Abstract
1. A combination of bilateral lesions within the nucleus parabrachialis medialis complex (n.p.b.m.) and bilateral vagotomy typically resulted in an apneustic respiratory pattern in decerebrate and paralysed cats. Integrated efferent phrenic nerve activity was recorded as an index of the respiratory rhythm.
2. Changes in components of this apneustic breathing cycle were evaluated in response to steady-state hypercapnia and hypoxia. The components evaluated were (a) the period of phrenic discharge (inspiratory time, TI), (b) the period of no detectable phrenic activity (expiratory time, TE), (c) the total duration of the apneustic respiratory cycle (TTOT, the sum of TI and TE), and (d) the average height of the integrated phrenic nerve activity (apneustic depth).
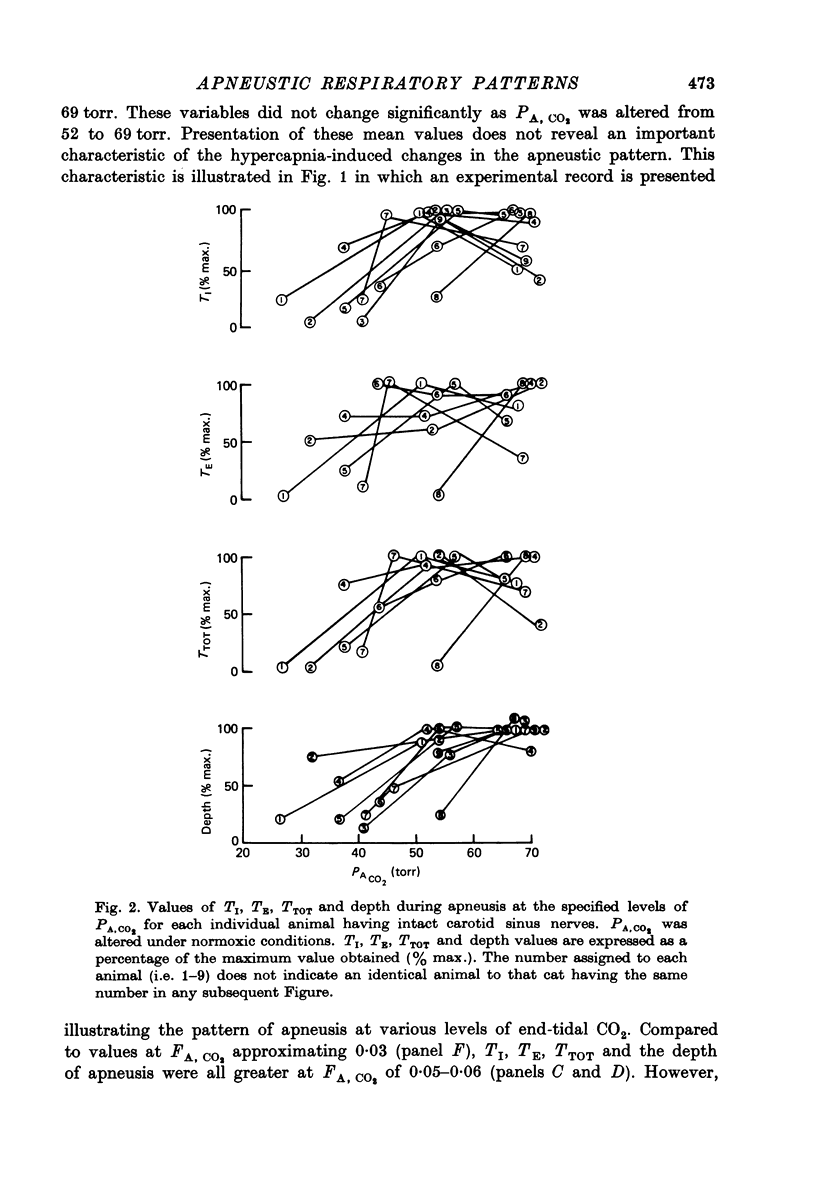
3. Elevations of PA, CO2 from values below 45 torr to 50-60 torr, under both hyperoxic and normoxic conditions, resulted in significant elevations of TI, TE, TTOT and depth. Further PA, CO2 elevations to approximately 70 torr caused no change, or frequently, a decrease in TI, TE and TTOT; the apneustic depth increased in most animals.
