Abstract
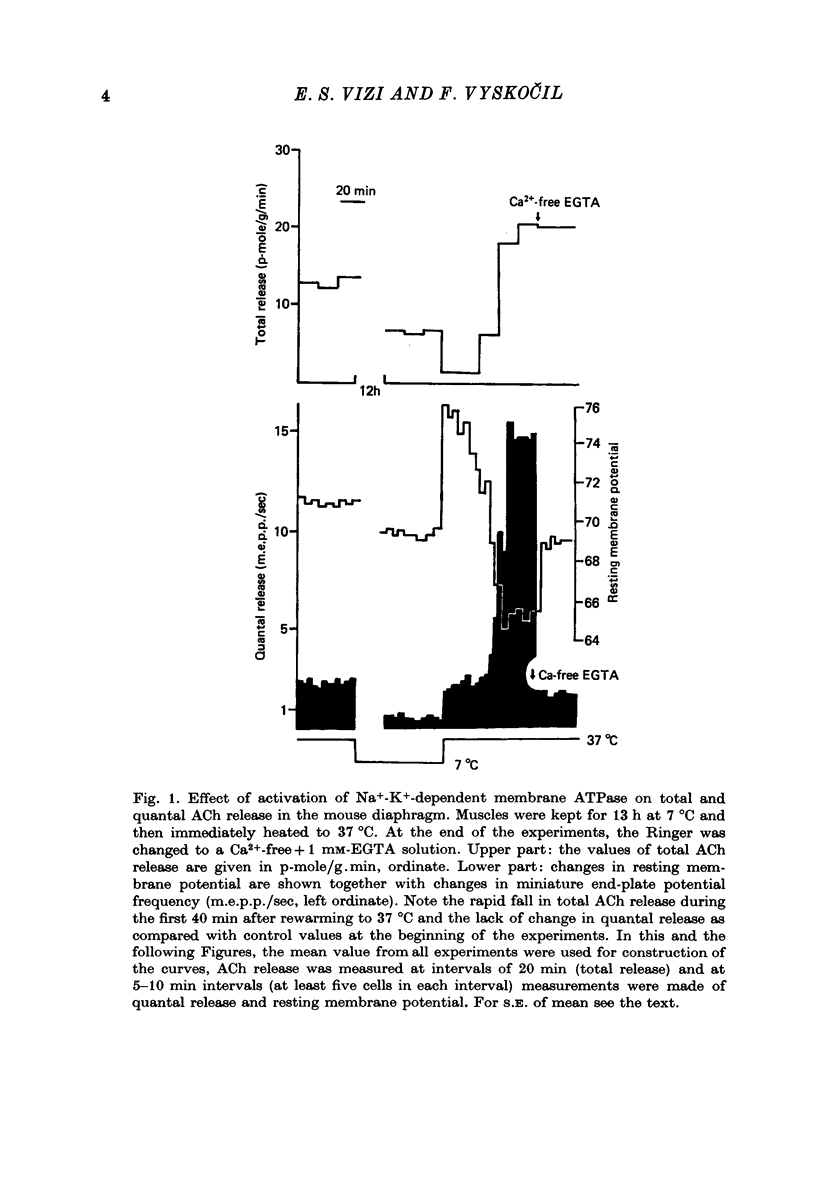
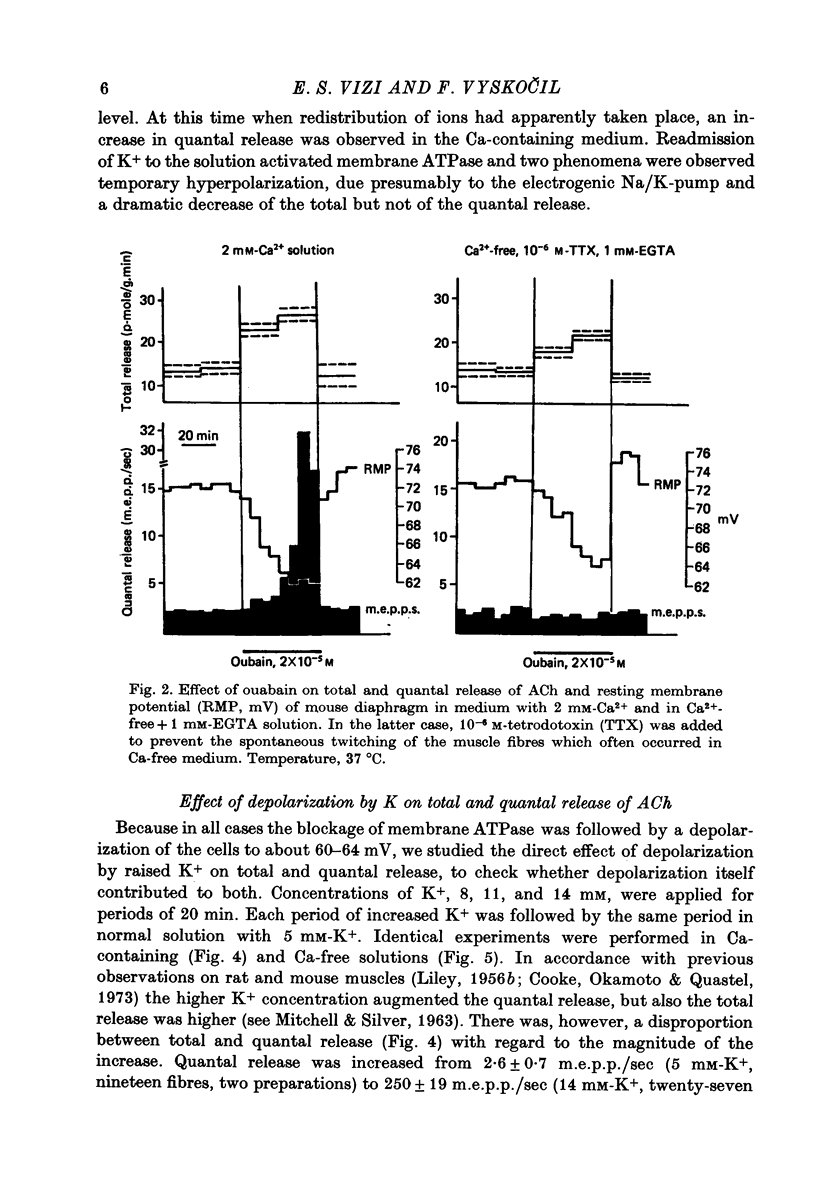
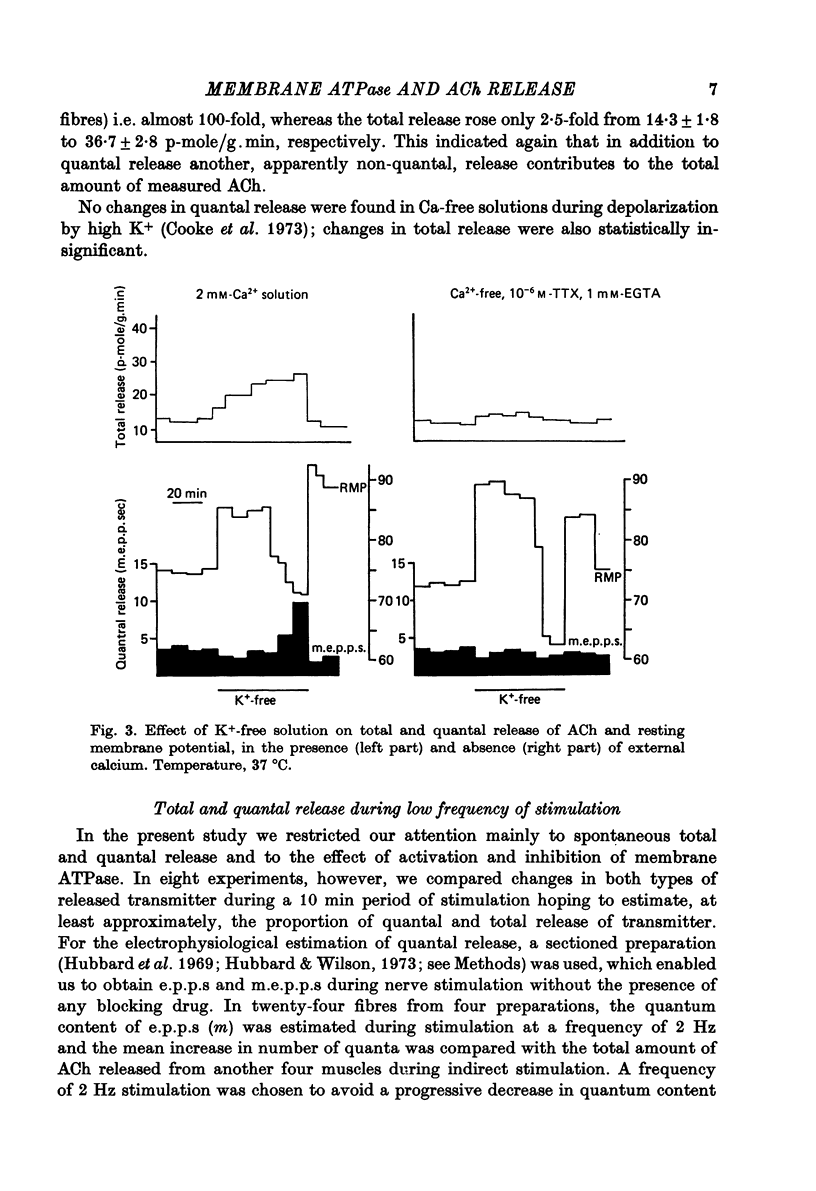
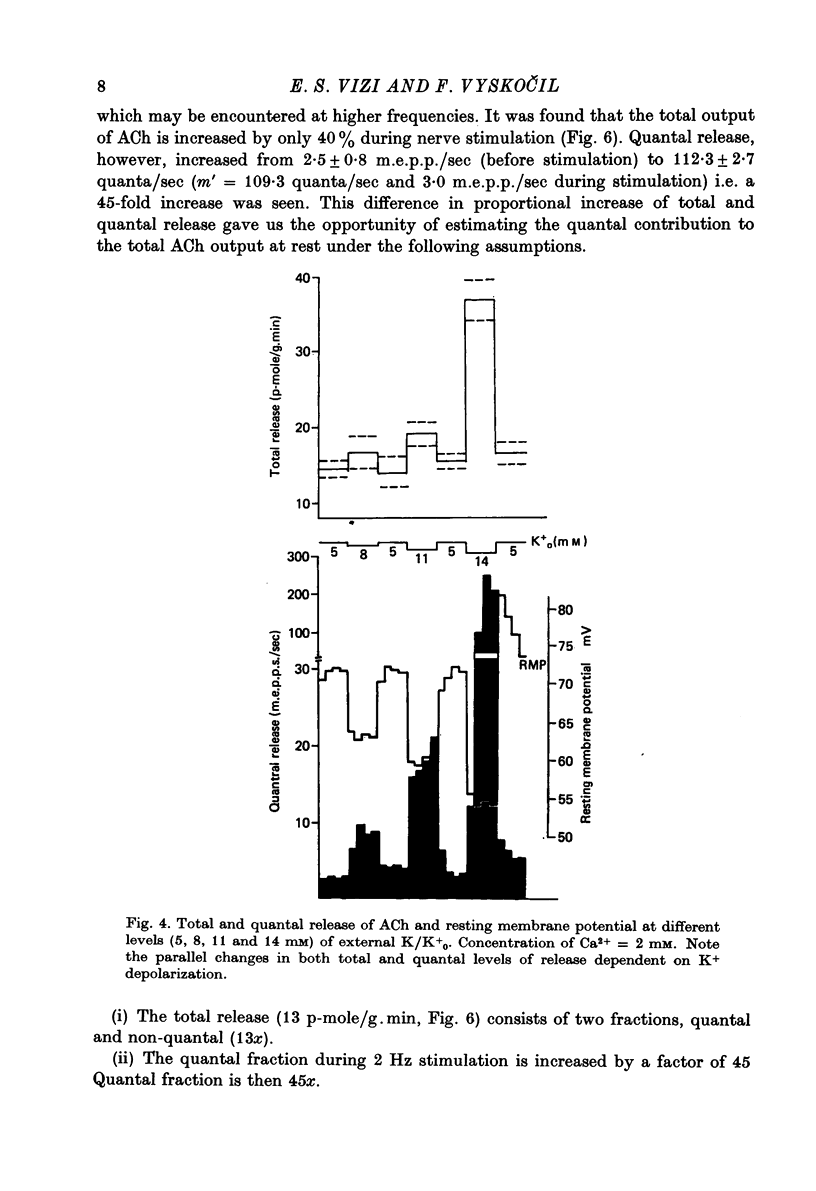
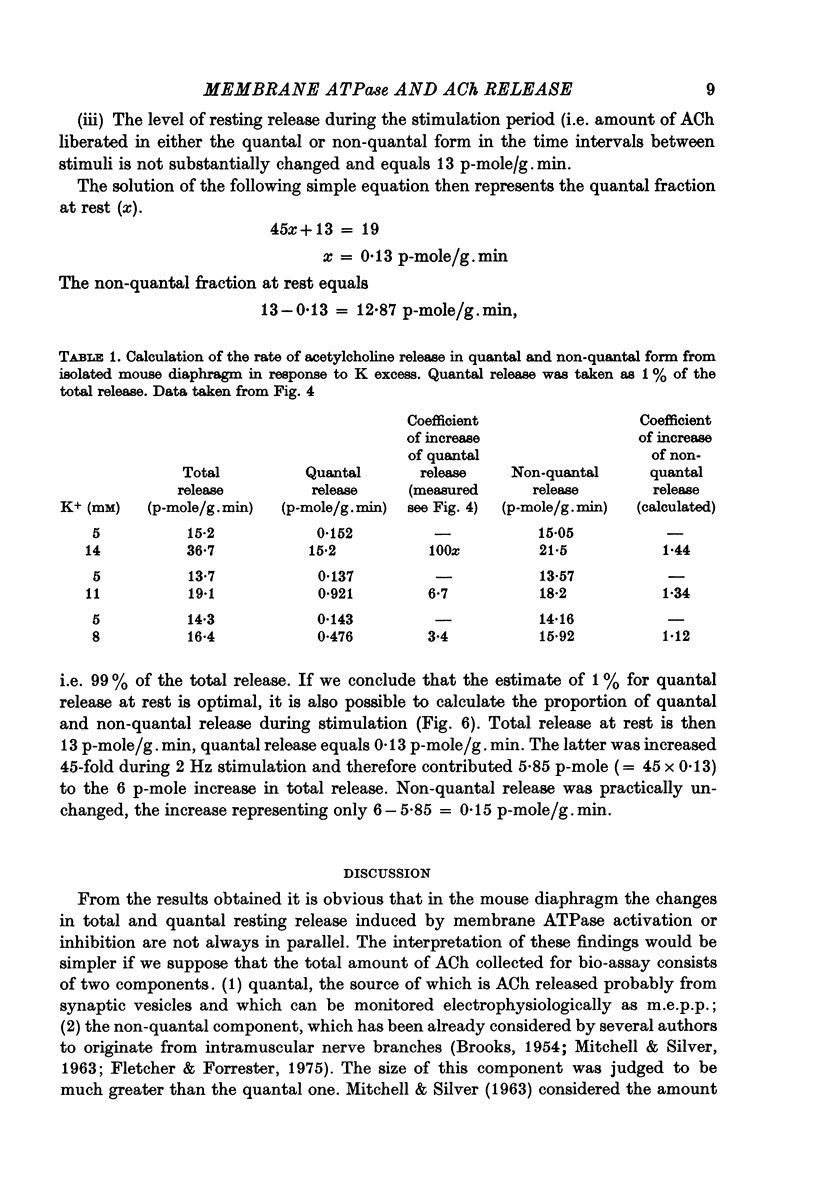
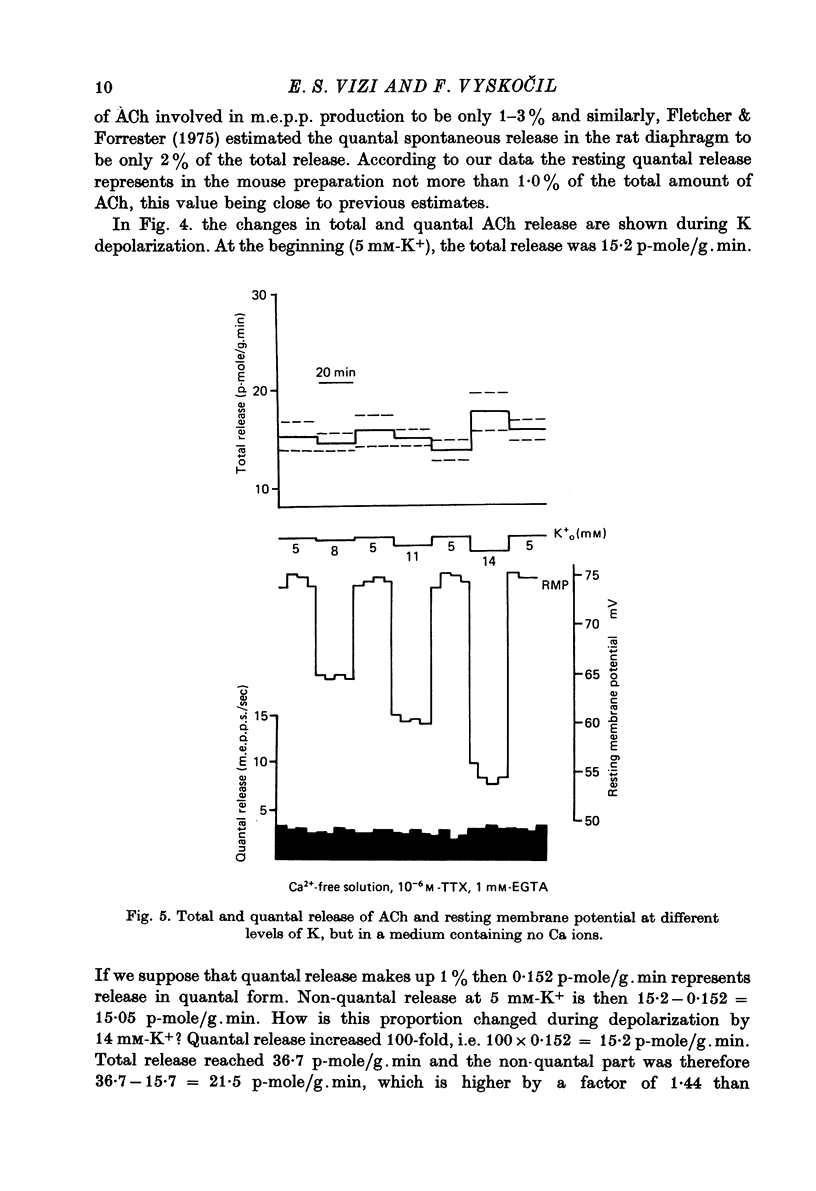
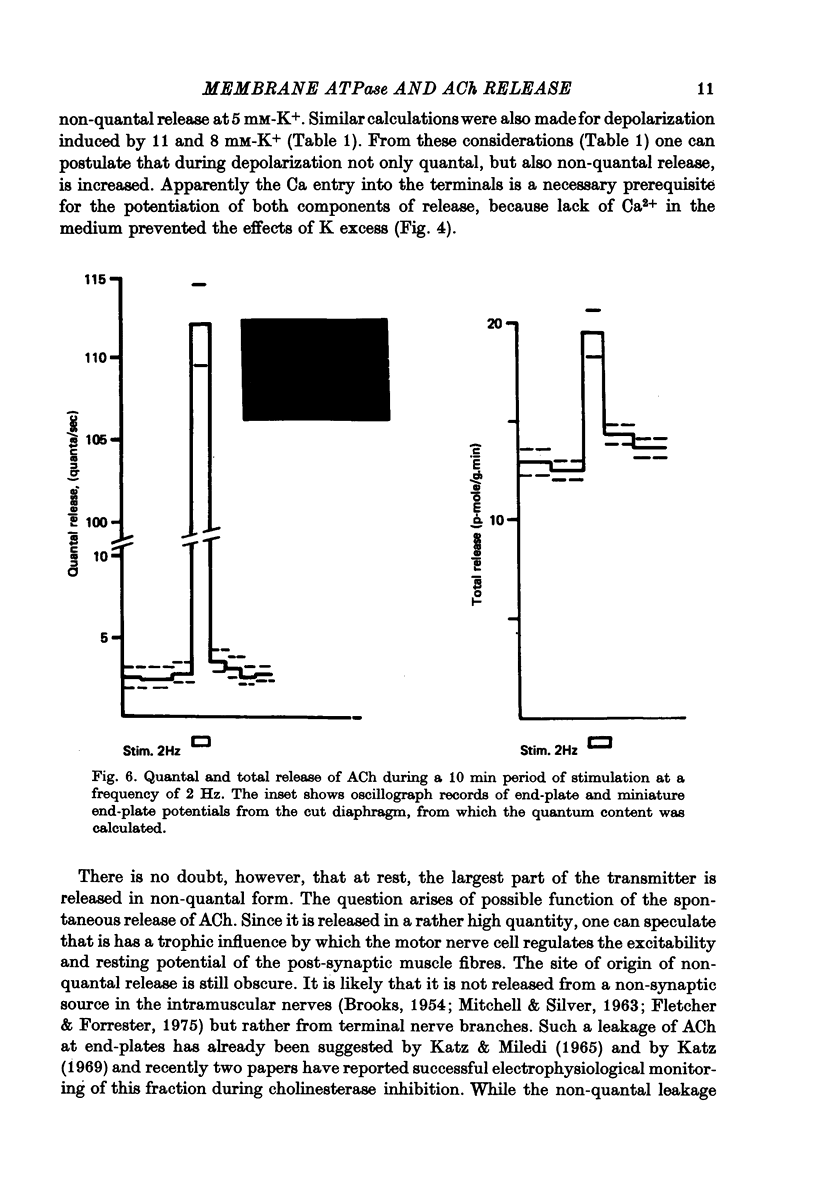
1. Acetylcholine (ACh) released from mouse diaphragm was gel filtrated and estimated by bio-assay and compared with electrophysiologically measured quantal release, expressed either as frequency of miniature end-plate potentials or quantum content of end-plate potentials. 2. Activation of Na+-K+-dependent membrane ATPase (membrane ATPase) in Na+-loaded muscles lowered the total amount of ACh released at rest to one tenth of the control value, but quantal release remained unchanged. 