Water retention in excess of total body sodium frequently occurs in patients with cardiac failure or cirrhosis and in pregnancy. In fact, hyponatraemia signifies a poor prognosis in cardiac failure and cirrhosis1,2. The mechanism for this hyponatraemia, however, has not been well defined. In the era when the antidiuretic hormone (ADH) bioassay was performed in ethanol-anaesthetized rats, consistent alterations in ADH could not be demonstrated in hyponatraemic patients with cirrhosis or heart failure. Therefore it was suggested that intrarenal, non-ADH-dependent, mechanisms accounted for the water retention in these oedematous disorders.
Another dilemma in understanding fluid balance in cardiac failure, cirrhosis and pregnancy relates to defining the afferent signals that could stimulate AVP release with resultant water retention. Expanded plasma volumes have been reported in all three conditions. This finding is typically associated with increased water excretion rather than water retention; thus, signals other than increased plasma volume must contribute to the water retention.
PREVIOUSLY PROPOSED MECHANISMS OF SODIUM AND WATER RETENTION
Backward versus forward cardiac failure
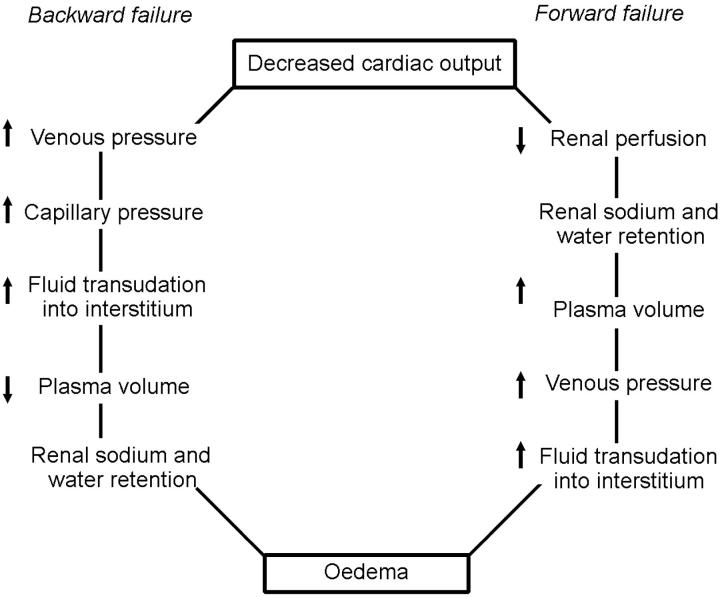
Two opposing theories have been proposed to explain the pathogenesis of water and sodium retention in cardiac failure (Figure 1)3,4,5. With backward failure3,4, pump failure produces an increase in venous pressure with resultant transudation of fluid from the intravascular compartment to the interstitial space, oedema formation and decreased plasma volume. The diminution of plasma volume then stimulates renal sodium and water retention. This hypothesis was not compatible with the observed expanded plasma volumes in heart-failure patients. In contrast, with the forward failure theory5, heart failure causes renal underperfusion with decreased sodium and water excretion and increased plasma volume.
Figure 1.
Backward and forward theories of heart failure [Modified by permission, from Peters JP, Am J Med 1952;12:66]
Overfill versus underfill hypotheses in cirrhosis
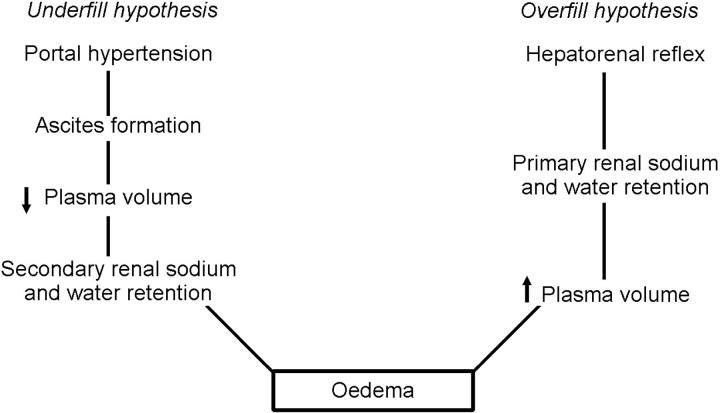
Overfill and underfill theories have been suggested to account for the water and sodium retention in cirrhosis (Figure 2)6,7. The underfill hypothesis proposes that portal hypertension produces transudation of fluid into the abdomen with ascites accumulation; this results in a decreased plasma volume which leads to renal sodium and water retention. In contrast, the overfill hypothesis suggests that a hepatorenal reflex induces primary renal water and sodium retention with expansion of plasma volume.