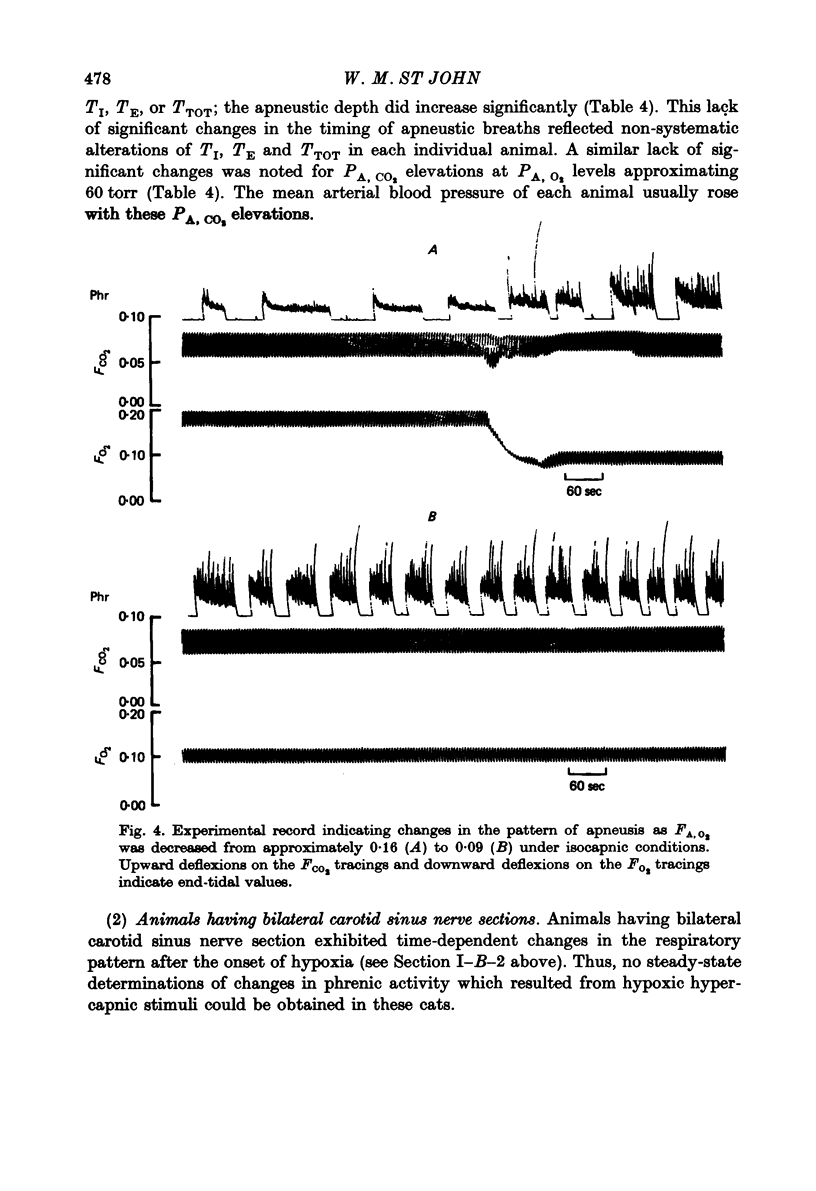
4. Diminutions in PA, O2 from normoxic to hypoxic levels at isocapnia typically caused an increase in apneustic depth and, concomitantly, significant decreases in TI, TE and TTOT.
5. Pharmacological stimulation of the carotid chemoreceptors by intracarotid administration of 1·0-20 μg NaCN produced a premature onset of phrenic nerve activity if delivered during the expiratory period. Such NaCN administrations, delivered during the inspiratory phase, resulted in an augmentation of the integrated phrenic discharge and a premature termination of phrenic activity. Carotid sinus nerve section eliminated the response to NaCN administration.
