Abstract
The cytotoxic necrotizing factor 1 (CNF1) from Escherichia coli has been shown to activate members of the Rho family by deamidation of glutamine 63. This amino acid is essential for hydrolysis of GTP, and any substitution results in a constitutively active Rho. Activation of Rho induces the formation of stress fibers, filopodia, and membrane ruffles due to activation of RhoA, Cdc42, and Rac, respectively. Here we show that the level of endogenous Rac decreased in CNF1-treated HEK293 and HeLa cells. The amount of mRNA remained unaffected, leaving the possibility that Rac is subject to proteolytic degradation. Treatment of cells with lactacystin, an inhibitor of the 26S proteasome, protected Rac from degradation. We have previously shown that CNF1 activates the c-Jun N-terminal kinase (JNK) only transiently in HeLa cells (M. Lerm, J. Selzer, A. Hoffmeyer, U. R. Rapp, K. Aktories, and G. Schmidt, Infect. Immun. 67:496-503, 1998). Here we show that CNF1-induced JNK activation is stabilized in the presence of lactacystin. The data indicate that Rac is degraded by a proteasome-dependent pathway in CNF1-treated cells.
Rho GTPases are key regulators of a wide variety of cellular functions, including regulation of actin structures, integrin signaling, and phospholipid signaling (18, 27). Furthermore, Rho proteins are implicated in endocytosis, secretion, control of transcription, cell cycle progression, and cell transformation (for reviews see references 2 and 24). Like all members of the Ras superfamily of small GTPases, Rho GTPases cycle between the GDP-bound inactive and GTP-bound active forms. In the cytoplasm, the GDP-bound form of Rho is complexed with the guanosine nucleotide dissociation inhibitor. The exchange of GDP for GTP is catalyzed by guanosine nucleotide exchange factors, whose activity may be triggered by an extracellular stimulus. Inactivation of Rho results from hydrolysis of the bound GTP, a process which is stimulated by GTPase-activating proteins (GAPs) (for a review see reference 17).
Dynamic reorganization of the actin cytoskeleton is involved in many cell functions, including cell motility, adhesion, and shape change. By modulating the ability of professional phagocytes to engulf bacteria, a process which is dependent on functional regulation of actin, many toxin-producing bacteria evade the host immune response. One important target for pathogens to interfere with the actin of the host cell is to modulate signaling of Rho GTPases. A wide variety of bacterial species synthesize protein toxins, which either activate or inactivate Rho GTPases (15). The cytotoxic necrotizing factor 1 (CNF1) from Escherichia coli deamidates members of the Rho family at glutamine 63/61, a residue which is critical for GTP hydrolysis (7, 25). Thus, the modification results in a constitutively activated Rho, which explains the strong stress fiber formation observed after CNF1 treatment. Also characteristic of CNF1-treated cells is the formation of filopodia and membrane ruffles, which is due to activation of Cdc42 and Rac, respectively (16). In this study, CNF1-induced modification of Rho GTPases in HEK293 cells was investigated. We found that the amount of Rac, but not the amount of Rho or Cdc42, decreased markedly in CNF1-treated cells. The levels of Rac mRNA remained constant. However, Rac was stabilized when CNF1-treated cells were incubated with lactacystin, an inhibitor of the 26S proteasome, indicating that deamidated Rac is subject to proteolytic degradation in CNF1-treated cells. This finding explains our recent observation that the c-Jun N-terminal kinase (JNK) is only transiently activated after CNF1 treatment (16). It is suggested that eukaryotic cells possess a switch-off mechanism for Rac constitutively activated by deamidation, which may be functional during the infection process of CNF-producing pathogens.
MATERIALS AND METHODS
Cell culture.
HEK293 and HeLa cells were cultivated in Dulbecco's modified Eagle's medium (12 mM l-glutamine) supplemented with 10% fetal calf serum, penicillin (4 mM), and streptomycin (4 mM) in a humidified atmosphere containing 5% CO2 at 37°C. For intoxication, the cells were treated with 500 ng of glutathione S-transferase (GST)-CNF1 per ml.
Actin staining.
Formaldehyde-fixed cells were washed intensively with phosphate-buffered saline. The cells were then incubated with rhodamine-conjugated phalloidin (1 U per coverslip) at room temperature for 1 h, washed again, and used for fluorescence microscopy (Kaiser's glycerol gelatin [Merck] was used as a bleaching preservative). For quantification of cells bearing lamellipodia and filopodia, 100 cells per coverslip were counted in two independent experiments.
