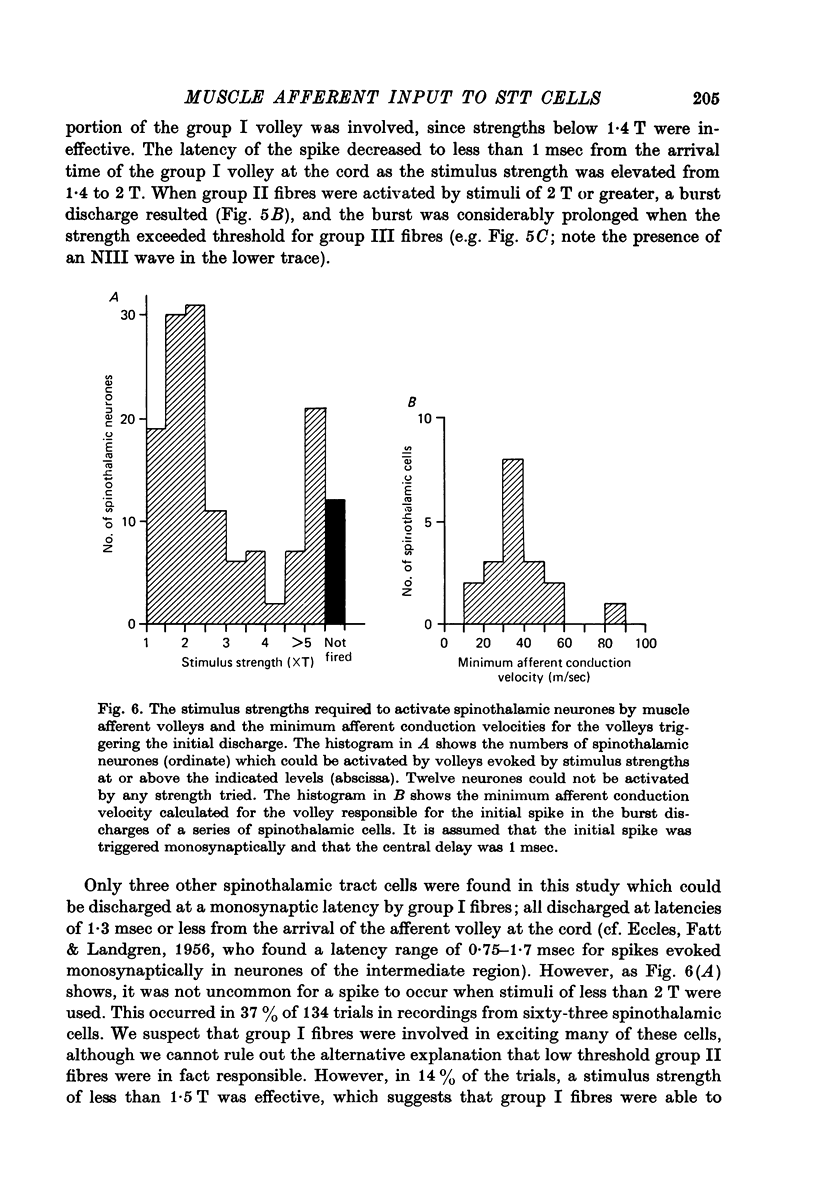