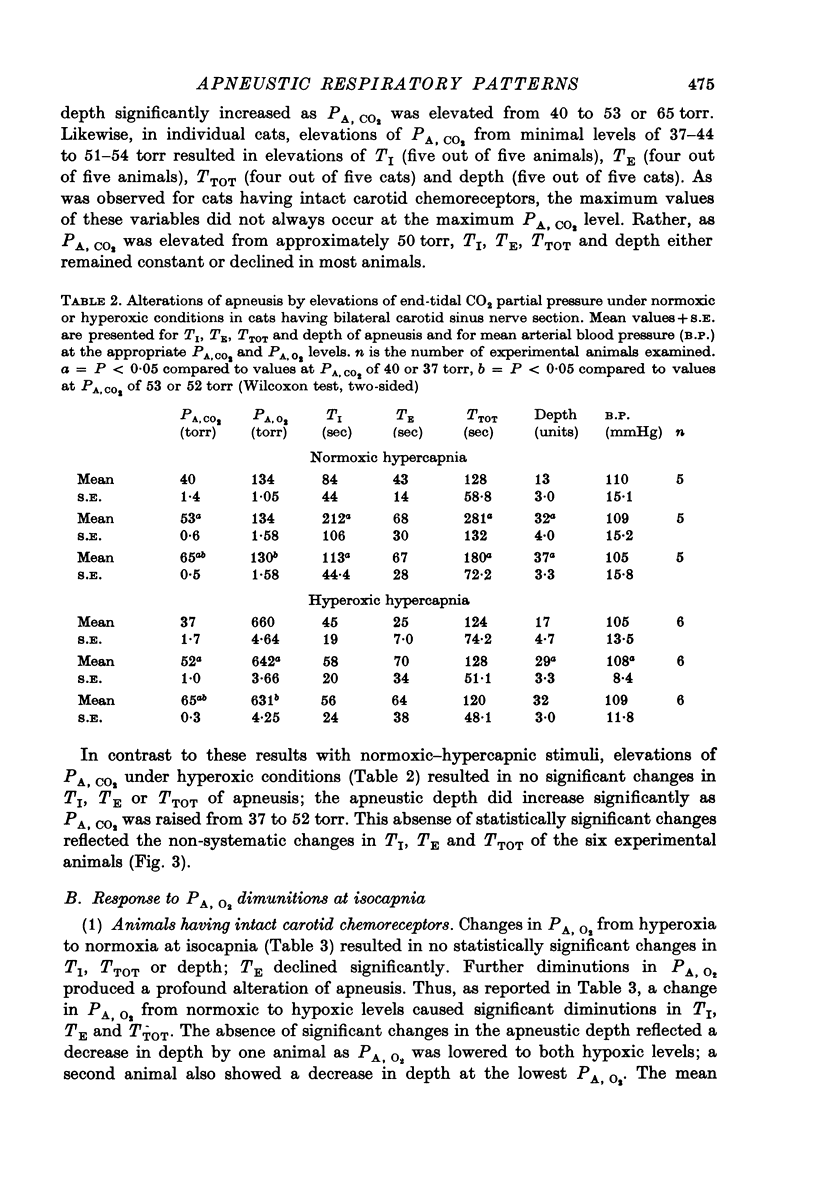
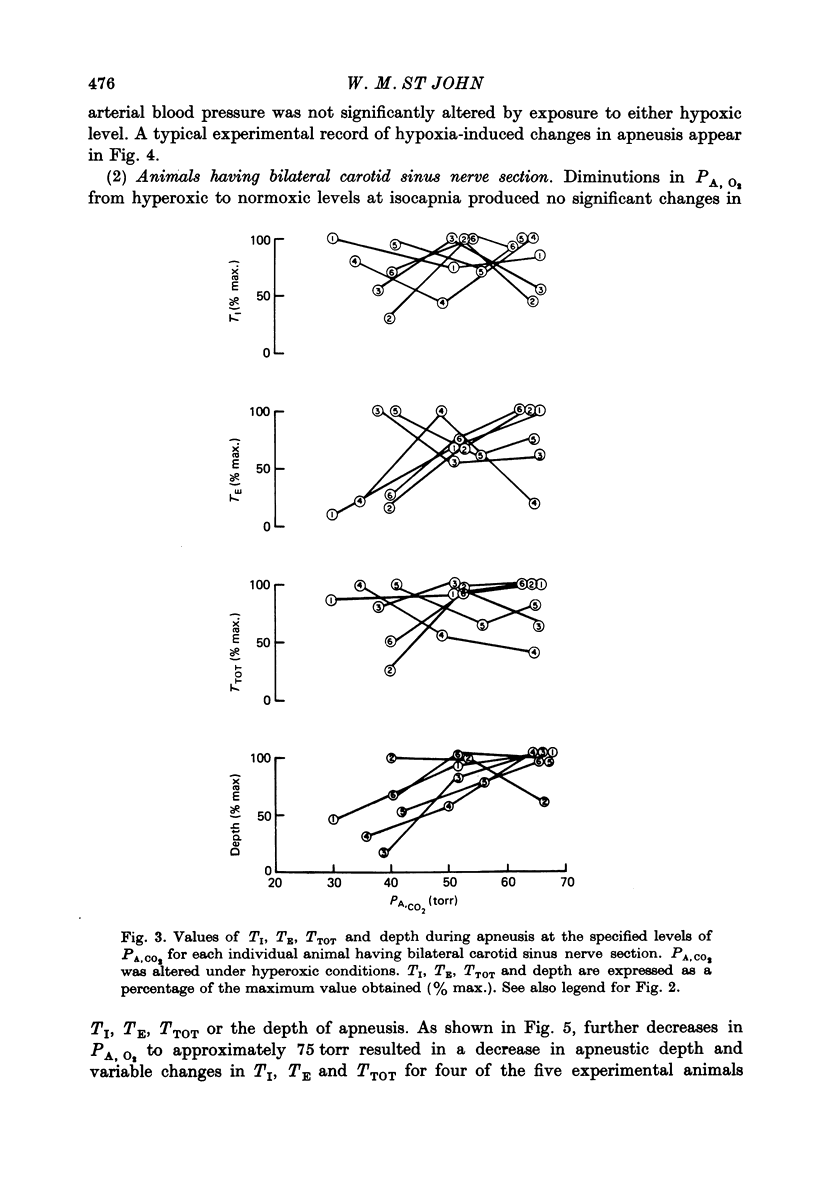
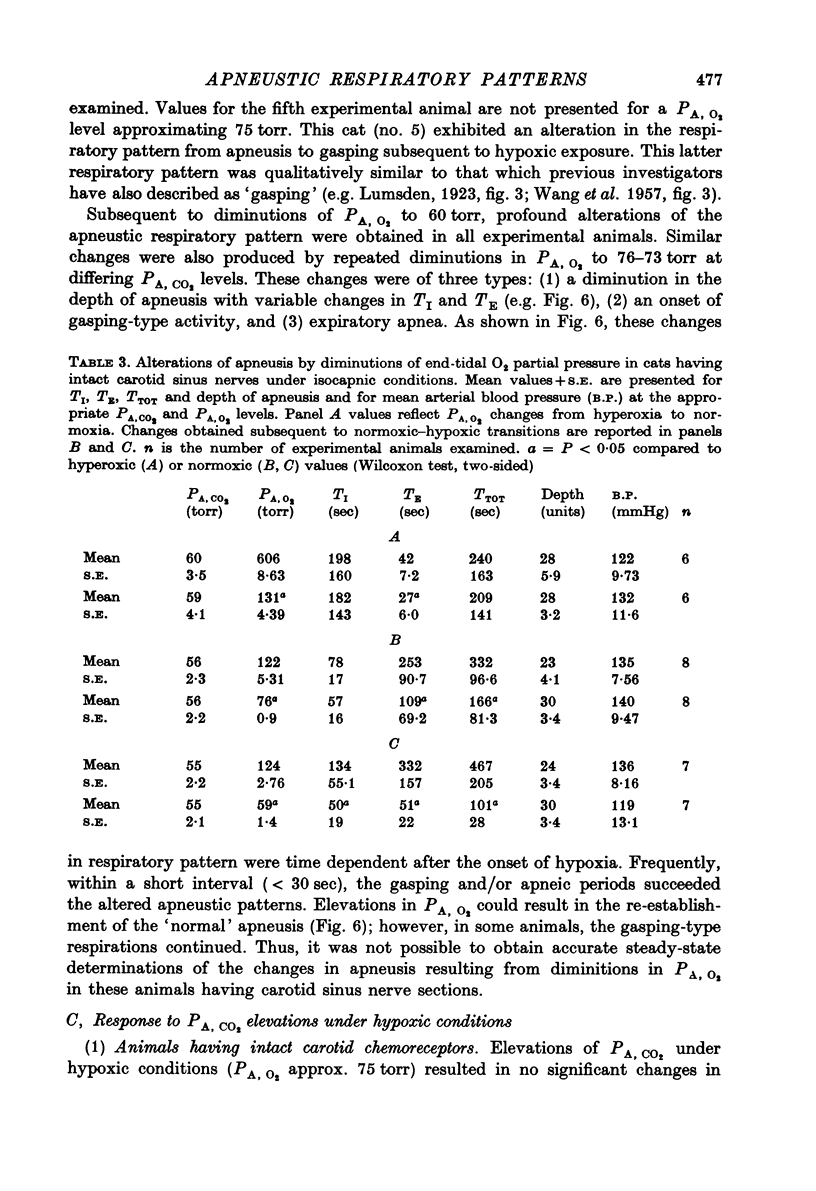
6. In experimental animals having bilateral carotid sinus nerve section, normoxic hypercapnia caused similar changes in the apneustic breathing pattern to those recorded in cats having intact carotid chemoreceptors. However, isocapnic hypoxia induced time-dependent changes in the pattern of phrenic discharge including diminutions in depth, an onset of gasping-type activity, or expiratory apnea.
7. In a few animals, bilateral n.p.b.m. lesions and bilateral vagotomy resulted in expiratory apnea which was continuous as long as ventilation with air was maintained. This expiratory apnea was replaced by an apneustic breathing pattern following diminutions of PA, O2 below 90 torr. This establishment of an apneustic breathing pattern by hypoxia was observed both in animals having intact, as well as sectioned, carotid sinus nerves. This expiratory apnea could also be terminated by a single apneustic inspiration following general somatic stimulation or, in cats having intact carotid chemoreceptors, following intracarotid NaCN administration.
8. It is concluded that hypercapnia and hypoxia produce differential alterations of the apneustic breathing pattern in decerebrate cats. Further, the hypoxia-induced changes are considered to represent the net result of carotid chemoreceptor stimulation and brain stem depression. The results of this study are considered in the context of proposed mechanisms for phase-switching of the respiratory cycle.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRECKENRIDGE C. G., HOFF H. E. Pontine and medullary regulation of respiration in the cat. Am J Physiol. 1950 Feb;160(2):385–394. doi: 10.1152/ajplegacy.1950.160.2.385. [DOI] [PubMed] [Google Scholar]