Protein preparation.
For toxin purification, a BL21 E. coli strain carrying pGEX-CNF1 was grown in minimal medium (40 mM Na2HPO4, 20 mM KH2PO4, 8 mM NaCl, 1 mM MgSO4, 100 μM CaCl2, 18 mM NH4Cl, 3 μM thiamine, 50 mM glucose, 3 nM ZnSO4, 2 nM MnCl2, 50 nM H3BO3, 1 nM NiCl2, 1 nM NaMoO4, 7 nM CdCl2, 0.5 nM CuCl2, 13 nM EDTA, 7 nM FeSO4). At an optical density of 0.5, 0.2 mM isopropyl-β-d-thiogalactopyranoside was added, and the culture was grown for an additional 4 h. Purification was performed as described in the protocol for GST-tagged proteins (Pharmacia). Since CNF1 is sensitive to thrombin cleavage, the GST fusion partner was not removed.
Western blot analysis.
HEK293 cells growing on petri dishes (diameter, 3 cm) were treated with 500 ng of full-length GST-CNF1 per ml and 30 μM lactacystin (Calbiochem, San Diego, Calif.) or 30 μM MG132 (Sigma, Steinheim, Germany) (data not shown) as indicated below, washed twice with phosphate-buffered saline, lysed in 30 μl of boiling sodium dodecyl sulfate (SDS) buffer (20 mM Tris-HCl, 200 mM glycine, 0.1% SDS), and boiled again immediately. After two cycles of boiling and vortexing, the samples were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto nitrocellulose. Rho GTPases were detected with specific antibodies (anti-Rac and anti-Cdc42 antibodies were obtained from Upstate [Lake Placid, N.Y.], and the anti-RhoA monoclonal antibody was obtained from Santa Cruz Biotechnology [Santa Cruz, Calif.]). Stained bands were quantified by using the ImageQuant 5.2 software.
Northern blot analysis.
HEK293 cells were treated with 500 ng of CNF1 per ml as indicated below before lysis. Northern blot analysis was performed as described previously (22) by using a Rac probe corresponding to full-length Rac labeled with digoxigenin.
Analysis of Rho mRNA expression.
In order to analyze expression of the diverse Rho species at the mRNA level, total RNA was isolated with a Qiagen RNA extraction kit (Qiagen, Hilden, Germany), and cDNA synthesis was performed with 4 μg of RNA for 50 min at 42°C (Invitrogen Life Technologies Super Script first-strand synthesis kit) in a total volume of 20 μl. Two microliters of the first-strand reaction mixture was subjected to standard PCR with Taq DNA polymerase (Roche Diagnostics GmbH). The sequences of the primers used for amplification of Rac1, RhoA, and glutamate dehydrogenase (GDH) mRNA were as follows: Rac1 mRNA (448-bp PCR product), 5′-CATCAAGTGTGTGGTGGTGGG-3′ and 5′-TTACAGCACCAATCTCCTTAG-3′; RhoA mRNA (582-bp PCR product), 5′-ATGGCTGCCATCCGGAAGAAA-3′ and 5′-TCACAAGACAAGGCAACCAGA-3; and GDH mRNA (392-bp PCR product), 5′-GTCTTCACCACCATGGAGAAGGCT-3′ and 5′-CATGCCAGTGAGCTTCCCGTTCA-3′. After PCR (30 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 2 min, and extension at 72°C for 2 min) the products were separated on 1.5% agarose gels and visualized by ethidium bromide staining. It was ensured by background experiments (i.e., by varying the amount of RNA template used for first-strand synthesis and by varying the number of PCR cycles) that reverse transcription (RT)-PCR-based quantification of Rho (Rac) mRNA expression was performed under nonsaturating conditions. Thus, by using a small amount of RNA (0.4 μg) and a rather low number of PCR cycles (30 cycles), we established suitable experimental conditions, under which the amount of the amplification product was not saturated. Lysate of MCF-7 (a human breast carcinoma cell line) was used as an internal control for the assay conditions.
Glucosylation with toxin B.