Figure 2.
Underfill and overfill hypotheses of oedema formation in cirrhosis [Modified by permission, from Schrier RW, J R Coll Physicians 1992;26:295]
Overfill versus underfill hypotheses in pregnancy
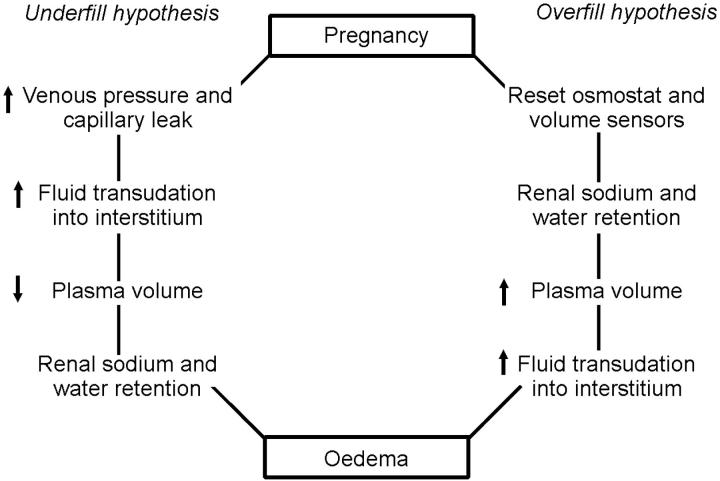
Overfill and underfill hypotheses of water and sodium retention in pregnancy have also been proposed (Figure 3)8. With the overfill hypothesis, the threshold for osmotic and volume regulation alters in pregnancy, with resultant renal sodium and water retention and increase in plasma volume. Against this theory are observations of hypo-osmolality and activation of the renin-angiotensin-aldosterone system in pregnancy—findings more consistent with the underfill hypothesis.
Figure 3.
Underfill and overfill hypotheses of oedema formation in pregnancy
Several developments have allowed these dilemmas in explaining the water retention in oedematous disorders to be more carefully studied. Specifically, a sensitive radio-immunoassay was developed to measure AVP9, and methods became available to more accurately measure intravascular volume—i.e. total plasma or blood volume. The mechanisms whereby AVP causes urinary concentration have been better elucidated with the cloning of the vasopressin V2 receptor on the basolateral membrane of the collecting duct10 and the collecting duct water channels (e.g. aquaporin-2)11. Lastly, pharmacological antagonists to the antidiuretic action of AVP were developed, first for intravenous and then for oral use12,13. These technological advances have provided the means to test a unifying hypothesis on the water retention of cardiac failure, cirrhosis and pregnancy.
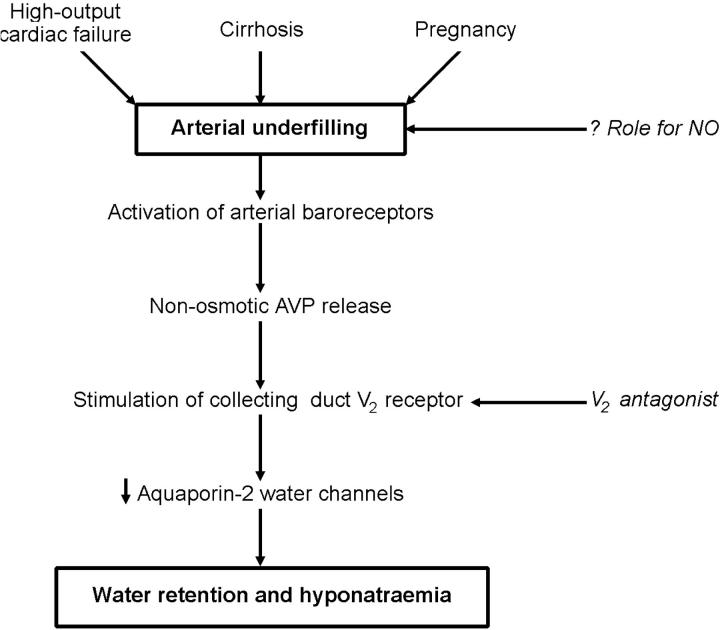
ARTERIAL UNDERFILLING HYPOTHESIS OF BODY FLUID VOLUME REGULATION
According to our unifying hypothesis, sodium and water retention is initiated by alteration of systemic and renal haemodynamics14,15,16. The focus is on the integrity of the arterial circulation. Approximately 85% of the plasma volume resides on the low-pressure venous side of the circulation, with the remaining 15% on the arterial side. Therefore, an expansion of total blood volume could occur with increases in the volume of the venous circulation, yet the kidney could sense arterial underfilling as the dominant afferent signal for renal sodium and water retention.