- Bertrand F., Hugelin A. Respiratory synchronizing function of nucleus parabrachialis medialis: pneumotaxic mechanisms. J Neurophysiol. 1971 Mar;34(2):189–207. doi: 10.1152/jn.1971.34.2.189. [DOI] [PubMed] [Google Scholar]
- Bradley G. W., von Euler C., Marttila I., Roos B. A model of the central and reflex inhibition of inspiration in the cat. Biol Cybern. 1975 Aug 8;19(2):105–116. doi: 10.1007/BF00364107. [DOI] [PubMed] [Google Scholar]
- Clark F. J., von Euler C. On the regulation of depth and rate of breathing. J Physiol. 1972 Apr;222(2):267–295. doi: 10.1113/jphysiol.1972.sp009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. I., Feldman J. L. Models of respiratory phase-switching. Fed Proc. 1977 Sep;36(10):2367–2374. [PubMed] [Google Scholar]
- Cohen M. I. Switching of the respiratory phases and evoked phrenic responses produced by rostral pontine electrical stimulation. J Physiol. 1971 Aug;217(1):133–158. doi: 10.1113/jphysiol.1971.sp009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge F. L. The importance of timing on the respiratory effects of intermittent carotid body chemoreceptor stimulation. J Physiol. 1972 Apr;222(2):319–333. doi: 10.1113/jphysiol.1972.sp009799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald R. S. Relationships between tidal volume and phrenic nerve activity during hypercapnia and hypoxia. Acta Neurobiol Exp (Wars) 1973;33(1):419–425. [PubMed] [Google Scholar]
- Haldane J. S., Meakins J. C., Priestley J. G. The respiratory response to anoxaemia. J Physiol. 1919 May 20;52(6):420–432. doi: 10.1113/jphysiol.1919.sp001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscoe S., Polosa C. Synchronization of respiratory frequency by somatic afferent stimulation. J Appl Physiol. 1976 Feb;40(2):138–148. doi: 10.1152/jappl.1976.40.2.138. [DOI] [PubMed] [Google Scholar]