For glucosylation, cell lysates were incubated with toxin B (1 μg/ml) in glucosylation buffer (10 mM HEPES [pH 7.4], 150 mM KCl, 10 μM UDP-[14C]glucose) for 30 min at 37°C. Glucosylated proteins were separated by SDS-PAGE and analyzed by phosphorimaging.
JNK assay.
HeLa or HEK293 cells growing on 3-cm-diameter petri dishes were treated with or without CNF1 (500 ng/ml) for 3 or 6 h and with 30 μM lactacystin (Calbiochem) or 30 μM MG132 (Sigma) (data not shown) for 6 h. Dimethyl sulfoxide (DMSO) was used as a solvent control. The JNK assay was performed as described previously (16).
RESULTS
Amount of Rac is reduced in CNF1-treated cells.
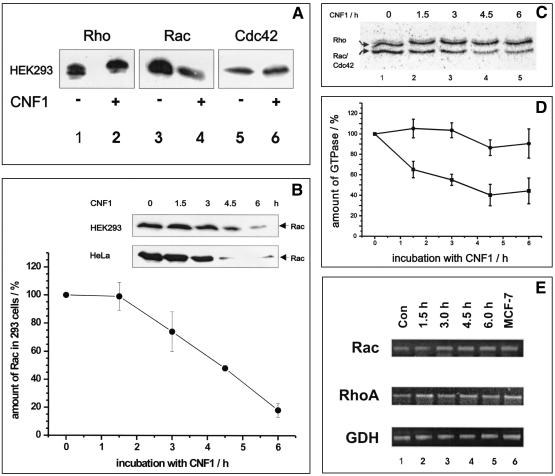
We showed previously that CNF1 activates the JNK only transiently, although the toxin constitutively activates Rho GTPases by deamidation (16). Therefore, we studied the fate of the CNF1 targets Rac and Cdc42, which are known to be involved in JNK activation, during the course of intoxication in HEK293 cells (human embryonic kidney cells). First, HEK293 cells were treated with CNF1 (500 ng/ml) overnight. To reduce proteolytic activity during cell lysis, we lysed the cells directly in SDS buffer at 95°C. Subsequently, proteins were separated by SDS-PAGE, transferred onto nitrocellulose, and probed for small GTPases with antibodies specific for RhoA, Rac, or Cdc42. As shown in Fig. 1A, the level of Rac was reduced, whereas the amount of RhoA was only slightly influenced and the level of Cdc42 was not influenced at all by CNF1 treatment. Next, we investigated the time course of the disappearance of Rac. Figure 1B shows the Rac levels at zero time and after 1.5, 3, 4.5, and 6 h of CNF1 treatment of HEK293 cells. After 4.5 h of CNF1 treatment, the amount of Rac was clearly reduced, and after 6 h more than 60% of the initial amount of Rac had disappeared.
FIG. 1.
(A) Detection of Rho proteins from CNF1-treated cells by Western blotting. HEK293 cells were treated with 500 ng of CNF1 per ml overnight and lysed in boiling SDS buffer. The crude lysates were separated by SDS-PAGE and transferred onto a nitrocellulose membrane. Rho GTPases were detected with specific antibodies. Typical results of three different experiments are shown. (B) Time course of Rac degradation in CNF1-treated cells. HEK293 and HeLa cells were incubated in the presence or absence of 500 ng of CNF1 per ml for different times. The cells were lysed in boiling SDS buffer, and the crude lysates were separated by SDS-PAGE. Proteins were transferred onto a nitrocellulose membrane and probed for Rac with a specific Rac antibody. Bands obtained in three independent experiments (HEK293 cells) were quantified by using the ImageQuant 5.2 software. The amount of Rac detected in lysates of HEK293 cells without CNF1 treatment (zero time) was defined as 100%. The means ± standard deviations of three independent experiments are shown. (C) Detection of Rho proteins from CNF1-treated cells by glucosylation with toxin B. HEK293 cells were treated with 500 ng of CNF1 per ml and lysed in lysis buffer. Lysates were treated with C. difficile toxin B in the presence of labeled UDP-[14C]glucose. Proteins were separated by SDS-PAGE. Gels were stained and dried, and glucosylated proteins were detected by phosphorimaging. Note that the upper band shifted to a higher molecular mass, indicating that there was deamidation of RhoA by CNF1. The lower band represents mostly Rac but also a small amount of Cdc42. Typical results of four different experiments are shown. (D) Quantification of Rho proteins. For quantification of the labeled RhoA and Rac-Cdc42 bands, the ImageQuant software (Amersham Pharmacia Biotech) was used. The means ± standard deviations of four different experiments are shown. (E) Analysis of Rho mRNA expression. In order to analyze expression of the diverse Rho species at the mRNA level, total RNA was isolated from untreated (Con; lane 1) and CNF-treated (lanes 2 to 5) cells and cDNA was synthesized. Two microliters of the first-strand reaction mixture was subjected to a standard PCR. GDH was used as the internal standard. After PCR the products were separated on 1.5% agarose gels and visualized by ethidium bromide staining. Lysate of MCF-7 (a human breast carcinoma cell line) was used as an internal control for the assay conditions (lane 6).
