Abstract
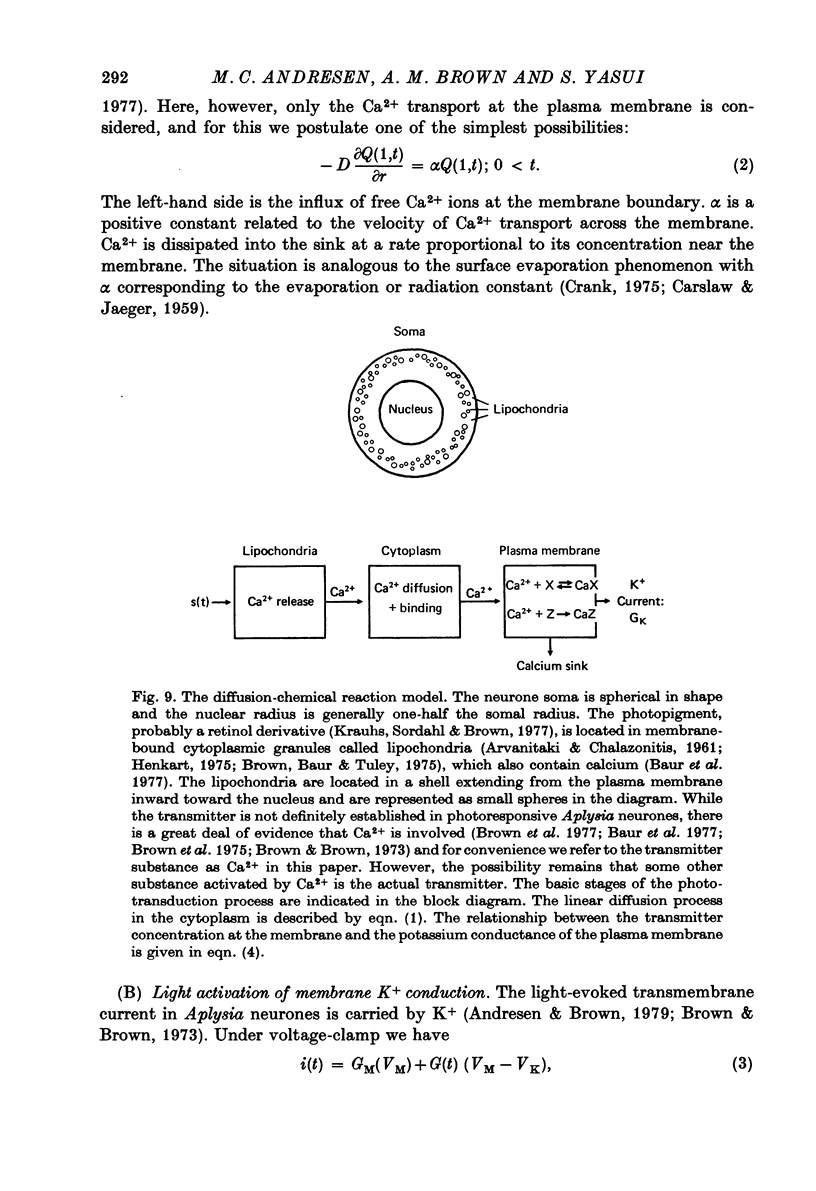
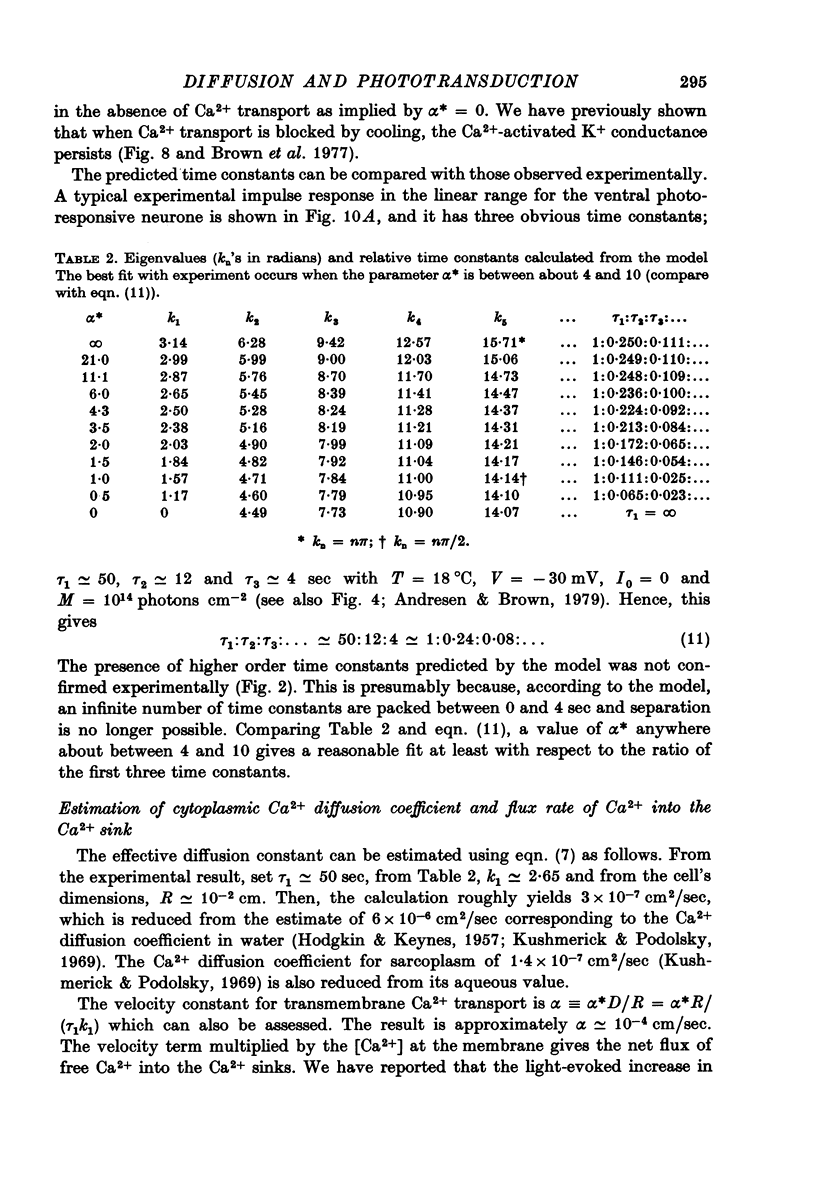
1. Membrane currents produced by flashes and steps of light (photo-current) were recorded from the ventral photoresponsive neurone of Aplysia californica. The effects of background illumination and changes in temperature were also examined. 2. The falling phase of the response wave form may be separated into two components with time constants of 10--12 sec and 50 sec. 3. Background illumination reduced the response amplitude to light impulses without appreciably altering the response wave form. 4. Lowering the temperature greatly reduced the amplitude of the photo-current with a Q10 of 2.91 (25--15 degrees C) and greatly prolonged the duration of the response. 5. Because of the relatively large distance between the plasma membrane and the pigmented cytoplasmic lipochondria where light is absorbed, a diffusion-based model with Ca as the internal-transmitter (Andresen & Brown, 1979) was developed. 6. In this model diffusion of Ca2+ released from the lipochondria upon photon absorption is slowed by the reversible uptake of Ca2+ at cytoplasmic binding sites. Ca2+ interacts with sites at the plasma membrane to increase GK and Ca2+ levels are subsequently restored by irreversible uptake processes. Ca2+ release and its adsorption and desorption from the more numerous plasma membrane binding sites were assumed to be instantaneous with respect to the duration of the light-evoked response. 7. The linearized model equations adequately predict the experimental response wave forms, the effects of temperature, and saturation of the steady-state amplitude--stimulus relationship. Aside from amplitude scaling, no curve-fitting was used. 8. The model also gives realistic values for the cytoplasmic diffusion coefficient of Ca and the net rate of Ca efflux required to restore dark Ca activity.