- Knox C. K. Characteristics of inflation and deflation reflexes during expiration of the cat. J Neurophysiol. 1973 Mar;36(2):284–295. doi: 10.1152/jn.1973.36.2.284. [DOI] [PubMed] [Google Scholar]
- Knox C. K., King G. W. Changes in the Breuer-Hering reflexes following rostral pontine lesion. Respir Physiol. 1976 Nov;28(2):189–206. doi: 10.1016/0034-5687(76)90038-4. [DOI] [PubMed] [Google Scholar]
- Lumsden T. Observations on the respiratory centres in the cat. J Physiol. 1923 Mar 21;57(3-4):153–160. doi: 10.1113/jphysiol.1923.sp002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint John W. M., Bond G. C., Pasley J. N. Integration of chemoreceptor stimuli by rostral brainstem respiratory areas. J Appl Physiol. 1975 Aug;39(2):209–214. doi: 10.1152/jappl.1975.39.2.209. [DOI] [PubMed] [Google Scholar]
- St John W. M., Glasser R. L., King R. A. Apneustic breathing after vagotomy in cats with chronic pneumotaxic center lesions. Respir Physiol. 1971 Jun;12(2):239–250. doi: 10.1016/0034-5687(71)90056-9. [DOI] [PubMed] [Google Scholar]
- St John W. M. Integration of peripheral and central chemoreceptor stimuli by pontine and medullary respiratory centers. Fed Proc. 1977 Sep;36(10):2421–2427. [PubMed] [Google Scholar]
- St John W. M., Wang S. C. Alteration from apneusis to more regular rhythmic respiration in decerebrate cats. Respir Physiol. 1977 Sep;31(1):91–106. doi: 10.1016/0034-5687(77)90068-8. [DOI] [PubMed] [Google Scholar]