To exclude the possibility that the antibody did not recognize deamidated Rac, we also tested the recombinant Q61E mutant, which is equivalent to deamidated Rac. The anti-Rac antibody used detected mutant and wild-type Rac with the same sensitivity (data not shown). Moreover, we determined the amount of Rho proteins by 14C glucosylation with Clostridium difficile toxin B, which modifies Rho, Rac, and Cdc42 by monoglucosylation at threonine-37 and threonine-35 (13) (Fig. 1C and D). Because HEK293 cells express only small amounts of Cdc42 (Fig. 1A), the two detectable bands in Fig. 1C corresponded mainly to RhoA (upper band) and Rac (lower band). The CNF1-induced upward shift of RhoA to an apparent higher molecular mass indicated that RhoA was quantitatively modified. In contrast to RhoA, whose level was only barely diminished, the level of Rac decreased drastically with the time of CNF1 intoxication. After 6 h of toxin treatment, the amount of Rac was reduced by about 80% in HEK293 cells (Fig. 1B and D).
Rac is degraded by a proteasome-dependent pathway.
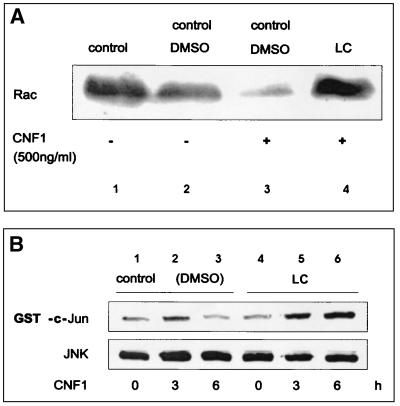
The reduction in the Rac level after CNF1 treatment can be explained by a reduction in the Rac mRNA level or by enhancement of proteolytic degradation of modified Rac. First, we tested whether the level of Rac mRNA changed after exposure of cells to CNF1. A Northern blot analysis with a Rac-specific probe was performed. We were not able to detect a reduction in the level of Rac mRNA during intoxication with CNF1 (data not shown). As a further control we analyzed the levels of Rac and Rho mRNA by quantitative RT-PCR with GDH mRNA as an internal control. It was shown by background experiments that the RT-PCR-based quantification of Rho (Rac) mRNA expression was performed under nonsaturating conditions (see Materials and Methods). This method again showed that there were constant levels of Rac mRNA, as well as Rho mRNA (Fig. 1E). Next, we tested whether Rac is degraded by a proteasome-dependent pathway. Several highly specific inhibitors of proteasome-induced degradation have been described, one of which is the fungal substance lactacystin (5). We tested whether lactacystin prevents the disappearance of Rac in CNF1-treated cells. Lactacystin was added to HEK293 cells immediately before addition of CNF1. The cells were incubated for 6 h prior to lysis. DMSO was used as a solvent control. As shown in Fig. 2A, treatment of the cells with lactacystin prevented a CNF1-induced decrease in the Rac level. The data indicate that Rac is stabilized when the proteasome is inhibited by lactacystin.
FIG. 2.