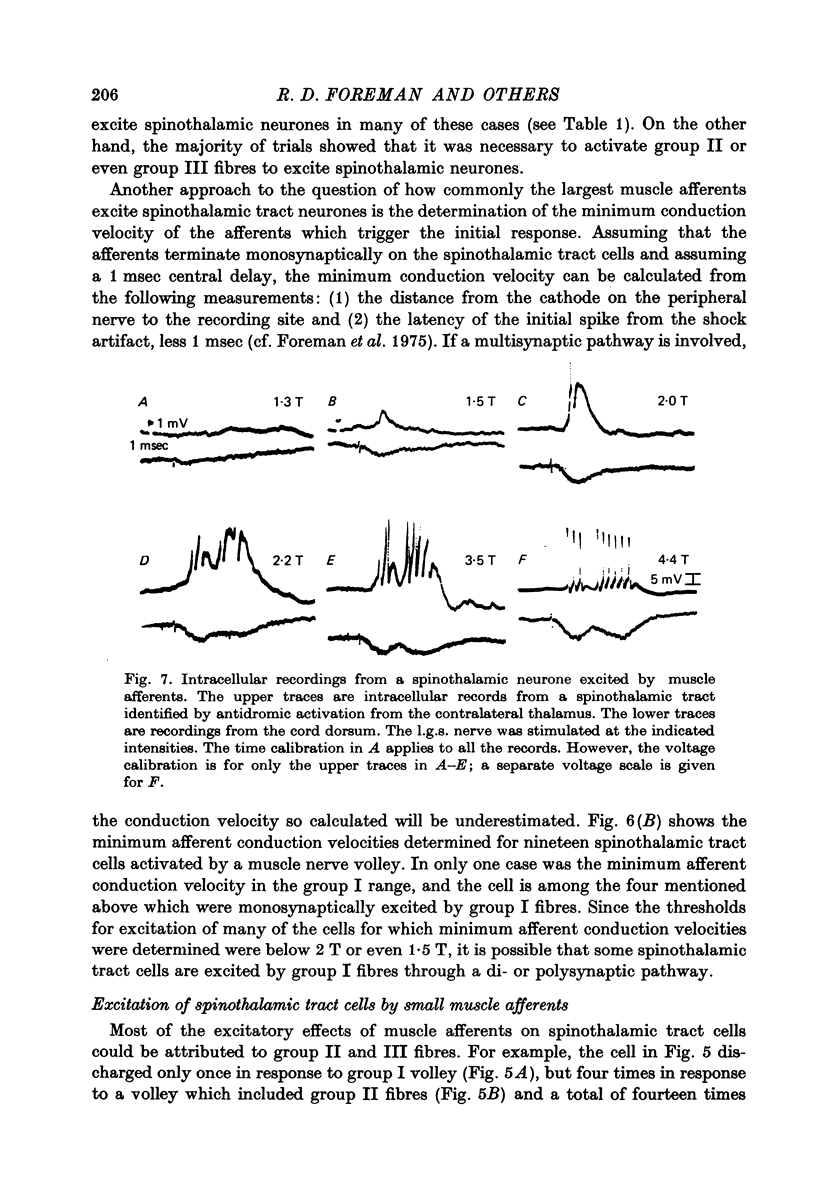