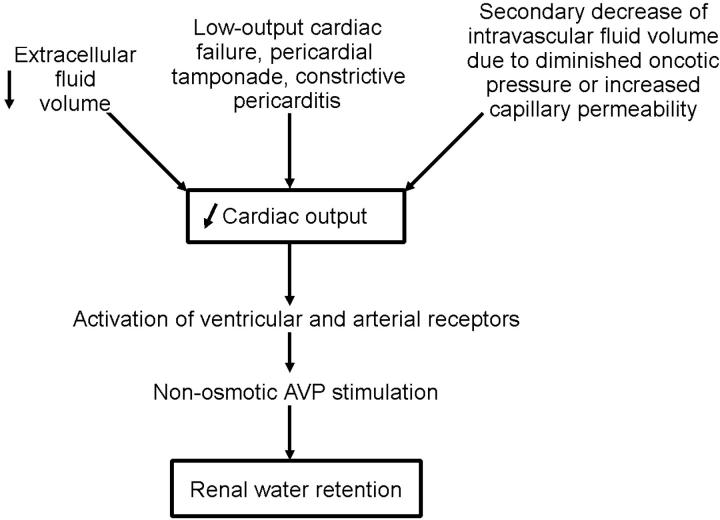
Low-output cardiac failure would result in arterial underfilling (Figure 4). By contrast, in pregnancy and cirrhosis, with their increased cardiac output, the arterial underfilling is a consequence of systemic arterial vasodilation (Figure 5). Such arterial underfilling is sensed by high-pressure baroreceptors in the ventricle, carotid artery and aortic arch and results in activation of the sympathetic nervous and renin-angiotensin-aldosterone systems as well as the non-osmotic release of AVP. The binding of AVP to its V2 receptor on the basolateral membrane of the collecting duct produces a short-term translocation of the water channel, aquaporin-2 (AQP2), from cytosolic storage vesicles to the apical membrane through a cAMP-mediated pathway. This trafficking process increases the water permeability of the apical membrane of collecting-duct cells, thereby promoting water retention. In the long term, AVP controls AQP2 gene expression through a cAMP response element on the AQP2 promoter. Such regulation determines the quantity of AQP2 channels available for modulation of the apical membrane's water permeability.
Figure 4.
Sequence of events in which a decrease in cardiac output initiates water retention [Modified by permission, from Schrier RW, Ann Intern Med 1990;113:155]
Figure 5.
Sequence of events in which peripheral arterial vasodilation initiates water retention [Modified by permission, from Schrier RW, J Am Soc Nephrol 1992;2:1549]
On this background, we now review recent advances in our understanding of altered water metabolism in cardiac failure, cirrhosis and pregnancy.
CARDIAC FAILURE
Before the development of a sensitive AVP radioimmunoassay, the results of plasma AVP concentrations in patients with hyponatraemia and heart failure were conflicting. However, with use of the radioimmunoassay technique, osmotically inappropriate high concentrations of plasma AVP were consistently reported in patients with hyponatraemia and heart failure9,17. The degree of hyponatraemia and hypo-osmolality occurring in heart failure was sufficient to maximally suppress AVP in normal subjects, and yet plasma AVP was not suppressed. Moreover, hypothalamic AVP mRNA expression was found to be increased in a rat coronary ligation model of heart failure18. Therefore, non-osmotic, baroreceptor-mediated, stimulation of AVP was incriminated in heart failure.