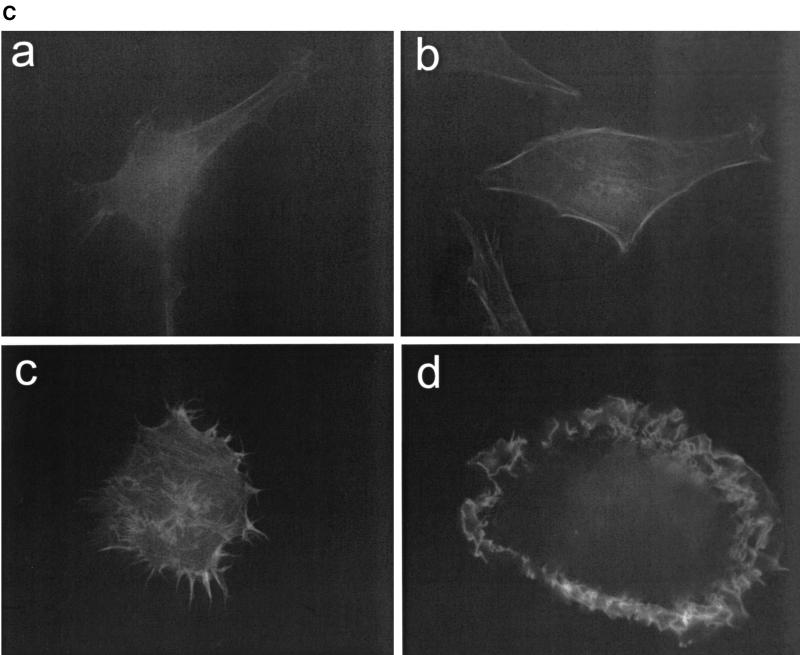
(A) Lactacystin inhibits degradation of Rac. HEK293 cells were treated with CNF1 (500 ng/ml) in the presence of DMSO or 30 μM lactacystin (LC). After 6 h, the cells were lysed in boiling SDS buffer, the crude lysates were separated by SDS-PAGE and transferred onto nitrocellulose, and Rac was detected with a specific anti-Rac antibody. (B) Lactacystin prevents the transient activation of JNK. HEK293 cells were treated with CNF1 for 3 and 6 h in the absence or presence of 30 μM lactacystin. The lysates were incubated with a JNK antibody and protein A beads to precipitate the endogenous JNK. The beads carrying the precipitated JNK were incubated with GST-c-Jun in the presence of [γ-32P]ATP. The samples were separated by SDS-PAGE and transferred onto nitrocellulose, and the radiolabeling of GST-c-Jun was determined with a PhosphorImager. The amount of precipitated JNK was determined by using the JNK antibody. (C) Lactacystin- and CNF1-treated HeLa cells exhibit a pronounced Rac phenotype. HeLa cells were grown on glass coverslips, fixed, and stained with rhodamine-conjugated phalloidin after 6 h of treatment with CNF1 (500 ng/ml) (c and d) in the presence of DMSO (a and c) or 30 μM lactacystin (b and d). CNF1 induced a phenotype characterized by formation of a large number of filopodia (present in 76% of the cells, 44% with lamellipodia). Lactacystin had no major effect on cell morphology. Treatment of cells with CNF and lactacystin caused a phenotype characterized by formation of lamellipodia (present in 98% of the cells, 46% with filopodia). Typical results of three independent experiments are shown.
Next, we studied the influence of lactacystin on CNF1-induced morphology changes. Since HEK293 cells are not suitable for morphological studies, we decided to investigate the morphological effects of CNF1 in HeLa cells. It is generally accepted that the formation of lamellipodia is regulated by Rac, whereas the formation of filopodia is reportedly governed by Cdc42 (21). As shown in Fig. 2C, lactacystin alone did not induce any major changes in the morphology of these cells. Combined treatment of HeLa cells with lactacystin and CNF1 resulted in the formation of lamellipodium-like structures in about 95% of the cells (46% with filopodia), whereas about 75% of the cells treated with CNF1 alone were characterized by a large number of filopodium-like structures (44% with lamellipodia). The morphological changes observed are in full agreement with the hypothesis that CNF1 caused degradation of Rac. Concomitantly, the phenotype of CNF1-activated Cdc42 dominated. In line with this notion, we also observed that in HeLa cells treatment with CNF1 caused a decrease in the amount of Rac (Fig. 1B). After addition of lactacystin, which inhibited the degradation of CNF1-activated Rac, the Rac phenotype (e.g., formation of lamellipodia) was pronounced.
In addition, another proteasome inhibitor tested (MG132) was also able to stabilize Rac during CNF1 intoxication. Moreover, CNF1 together with MG132 led to a more pronounced Rac phenotype in HeLa cells (data not shown), as we found for CNF1 with lactacystin.