Full text
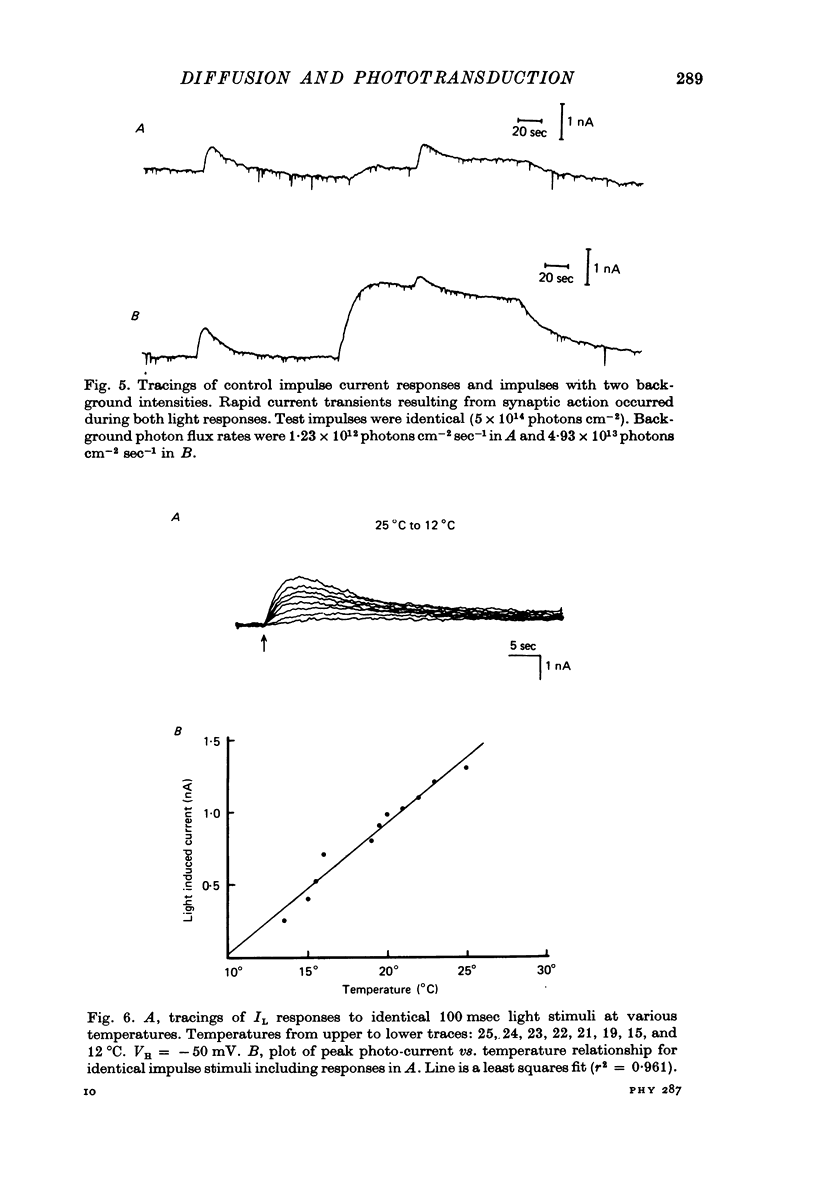
PDF

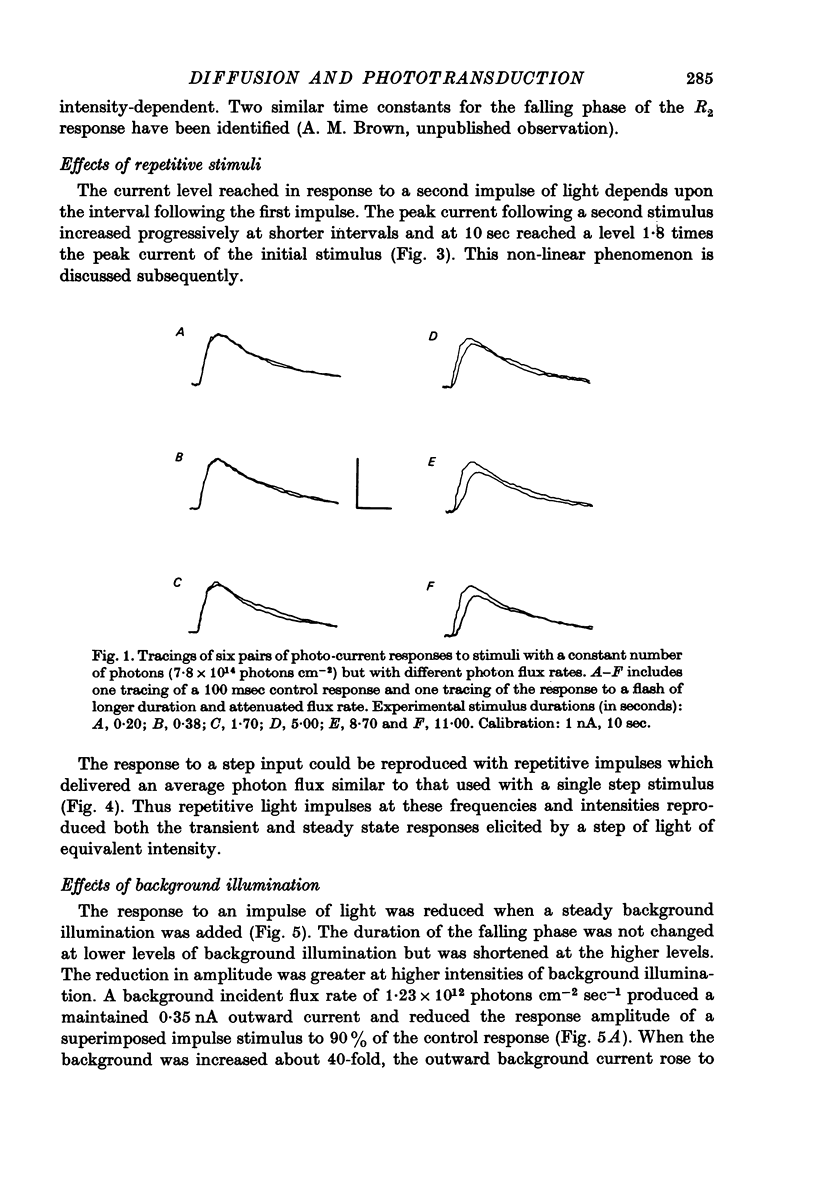
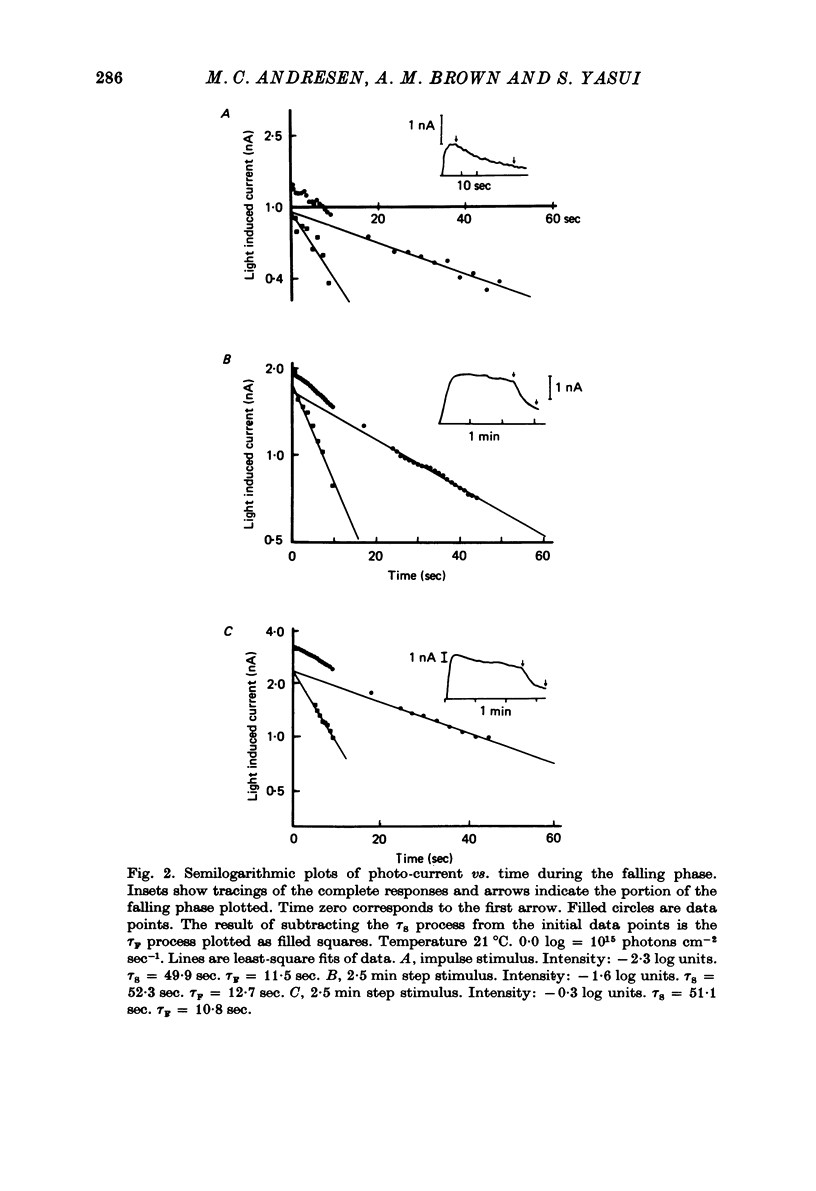
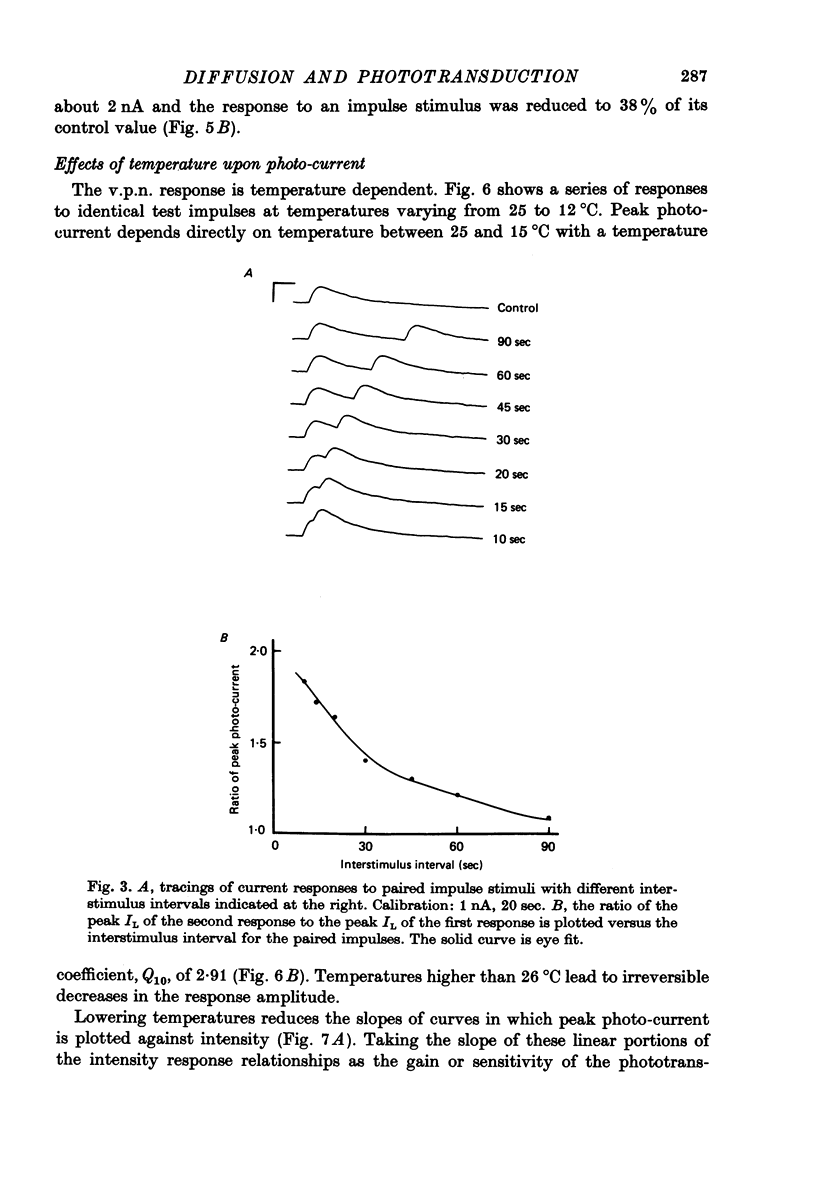
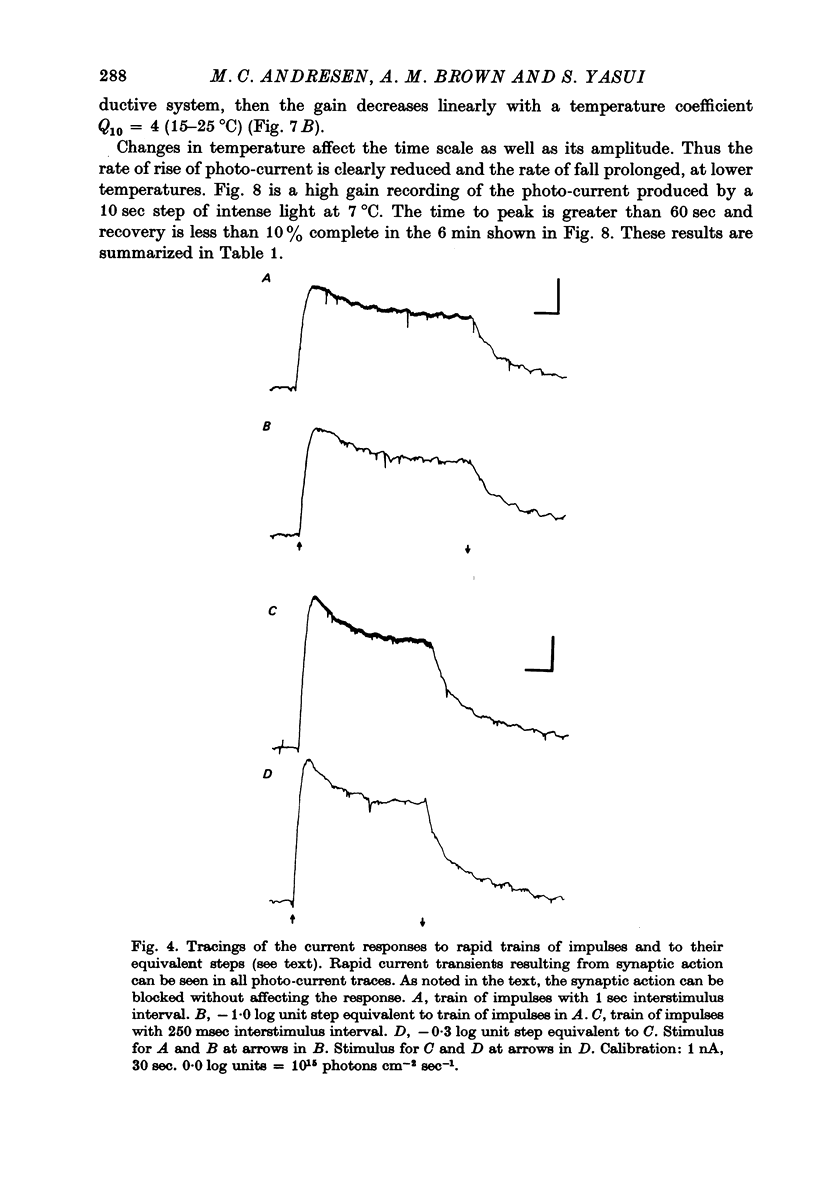
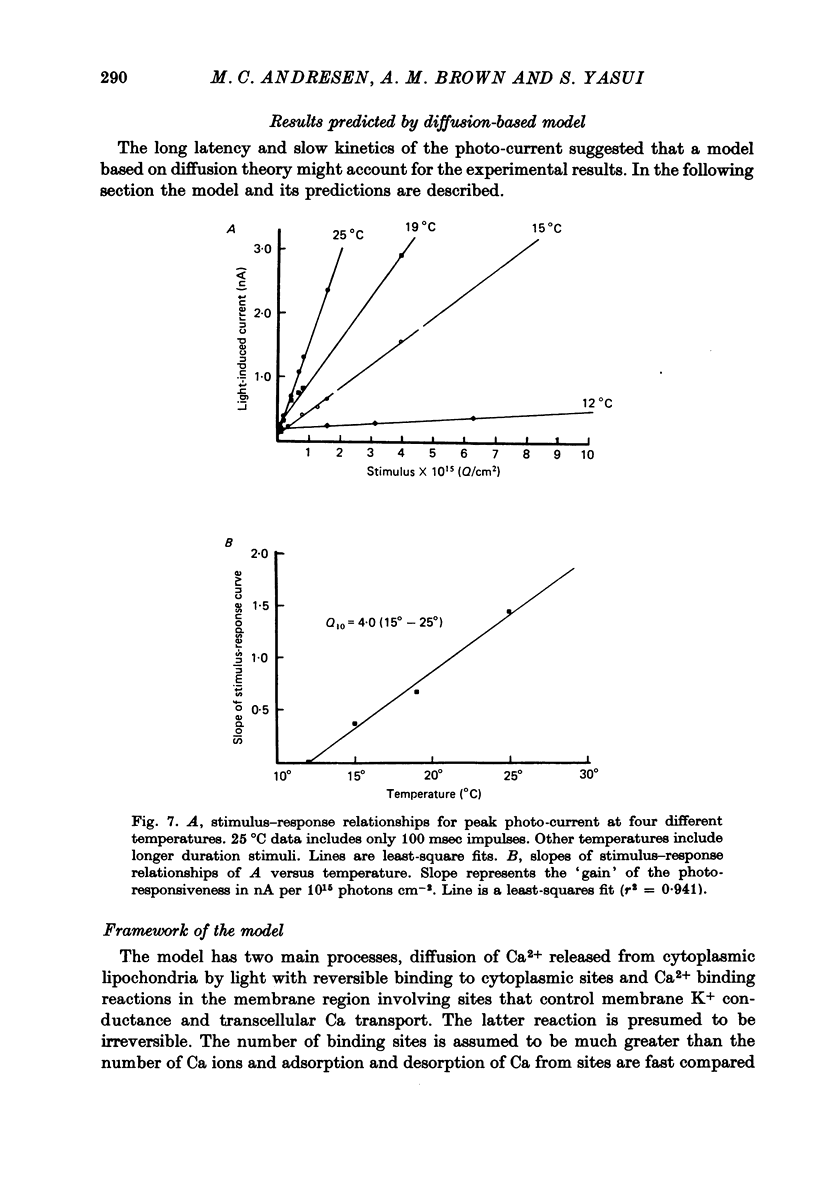
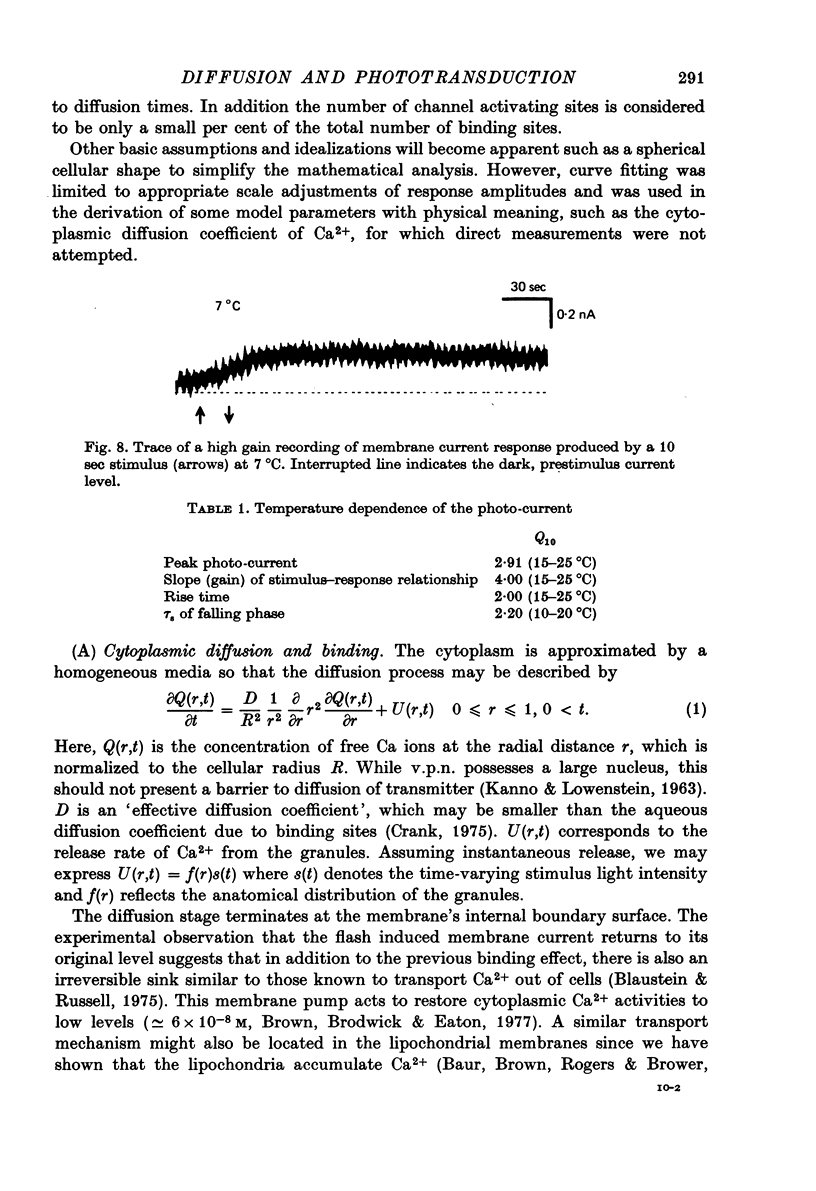






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andresen M. C., Brown A. M. Photoresponses of a sensitive extraretinal photoreceptor in Aplysia. J Physiol. 1979 Feb;287:267–282. doi: 10.1113/jphysiol.1979.sp012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. Kinetics and energetics of calcium efflux from intact squid giant axons. J Physiol. 