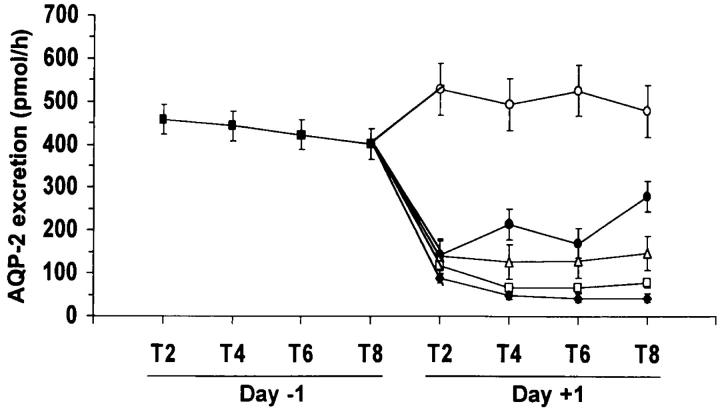
The cloning of the vasopressin V2 receptor10 and the collecting-duct water channel AQP211, and the development of nonpeptide V2 receptor antagonists12,13, allowed examination of water metabolism in heart failure at a molecular level. Our laboratory demonstrated a significant upregulation of kidney AQP2 mRNA and protein expression in heart-failure rats with hyponatraemia19. This effect was associated with an increase in plasma AVP as measured by radioimmunoassay. Administration of an oral nonpeptide V2 receptor antagonist reversed the impairment of water excretion and corrected hyponatraemia in heart-failure patients (Abraham WT, Martin P-Y, Xu L, Schrier RW. Unpublished). These effects of V2 antagonism in heart-failure patients were associated with diminished urinary excretion of AQP2 water channels (Figure 6)20. In this regard, urinary AQP2 excretion has been found a reliable marker of apical membrane AQP2 in the collecting duct21.
Figure 6.
Effect of VPA-985 administration on urinary AQP2 excretion of heart failure patients according to dose groups. (X axis represents the collection periods. Day - 1 is the baseline observation and day + 1 is the study period. ▪ Baseline; ○ Placebo; • 30 mg; ▵ 75 mg; □ 150 mg; ♦ 250 mg) [Reprinted by permission, from Ref. 20]
CIRRHOSIS
As in heart failure, the rat bioassay for antidiuretic hormone did not incriminate AVP in the water retention of cirrhosis; but use of radioimmunoassay techniques revealed in appropriately high plasma AVP concentrations in hyponatraemic patients with cirrhosis22. Upregulation of hypothalamic AVP mRNA expression was also demonstrated in carbon-tetrachloride-induced cirrhosis in rats23. Increased renal expression of AQP2 mRNA and protein has been reported in cirrhotic rats24 along with enhanced trafficking of AQP2 to the apical membrane of the collecting duct in cirrhotic animals25. Moreover, administration of V2 receptor antagonists has been found to improve solute-free water excretion in rats with cirrhosis and ascites26,27. Kappa opioid agonists, which interfere with the central release of AVP, likewise increase renal water excretion in experimental cirrhosis27,28,29. Finally, a beneficial effect from V2 receptor antagonists, in enhancing solute-free water excretion, has been reported in cirrhotic patients13.
A proposed stimulus for the non-osmotic release of AVP in cirrhosis is arterial underfilling secondary to splanchnic vasodilation—the ‘peripheral arterial vasodilation hypothesis’14. Our laboratory sought to determine whether the diminished systemic vascular resistance and the hyperdynamic circulation and altered water metabolism of cirrhosis could be reversed by inhibition of nitric oxide (NO). After administration of the nonspecific NO synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME) for seven days the hyperdynamic circulation of cirrhotic rats became normal30; and the same dose of L-NAME was associated with improved renal water and sodium excretion, correction of hyponatraemia and decreased ascites31. With L-NAME, plasma AVP in the cirrhotic rats returned to control concentrations, as did plasma renin activity and aldosterone concentration31. These results implicate nitric oxide as an important mediator of peripheral arterial vasodilation and altered water metabolism in cirrhosis.
