T. M., J. D. Fetherston, and R. D. Perry. 1994. Pleiotropic effects of a Yersinia pestis fur mutation. J. Bacteriol. 176:7614-7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Staggs, T. M., and R. D. Perry. 1991. Identification and cloning of a fur regulatory gene in Yersinia pestis. J. Bacteriol. 173:417-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suo, Z., C. T. Walsh, and D. A. Miller. 1999. Tandem heterocyclization activity of the multidomain 230 kDa HMWP2 subunit of Yersinia pestis yersiniabactin synthetase: interaction of the 1-1382 and 1383-2035 fragments. Biochemistry 38:14023-14035. [DOI] [PubMed] [Google Scholar]
- 72.Surgalla, M. J., and E. D. Beesley. 1969. Congo red-agar plating medium for detecting pigmentation in Pasteurella pestis. Appl. Microbiol. 18:834-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thompson, J. M., H. A. Jones, and R. D. Perry. 1999. Molecular characterization of the hemin uptake locus (hmu) from Yersinia pestis and analysis of hmu mutants for hemin and hemoprotein utilization. Infect. Immun. 67:3879-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195-199. [PubMed] [Google Scholar]
- 75.Weinberg, E. D., and G. A. Weinberg. 1995. The role of iron in infection. Curr. Opin. Infect. Dis. 8:164-169. [Google Scholar]
- 76.Wyckoff, E. E., J. A. Stoebner, K. E. Reed, and S. M. Payne. 1997. Cloning of a Vibrio cholerae vibriobactin gene cluster: identification of genes required for early steps in siderophore biosynthesis. J. Bacteriol. 179:7055-7062. [DOI] [PMC free article] [PubMed] [Google Scholar]