1976 Jul;259(1):103–144. doi: 10.1113/jphysiol.1976.sp011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur P. S., Jr Lipochondria and the light response of Aplysia giant neurons,. J Neurobiol. 1977 Jan;8(1):19–42. doi: 10.1002/neu.480080103. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. Reconstruction of the electrical responses of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):759–791. doi: 10.1113/jphysiol.1974.sp010733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Russell J. M. Sodium-calcium exchange and calcium-calcium exchange in internally dialyzed squid giant axons. J Membr Biol. 1975 Jul 24;22(3-4):285–312. doi: 10.1007/BF01868176. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Baur P. S., Jr, Tuley F. H., Jr Phototransduction in aplysia neurons: calcium release from pigmented granules is essential. Science. 1975 Apr 11;188(4184):157–160. doi: 10.1126/science.1114346. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Brodwick M. S., Eaton D. C. Intracellular calcium and extra-retinal photoreception of Aplysia Giant neurons. J Neurobiol. 1977 Jan;8(1):1–18. doi: 10.1002/neu.480080102. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Brown H. M. Light response of a giant Aplysia neuron. J Gen Physiol. 1973 Sep;62(3):239–254. doi: 10.1085/jgp.62.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONE R. A. THE RAT ELECTRORETINOGRAM. II. BLOCH'S LAW AND THE LATENCY MECHANISM OF THE B-WAVE. J Gen Physiol. 1964 Jul;47:1107–1116. doi: 10.1085/jgp.47.6.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G., HODGKIN A. L. CHANGES IN TIME SCALE AND SENSITIVITY IN THE OMMATIDIA OF LIMULUS. J Physiol. 