PREGNANCY
Normal pregnancy is associated with a 30-50% increase in total plasma and extracellular fluid volumes and a substantial rise in cardiac output. Hyponatraemia is also the rule. Some investigators have suggested that such hyponatraemia is due to resetting of an ‘osmostat’ whereby the osmotic regulation of vasopressin is normal but occurs around a lower plasma osmolality32. In contrast, the peripheral arterial vasodilation hypothesis suggests that arterial underfilling, due to a decrease in systemic vascular resistance, stimulates non-osmotic release of AVP with subsequent water retention and hyponatraemia33.
By day 7 of gestation in rats, plasma osmolality has declined significantly and it then remains low throughout pregnancy (normal gestation 21-22 days)34. AVP concentrations, however, are not suppressed by this hypo-osmolality; in fact they tend to rise progressively in pregnancy34. Papillary AQP2 mRNA and protein rose early in pregnancy and remained above the non-pregnant level throughout. The effect of the non-peptide V2 receptor antagonist OPC-31260 was then investigated. Papillary AQP2 mRNA was significantly suppressed by administration of OPC-31260 to rats at day 14 of pregnancy. In fact, the renal papillary concentration of AQP2 fell to the level of expression seen in non-pregnant rats receiving OPC-31260. These results therefore indicate that an increase in AQP2 water channels contributes to the water retention in pregnancy through a vasopressin V2 receptor-mediated effect.
In human pregnancy, mean arterial pressure decreases by six weeks' gestation in association with an increase in cardiac output and plasma volume and a decrease in systemic vascular resistance35. The initiators of such physiological changes are not fully known. To evaluate the potential role of nitric oxide in mediating the peripheral arterial vasodilation in pregnancy, we investigated the constitutive NO synthase isoforms in the vasculature and hypothalamus of pregnant rats on day 20 of gestation and age-matched non-pregnant controls36. Neuronal NO synthase protein and mRNA were higher in the hypothalamus of pregnant rats, and endothelial NO synthase protein expression was also higher in both conductance arteries (aorta) and resistance arteries (mesenteric). These increases were associated with raised plasma AVP concentrations and by increased hypothalamic mRNA for AVP.
The effect of nitric oxide inhibition on systemic and renal haemodynamics was then studied37. In pregnant rats at day 14 of gestation, cardiac output was significantly higher than in non-pregnant controls as systemic vascular resistance (SVR) decreased and mean arterial pressure was unchanged. At day 14 of gestation, pregnant rats also had higher glomerular filtration rates (GFR) and renal plasma flow (RPF) than non-pregnant rats. When pregnant rats were given L-NAME from day 7 through 14 of gestation, values for cardiac output, SVR, GFR and RPF did not differ from those in non-pregnant animals. This study therefore indicated that, as in human pregnancy, primary peripheral vasodilation occurs early35 and that the hyperdynamic circulation and glomerular hyperfiltration of normal rat mid-term pregnancy can be chronically reversed by NO synthase inhibition37. Despite these important systemic and renal haemodynamic changes with NO synthase inhibition in pregnancy, we were unable to reverse the hyponatraemia and water retention associated with normal rat pregnancy. This result suggested that pregnancy is associated with a stimulus for thirst and that the resultant polydipsia contributes to the hypo-osmolality of pregnancy.
CONCLUSION
In summary, we have proposed that water retention in cardiac failure, cirrhosis and pregnancy can be understood in the context of our unifying hypothesis of body fluid volume expansion. In our hypothesis, decreased cardiac output or peripheral arterial vasodilation stimulates renal sodium and water retention. Our laboratory has tested the hypothesis by cellular and molecular methods in animal models and human beings.
In cardiac failure, cirrhosis and pregnancy, techniques reveal high plasma AVP concentrations at plasma osmolalities that would normally suppress AVP to undetectable levels. Increased expression of kidney AQP2 water channels has also been demonstrated in cardiac failure, cirrhosis and pregnancy. Water retention and hyponatraemia can be reversed by V2 receptor antagonists in cirrhosis and cardiac failure. Such antagonists also increase water excretion in pregnancy and clinical studies have shown that V2 receptor antagonism is effective in patients with cardiac failure and cirrhosis.
Nitric oxide has been implicated as an important mediator of peripheral arterial vasodilation, leading to arterial underfilling in cirrhosis and pregnancy. Inhibition of NO synthases with L-NAME reverses the hyperdynamic circulations associated with these conditions. Moreover, NO synthase inhibition in cirrhosis reverses the impairment in sodium and water excretion.