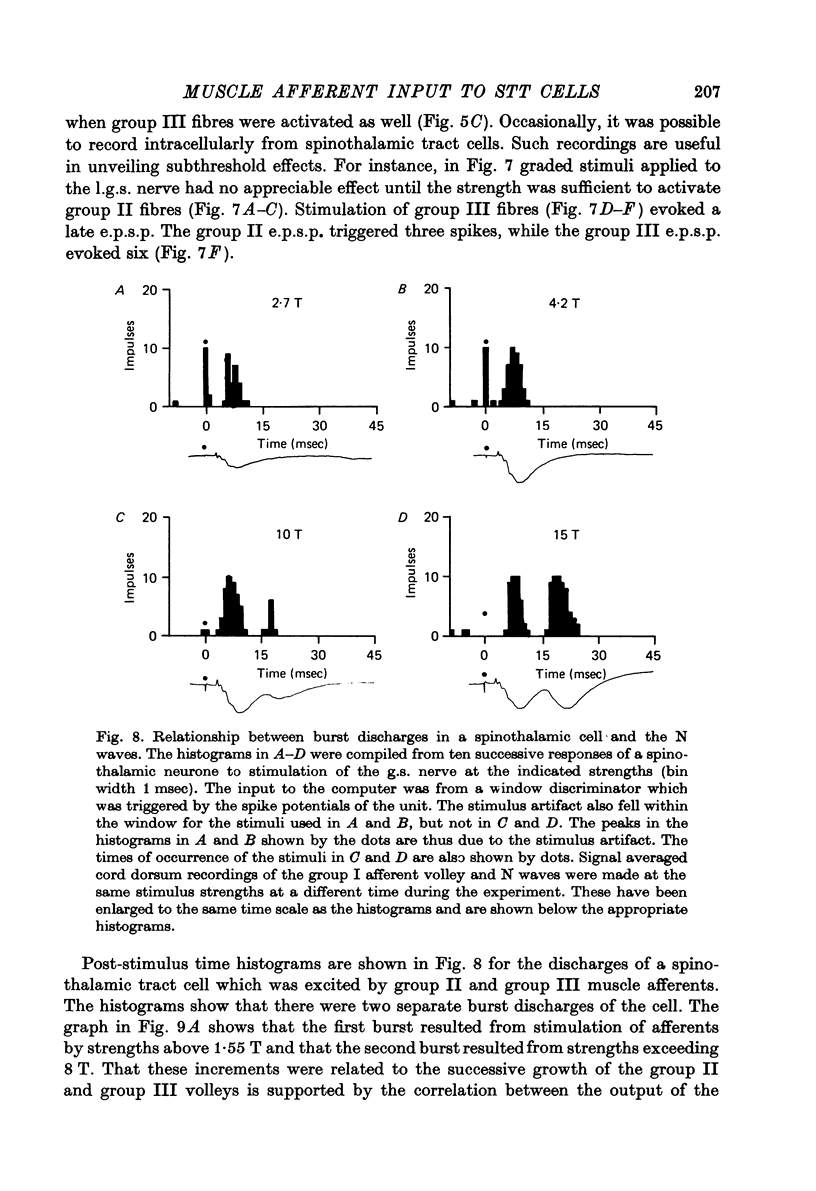
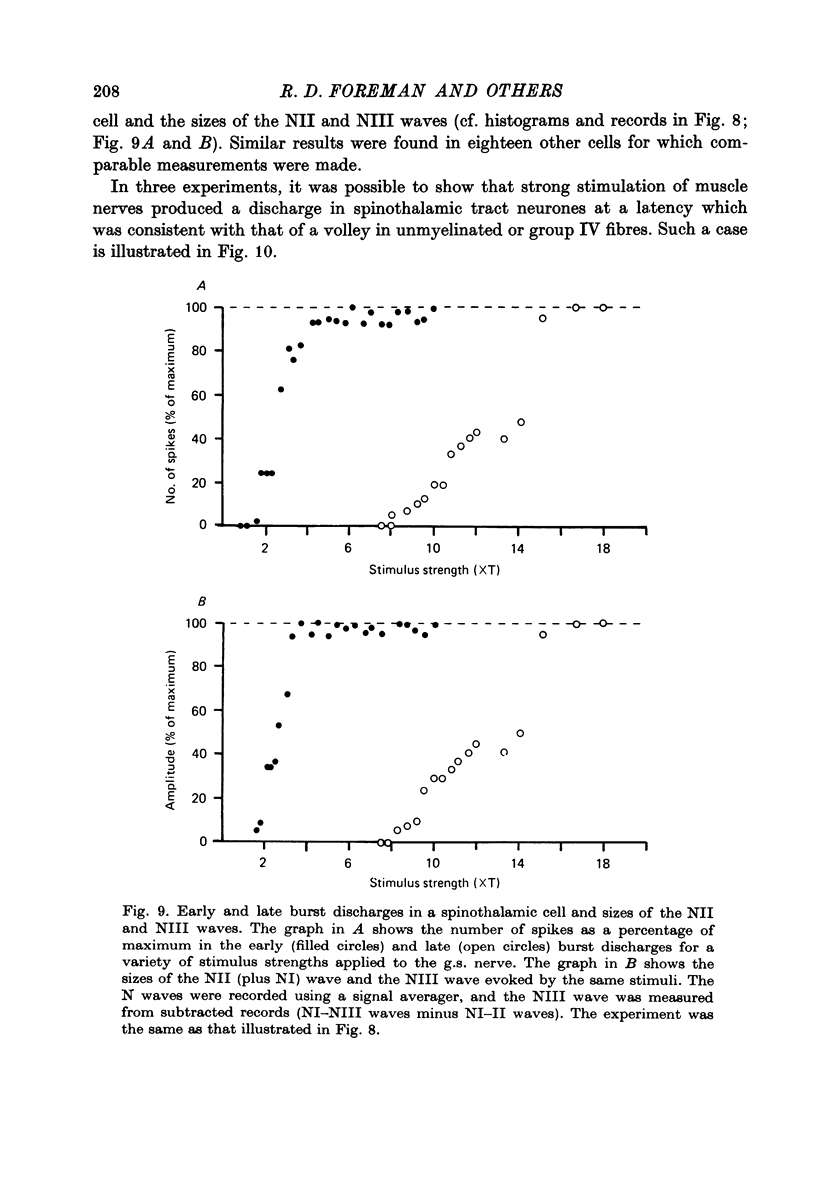