1964 Aug;172:239–263. doi: 10.1113/jphysiol.1964.sp007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A. The visual process: Excitatory mechanisms in the primary receptor cells. Annu Rev Biophys Bioeng. 1972;1:131–158. doi: 10.1146/annurev.bb.01.060172.001023. [DOI] [PubMed] [Google Scholar]
- Henkart M. Light-induced changes in the structure of pigmented granules in aplysia neurons. Science. 1975 Apr 11;188(4184):155–157. doi: 10.1126/science.1114345. [DOI] [PubMed] [Google Scholar]
- KANNO Y., LOEWENSTEIN W. R. A STUDY OF THE NUCLEUS AND CELL MEMBRANES OF OOCYTES WITH AN INTRA-CELLULAR ELECTRODE. Exp Cell Res. 1963 Jun;31:149–166. doi: 10.1016/0014-4827(63)90164-2. [DOI] [PubMed] [Google Scholar]
- Krauhs J. M., Sordahl L. A., Brown A. M. Isolation of pigmented granules involved in extra-retinal photoreception in Aplysia californica neurons. Biochim Biophys Acta. 1977 Nov 15;471(1):25–31. doi: 10.1016/0005-2736(77)90389-3. [DOI] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. VISUAL ADAPTATION. Proc R Soc Lond B Biol Sci. 1965 Mar 16;162:20–46. doi: 10.1098/rspb.1965.0024. [DOI] [PubMed] [Google Scholar]