CNF1-induced activation of JNK is stabilized in the presence of lactacystin.
We have previously shown that the JNK is transiently activated by CNF1 (16). To study whether the degradation of Rac in CNF1-treated cells is responsible for this transient effect, we examined the influence of the proteasome inhibitor lactacystin on JNK activity in CNF1-treated HEK293 cells. Cells were treated with CNF1 in the absence and presence of lactacystin, and then endogenous JNK was immunoprecipitated and probed for its kinase activity with GST-c-Jun in the presence of [γ-32P]ATP. The amount of radiolabeled GST-c-Jun was determined by SDS-PAGE and phosphorimaging (Fig. 2B). As shown previously for HeLa cells (16), HEK293 cells responded to CNF1 with a transient rise in JNK activity. The activation of JNK peaked after 3 h, and after 6 h the activation state was below the control levels. In the presence of lactacystin, however, JNK activity even increased slightly after 6 h. This indicated that the transient JNK activation observed in the presence of CNF1 was due to initial activation of Rac followed by degradation of the GTPase via proteasomal degradation.
DISCUSSION
The E. coli toxin CNF1 deamidates Rho proteins at glutamine 61/63 and thereby blocks their intrinsic activity, as well as the GAP-stimulated GTPase activity. Recently, we have shown that the JNK is only transiently activated by CNF1, although Rac and Cdc42, which activate the kinase, are constitutively activated by the toxin. This study showed that CNF1-activated Rac is degraded in HEK293 and HeLa cells by a proteasome-dependent pathway.
Virtually all proteins degraded by the 26S proteasome are labeled with ubiquitin (14). Ubiquitin is a small protein (about 8 kDa) which is transferred onto lysine residues of the target protein by specific ubiquitin ligases (14). Subsequently, another ubiquitin molecule is linked to the first and so on. Interestingly, a recent report showed that Rac3 interacts with the E3 ubiquitination subunit Cullin-1 only in the constitutively active form (26). Moreover, RhoB has been reported to have rapid turnover due to ubiquitin-mediated destruction by the 26S proteasome (4). In the present study we observed proteasome-dependent degradation of Rac; however, we were not able to detect an upward shift of Rac by SDS-PAGE, which is expected after ubiquitination of a protein. Moreover, we were not able to detect ubiquitinated Rac in the lysates of CNF1- and lactacystin-treated cells by Western blot analysis using a ubiquitin-specific antibody. Recently, it was reported that targeting of substrates to the proteasome is not dependent on ubiquitination but occurs after noncovalent interaction with a specific protein factor known as antizyme (10, 20, 23). It is tempting to speculate that Rac is degraded by this antizyme-proteasome pathway, because no shift in molecular mass is detectable after precipitation of the activated Rac. Moreover, one can say that cells may possess a mechanism for rapid degradation of Rac to prevent the effects of prolonged activation of the GTPase (11, 29). It has previously been reported that the levels of Rho decrease after ADP ribosylation by the C3 exoenzyme from Clostridium botulinum (19). The reason for degradation of C3-modified RhoA remains unclear. Our previous studies of degradation of ADP-ribosylated RhoA showed that this degradation could also be blocked by lactacystin (3). This finding supports the possibility of a more general role of proteasomal degradation as a regulatory mechanism in cells answering to modified Rho proteins. A change in conformation, overall structure, or stability of modified Rho might favor the degradation. However, the overall structure or stability of Rac is not altered significantly after deamidation by CNF1 (16). Moreover, another major difference concerning the functional consequences of GTPase modification by C3 and CNF1 is the inactivation of GTPase by the first bacterial enzyme and the activation of GTPase by the second bacterial enzyme. Because fine-tuning of the activation state of Rho proteins is essential for the regulatory function of the GTPases, cells most likely depend on degradation of persistently active proteins. The reason why deamidated Rac but not deamidated Rho or Cdc42 is degraded in HeLa and HEK293 cells remains to be determined.