- St John W. M., Wang S. C. Integration of chemoreceptor stimuli by caudal pontile and rostral medullary sites. J Appl Physiol. 1976 Nov;41(5 Pt 1):612–622. doi: 10.1152/jappl.1976.41.5.612. [DOI] [PubMed] [Google Scholar]
- Stella G. On the mechanism of production, and the physiological significance of "apneusis". J Physiol. 1938 Jun 14;93(1):10–23. doi: 10.1113/jphysiol.1938.sp003621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella G. The dependence of the activity of the "apneustic centre" on the carbon dioxide of the arterial blood. J Physiol. 1938 Aug 15;93(3):263–275. doi: 10.1113/jphysiol.1938.sp003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella G. The reflex response of the "apneustic" centre to stimulation of the chemo-receptors of the carotid sinus. J Physiol. 1939 Apr 14;95(3):365–372. doi: 10.1113/jphysiol.1939.sp003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang P. C. Brain stem control of respiratory depth and rate in the cat. Respir Physiol. 1967 Dec;3(3):349–366. doi: 10.1016/0034-5687(67)90064-3. [DOI] [PubMed] [Google Scholar]
- von Euler C., Marttila I., Remmers J. E., Trippenbach T. Effects of lesions in the parabrachial nucleus on the mechanisms for central and reflex termination of inspiration in the cat. Acta Physiol Scand. 1976 Mar;96(3):324–337. doi: 10.1111/j.1748-1716.1976.tb10203.x. [DOI] [PubMed] [Google Scholar]
- von Euler C. The functional organization of the respiratory phase-switching mechanisms. Fed Proc. 1977 Sep;36(10):2375–2380. [PubMed] [Google Scholar]
- von Euler C., Trippenbach T. Excitability changes of the inspiratory "off-switch" mechanism tested by electrical stimulation in nucleus parabrachialis in the cat. Acta Physiol Scand. 1976 Jun;97(2):175–188. doi: 10.1111/j.1748-1716.1976.tb10250.x. [DOI] [PubMed] [Google Scholar]