Abstract
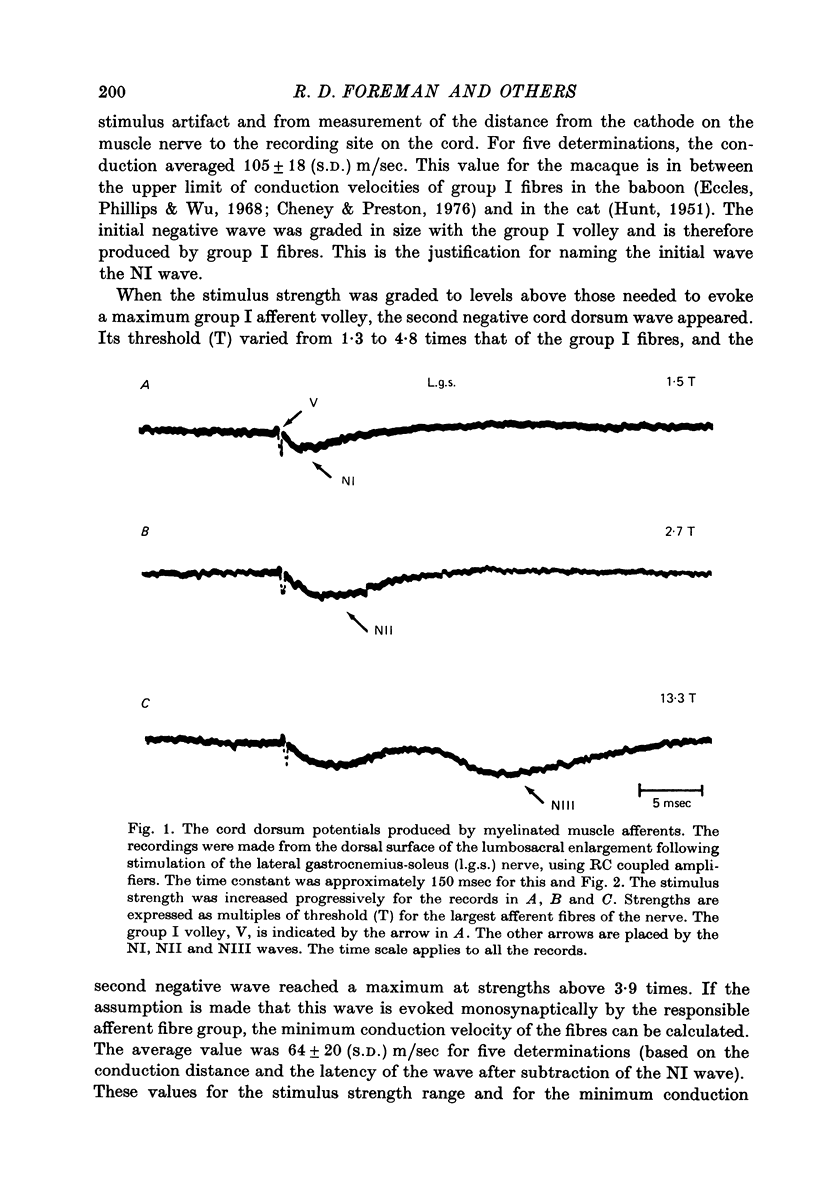