References
- 1.Lee WH, Packer M. Prognostic importance of serum sodium concentration and its modification by converting-enzyme inhibition in patients with severe chronic heart failure. Circulation 1986;73: 257-67 [DOI] [PubMed] [Google Scholar]
- 2.Arroyo V, Rodes J, Gutierrez-Lizarraga MA, Revert L. Prognostic value of spontaneous hyponatremia in cirrhosis with ascites. Am J Dig Dis 1976;21: 249-56 [DOI] [PubMed] [Google Scholar]
- 3.Starling EH. On the absorption of fluid from the connective tissue spaces. J Physiol (Lond) 1896;19: 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hope JA. A Treatise on the Diseases of the Heart and Blood Vessels. London: William Kid, 1832
- 5.Mackenzie J. Disease of the Heart, (3rd edn). London: Oxford University Press, 1913
- 6.Lieberman FL, Denison EK, Reynolds TB. The relationship of plasma volume, portal hypertension, ascites, and renal sodium retention in cirrhosis; the overflow theory of ascites formation. Ann N Y Acad Sci 1970;170: 202-6 [Google Scholar]
- 7.Papper S. The role of the kidney in Laennec's cirrhosis of the liver. Medicine 1958;37: 299-316 [DOI] [PubMed] [Google Scholar]
- 8.Schrier RW, Durr J. Pregnancy: an overfill or underfill state. Am J Kidney Dis 1987;9: 284-9 [DOI] [PubMed] [Google Scholar]
- 9.Szatalowicz VL, Arnold PE, Chaimovitz C, et al. Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure. N Eng J Med 1981;305: 263-6 [DOI] [PubMed] [Google Scholar]
- 10.Lolait SJ, O'Carroll AM, McBride OW, et al. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature 1992;357: 336-9 [DOI] [PubMed] [Google Scholar]
- 11.Sasaki S, Fushimi K, Saito H, et al. Cloning, characterization, and chromosomal mapping of human aquaporin of collecting duct. J Clin Invest 1994;93: 1250-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamura Y, Ogawa H, Yamashita H, et al. Characterization of a novel aquaretic agent, OPC-31260, as an orally effective, nonpeptide vasopressin V2 receptor antagonist. Br J Pharmacol 1992;105: 787-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue T, Ohnishi A, Matsuo A, et al. Therapeutic and diagnostic potential of a vasopressin-2 antagonist for impaired water handling in cirrhosis. Clin Pharmacol Ther 1998;63: 561-70 [DOI] [PubMed] [Google Scholar]
- 14.Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology 1988;8: 1151-7 [DOI] [PubMed] [Google Scholar]
- 15.Schrier RW. Pathogenesis of sodium and water retention in high-output and low-output cardiac failure, nephrotic syndrome, cirrhosis, and pregnancy. First of two parts. N Engl J Med 1988;319: 1065-72 [DOI] [PubMed] [Google Scholar]
- 16.Schrier RW. Pathogenesis of sodium and water retention in high-output and low-output cardiac failure, nephrotic syndrome, cirrhosis, and pregnancy. Second of two parts. N Eng J Med 1988;319: 1127-34 [DOI] [PubMed] [Google Scholar]
- 17.Pruszczynski W, Vahanian A, Ardaillou R, Acar J. Role of antidiuretic hormone in impaired water excretion of patients with congestive heart failure. J Clin Endocrinol Metab 1984;58: 599-602 [DOI] [PubMed] [Google Scholar]
- 18.Kim JK, Michel J-B, Soubrier F, et al. Arginine vasopressin gene expression in chronic cardiac failure in rats. Kidney Int 1990;38: 818-22 [DOI] [PubMed] [Google Scholar]
- 19.Xu D-L, Martin P-Y, Ohara M, et al. Upregulation of aquaporin-2 water channel expression in chronic heart failure. J Clin Invest 1997;99: 1500-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin P-Y, Abraham WT, Xu L, et al. Selective V2-receptor vasopressin antagonism decreases urinary aquaporin-2 excretion in patients with chronic heart failure. J Am Soc Nephrol 1999;10: 2165-70 [DOI] [PubMed] [Google Scholar]