Rho proteins are the selective targets for various bacterial toxins, which covalently modify the GTPases by ADP ribosylation, glucosylation, deamidation, and transglutamination (1). In addition, it was shown recently that various bacterial effectors which are delivered into mammalian cells by type III secretion machinery affect Rho GTPases not by modifying but by modulating their functions. For example, several pathogens, including Pseudomonas aeruginosa and Salmonella and Yersinia species, produce GAP-like proteins, which terminate the active state of Rho GTPases (8, 9, 28). Moreover, Salmonella produces a noncovalently acting activator of Rho GTPases (SopE/SopE2) (12), as well as an inactivator (SptP) (8), probably to switch on and off cytoskeletal changes in the host target cell in a space- and time-dependent manner according to the requirements of the pathogen. For example, cellular engulfment of pathogens is facilitated by increased membrane ruffling caused by activation of Rac. After uptake, the bacteria might need down-regulation of Rac activity for further development. Also, CNF1 was shown to induce uptake of bacteria by nonprofessional phagocytosis of eukaryotic host cells (6). CNF1 activates Rho proteins constitutively; therefore, termination of the active state of Rho GTPases is possible only by degradation. It is exciting to speculate that the degradation of the deamidated Rho GTPases is functionally important for the host-pathogen interaction and for survival of the pathogen.
Taken together, the data indicate that Rac, which is constitutively activated by CNF1, is subject to enhanced degradation. It remains to be determined whether this regulation of Rac is important not only under pathophysiological conditions but also under physiological conditions causing activation of the GTPase.
Acknowledgments
We thank Iris Misicka and Angelika Uhl for excellent technical assistance.
This work was supported by Sonderforschungsbereich (SFB) 388.
Editor: D. L. Burns
REFERENCES
- 1.Aktories, K., G. Schmidt, and F. Hofmann. 2000. GTPases targeted by bacterial toxins, p. 311-331. In A. Hall (ed.), GTPases. Oxford University Press, Oxford, United Kingdom.
- 2.Aspenstrom, P. 1999. Effectors for the Rho GTPases. Curr. Opin. Cell Biol. 11:95-102. [DOI] [PubMed] [Google Scholar]
- 3.Barth, H., C. Olenik, P. Sehr, G. Schmidt, K. Aktories, and D. K. Meyer. 1999. Neosynthesis and activation of Rho by Escherichia coli cytotoxic necrotizing factor (CNF1) reverse cytopathic effects of ADP-ribosylated Rho. J. Biol. Chem. 274:27407-27414. [DOI] [PubMed] [Google Scholar]
- 4.Engel, M. E., P. K. Datta, and H. L. Moses. 1998. RhoB is stabilized by transforming growth factor β and antagonizes transcriptional activation. J. Biol. Chem. 273:9921-9926. [DOI] [PubMed] [Google Scholar]
- 5.Fenteany, G., and S. L. Schreiber. 1998. Lactacystin, proteasome function and cell fate. J. Biol. Chem. 273:8545-8548. [DOI] [PubMed] [Google Scholar]
- 6.Fiorentini, C., L. Falzano, R. Rivabene, A. Fabbri, and W. Malorni. 1999. N-acetylcysteine protects epithelial cells against the oxidative imbalance due to Clostridium difficile toxin. FEBS Lett. 453:124-128. [DOI] [PubMed] [Google Scholar]
- 7.Flatau, G., E. Lemichez, M. Gauthier, P. Chardin, S. Paris, C. Fiorentini, and P. Boquet. 1997. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature 387:729-733. [DOI] [PubMed] [Google Scholar]
- 8.Galan, J. E., and Y. Fu. 2000. Modulation of actin cytoskeleton by salmonella GTPase activating protein SptP. Methods Enzymol. 325:496-504. [DOI] [PubMed] [Google Scholar]
- 9.Goehring, U.-M., G. Schmidt, K. J. Pederson, K. Aktories, and J. T. Barbieri. 1999. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J. Biol. Chem. 274:36369-36372. [DOI] [PubMed] [Google Scholar]