3. Inhibition of membrane ATPase by 2 X 10(-5) M-ouabain or by K-free solution led to an increase in total release and to a delayed progressive increase in quantal release. When Ca2+ was removed only the total release was enhanced. 4. Depolarization of the diaphragm by 8, 11 and 14 mM-K increased both total and quantal release only in the presence of Ca2+ in the perfusion medium. When Ca2+ was removed, no significant increase in release was observed. 5. The total and quantal release in response to 2 Hz stimulation of the preparation was increased 1.4 and 45 times, respectively. It is concluded that the total amount of ACh released at rest consists of two fractions, quantal and non-quantal, the former representing about 1% of the total release.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARSTAD J. A. Presynaptic effect of the neuro-muscular transmitter. Experientia. 1962 Dec 15;18:579–580. doi: 10.1007/BF02172193. [DOI] [PubMed] [Google Scholar]
- BROOKS V. B. The action of botulinum toxin on motor-nerve filaments. J Physiol. 1954 Mar 29;123(3):501–515. doi: 10.1113/jphysiol.1954.sp005067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Okamoto K., Quastel D. M. The role of calcium in depolarization-secretion coupling at the motor nerve terminal. J Physiol. 1973 Jan;228(2):459–497. doi: 10.1113/jphysiol.1973.sp010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P., Forrester T. The effect of curare on the release of acetylcholine from mammalian motor nerve terminals and an estimate of quantum content. J Physiol. 1975 Sep;251(1):131–144. doi: 10.1113/jphysiol.1975.sp011084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F., Miyamoto M. Reduction of transmitter release by D-tubocurarine. Nature. 