- 21.Rai T, Sekine K, Kanno K, et al. Urinary excretion of aquaporin-2 water channel protein in human and rat. J Am Soc Nephrol 1997;8: 1357-62 [DOI] [PubMed] [Google Scholar]
- 22.Bichet D, Szatalowicz V, Chaimovitz C, Schrier RW. Role of vasopressin in abnormal water excretion in cirrhotic patients. Ann Intern Med 1982;96: 413-7 [DOI] [PubMed] [Google Scholar]
- 23.Kim JK, Summer SN, Howard RL, Schrier RW. Vasopressin gene expression in rats with experimental cirrhosis. Hepatology 1993;17: 143-7 [PubMed] [Google Scholar]
- 24.Aahina Y, Izumi N, Enomoto N, Sasaki S, et al. Increased gene expression of water channel in cirrhotic rat kidneys. Hepatology 1995;21: 169-733 [PubMed] [Google Scholar]
- 25.Fernandez-Llama P, Jimenez W, Bosch-Marce M, et al. Dysregulation of renal aquaporins and Na-Cl cotransporter in CC14-induced cirrhosis. Kidney Int 2000;58: 216-28 [DOI] [PubMed] [Google Scholar]
- 26.Claria J, Jiminez W, Arroyo V, Guarner F, et al. Blockade of the hydroosmotic effect of vasopressin normalizes water excretion in cirrhotic rats. Gastroenterology 1989;97: 1294-9 [DOI] [PubMed] [Google Scholar]
- 27.Bosch-Marce M, Poo JL, Jiminez W, et al. Comparison of two aquaretic drugs (niravoline and OPC-31260) in cirrhotic rats with ascites and water retention. J Pharmacol Exp Therap 1999;289: 194-201 [PubMed] [Google Scholar]
- 28.Jimenez W, Angeli P, Leivas A, et al. Aquaretic effect of the kappa-opioid agonist RU 51599 in cirrhotic rats with ascites and water retention. Gastroenterology 1995;109: 217-23 [DOI] [PubMed] [Google Scholar]
- 29.Moreau R, Cailmail S, Hamon G, Lebrec D. Renal and haemodynamic responses to a novel kappa opioid receptor agonist, niravoline (RU 51,599), in rats with cirrhosis. J Gastroenterol Hepatol 1996;11: 857-63 [DOI] [PubMed] [Google Scholar]
- 30.Niederberger M, Martin P-Y, Gines P, Morris K, et al. Normalization of nitric oxide production corrects arterial vasodilation and hyperdynamic circulation in cirrhotic rats. Gastroenterology 1995;109: 1624-30 [DOI] [PubMed] [Google Scholar]
- 31.Martin P-Y, Ohara M, Gines P, et al. Nitric oxide synthase (NOS) inhibition for one week improves renal sodium and water excretion in cirrhotic rats with ascites. J Clin Invest 1998;101: 235-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durr JA, Stamoutsos B, Lindheimer MD. Osmoregulation during pregnancy in the rat. Evidence for resetting of the threshold for vasopressin secretion during gestation. J Clin Invest 1981;68: 337-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schrier RW, Briner VA. Peripheral arterial vasodilation hypothesis of sodium and water retention in pregnancy: implications for pathogenesis of preeclampsia—eclampsia. Obstet Gynecol 1991;77: 632-9 [PubMed] [Google Scholar]
- 34.Ohara M, Martin P-Y, Xu D-L, et al. Upregulation of aquaporin 2 water channel expression in pregnant rats. J Clin Invest 1998;101: 1076-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chapman AB, Abraham WT, Zamudio S, et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int 1998;54: 1056-63 [DOI] [PubMed] [Google Scholar]
- 36.Xu D-L, Martin P-Y, St. Jon J, et al. Upregulation of endothelial and neuronal constitutive nitric oxide synthase in pregnant rats. Am J Physiol 1996;271: R1739-R1745 [DOI] [PubMed] [Google Scholar]
- 37.Cadnapaphornchai MA, Ohara M Morris KG Jr, et al. Chronic NOS inhibition reverses systemic vasodilation and glomerular hyperfiltration in pregnancy. Am J Physiol Renal Physiol 2001;280: F592-F598 [DOI] [PubMed] [Google Scholar]