- 10.Gruendler, C., Y. Lin, J. Farley, and T. Wang. 2001. Proteasomal degradation of Smad1 induced by bone morphogenetic proteins. J. Biol. Chem. 276:46533-46543. [DOI] [PubMed] [Google Scholar]
- 11.Habets, G. M., E. H. M. Scholtes, D. Zuydgeest, R. A. Van der Kammen, J. C. Stam, A. Berns, and J. G. Collard. 1994. Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell 77:537-549. [DOI] [PubMed] [Google Scholar]
- 12.Hardt, W.-D., L.-M. Chen, K. E. Schuebel, X. R. Bustelo, and J. E. Galán. 1998. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93:815-826. [DOI] [PubMed] [Google Scholar]
- 13.Just, I., J. Selzer, M. Wilm, C. Von Eichel-Streiber, M. Mann, and K. Aktories. 1995. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375:500-503. [DOI] [PubMed] [Google Scholar]
- 14.Kornitzer, D., and A. Ciechanover. 2000. Modes of regulation of ubiquitin-mediated protein degradation. J. Cell. Physiol. 182:1-11. [DOI] [PubMed] [Google Scholar]
- 15.Lerm, M., G. Schmidt, and K. Aktories. 2000. Bacterial protein toxins targeting Rho GTPases. FEMS Microbiol. Lett. 188:1-6. [DOI] [PubMed] [Google Scholar]
- 16.Lerm, M., J. Selzer, A. Hoffmeyer, U. R. Rapp, K. Aktories, and G. Schmidt. 1999. Deamidation of Cdc42 and Rac by Escherichia coli cytotoxic necrotizing factor 1 (CNF1): activation of c-Jun-N-terminal kinase in HeLa cells. Infect. Immun. 67:496-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackay, D. J. G., and A. Hall. 1998. Rho GTPases. J. Biol. Chem. 273:20685-20688. [DOI] [PubMed] [Google Scholar]
- 18.Malcolm, K. C., A. H. Ross, R.-G. Qiu, M. Symons, and J. H. Exton. 1994. Activation of rat liver phospholipase D by the small GTP-binding protein RhoA. J. Biol. Chem. 269:25951-25954. [PubMed] [Google Scholar]
- 19.Meacci, E., V. Vasta, J. P. Moorman, D. A. Bobak, P. Bruni, J. Moss, and M. Vaughan. 1999. Effect of Rho and ADP-ribosylation factor GTPases on phospholipase D activity in intact human adenocarcinoma A549 cells. J. Biol. Chem. 274:18605-18612. [DOI] [PubMed] [Google Scholar]
- 20.Murakami, Y., S. Matsufuji, S. Hayashi, N. Tanahashi, and K. Tanaka. 1999. ATP-dependent inactivation and sequestration of ornithine decarboxylase by the 26S proteasome are prerequisites for degradation. Mol. Cell. Biol. 19:7216-7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nobes, C. D., and A. Hall. 1995. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81:53-62. [DOI] [PubMed] [Google Scholar]
- 22.Olenik, C., H. Barth, I. Just, K. Aktories, and D. K. Meyer. 1997. Gene expression of the small GTP-binding proteins RhoA, RhoB, Rac1, and Cdc42 in adult rat brain. Mol. Brain Res. 52:263-269. [DOI] [PubMed] [Google Scholar]
- 23.Pickart, C. M. 1997. Targeting of substrates to the 26S proteasome. FASEB J. 11:1055-1066. [DOI] [PubMed] [Google Scholar]
- 24.Ridley, A. J. 1996. Rho: theme and variations. Curr. Biol. 6:1256-1264. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt, G., P. Sehr, M. Wilm, J. Selzer, M. Mann, and K. Aktories. 1997. Gln63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor 1. Nature 387:725-729. [DOI] [PubMed] [Google Scholar]
- 26.Senadheera, D., L. Haataja, J. Groffen, and N. Heisterkamp. 2001. The small GTPase Rac interacts with ubiquitination complex proteins Cullin-1 and Cdc23. Int. J. Mol. Med. 8:127-133. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqi, A. R., J. L. Smith, A. H. Ross, R.-G. Qiu, M. Symons, and J. H. Exton. 1995. Regulation of phospholipase D in HL60 cells. J. Biol. Chem. 270:8466-8473. [DOI] [PubMed] [Google Scholar]
- 28.von Pawel-Rammingen, U., M. V. Telepnev, G. Schmidt, K. Aktories, H. Wolf-Watz, and R. Rosqvist. 2000. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol. Microbiol. 36:737-748. [DOI] [PubMed] [Google Scholar]
- 29.Zhuge, Y., and J. Xu. 2001. Rac1 mediates type I collagen-dependent MMP-2 activation. J. Biol. Chem. 276:16248-16256. [DOI] [PubMed] [Google Scholar]