1969 Aug 2;223(5205):531–533. doi: 10.1038/223531a0. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F. Neuromuscular transmission in a mammalian preparation in the absence of blocking drugs and the effect of D-tubocurarine. J Physiol. 1973 Jan;228(2):307–325. doi: 10.1113/jphysiol.1973.sp010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERNAN R. P. Membrane potential changes during sodium transport in frog sartorius muscle. Nature. 1962 Mar 10;193:986–987. doi: 10.1038/193986a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Transmitter leakage from motor nerve endings. Proc R Soc Lond B Biol Sci. 1977 Feb 11;196(1122):59–72. doi: 10.1098/rspb.1977.0029. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. F., Silver A. The spontaneous release of acetylcholine from the denervated hemidiaphragm of the rat. J Physiol. 1963 Jan;165(1):117–129. doi: 10.1113/jphysiol.1963.sp007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S. The inhibitory action of noradrenaline and adrenaline on acetylcholine output by guinea-pig ileum longitudinal muscle strip. Br J Pharmacol. 1969 Jan;35(1):10–28. doi: 10.1111/j.1476-5381.1969.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S., Zar M. A. The mechanism of acetylcholine release from parasympathetic nerves. J Physiol. 1971 Jul;215(3):819–848. doi: 10.1113/jphysiol.1971.sp009500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDIC M., STRAUGHAN D. W. ANTIDROMIC ACTIVITY IN THE RAT PHRENIC NERVE-DIAPHRAGM PREPARATION. J Physiol. 1964 Sep;173:130–148. doi: 10.1113/jphysiol.1964.sp007447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOU J. C. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta. 1957 Feb;23(2):394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- SOMOGYI J. UBER DIE WIRKUNG DER CA-IONEN AUF DIE DURCH NA+ UND K+ AKTIVIERBARE ADENOSINTRIPHOSPHATASE DES HIRNGEWEBES. Hoppe Seylers Z Physiol Chem. 1964;336:264–270. doi: 10.1515/bchm2.1964.336.1.264. [DOI] [PubMed] [Google Scholar]
- Sato M., Akaike N., Nishi R. Membrane potentials of frog sartorius muscle fibers, in which potassium ions were replaced by sodium. Kumamoto Med J. 1967 Mar 31;20(1):39–55. [PubMed] [Google Scholar]
- Schwartz A., Lindenmayer G. E., Allen J. C. The sodium-potassium adenosine triphosphatase: pharmacological, physiological and biochemical aspects. Pharmacol Rev. 1975 Mar;27(01):3–134. [PubMed] [Google Scholar]
- Tamai T., Kagiyama S. Studies of cat heart muscle during recovery after prolonged hypothermia. Hyperpolarization of cell membranes and its dependence on the sodium pump with electrogenic characteristics. Circ Res. 1968 Mar;22(3):423–433. doi: 10.1161/01.res.22.3.423. [DOI] [PubMed] [Google Scholar]
- Taylor G. S., Paton D. M., Daniel E. E. Characteristics of electrogenic sodium pumping in rat myometrium. J Gen Physiol. 1970 Sep;56(3):360–375. doi: 10.1085/jgp.56.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi E. S. Na+-K+-activated adenosinetriphosphatase as a trigger in transmitter release. Neuroscience. 1978;3(4-5):367–384. doi: 10.1016/0306-4522(78)90040-4. [DOI] [PubMed] [Google Scholar]
- Vizi E. S. Stimulation, by inhibition of (Na + -K + -Mg 2+ )-activated ATP-ase, of acetylcholine release in cortical slices from rat brain. J Physiol. 1972 Oct;226(1):95–117. doi: 10.1113/jphysiol.1972.sp009975. [DOI] [PMC free article] [PubMed] [Google Scholar]