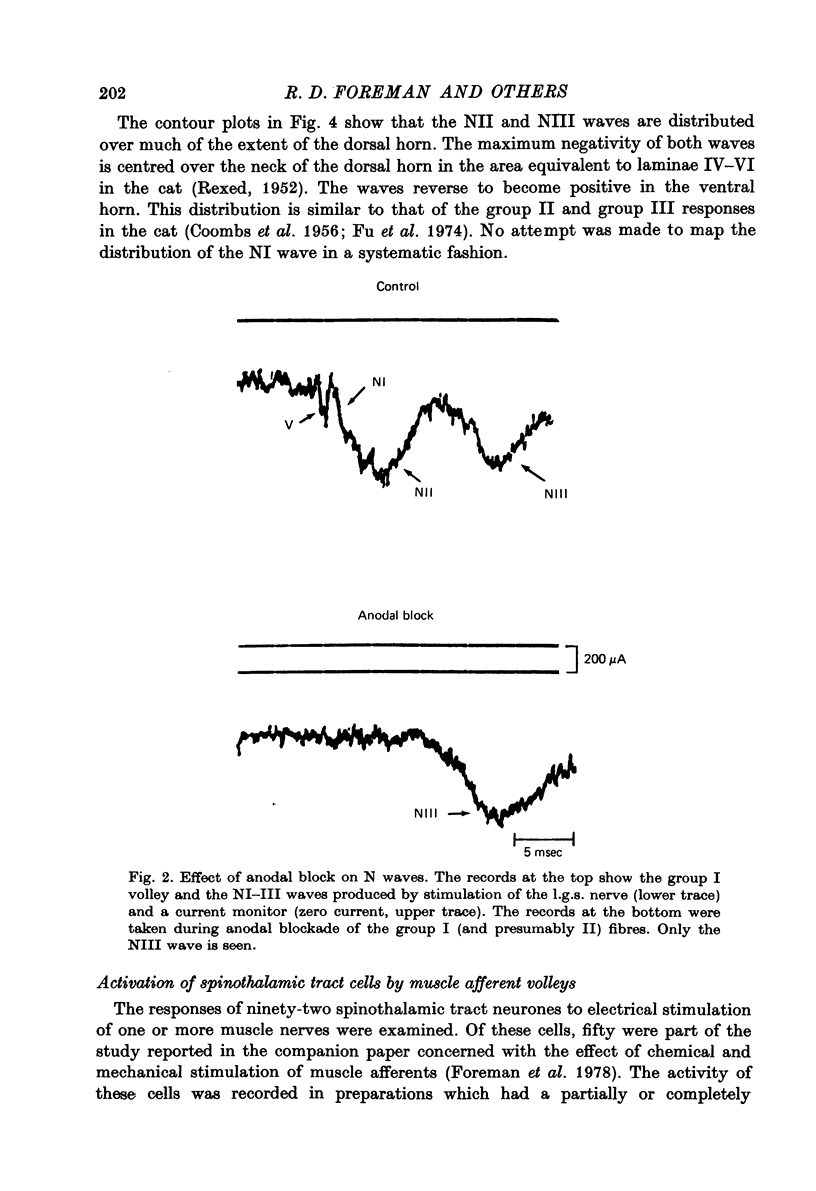
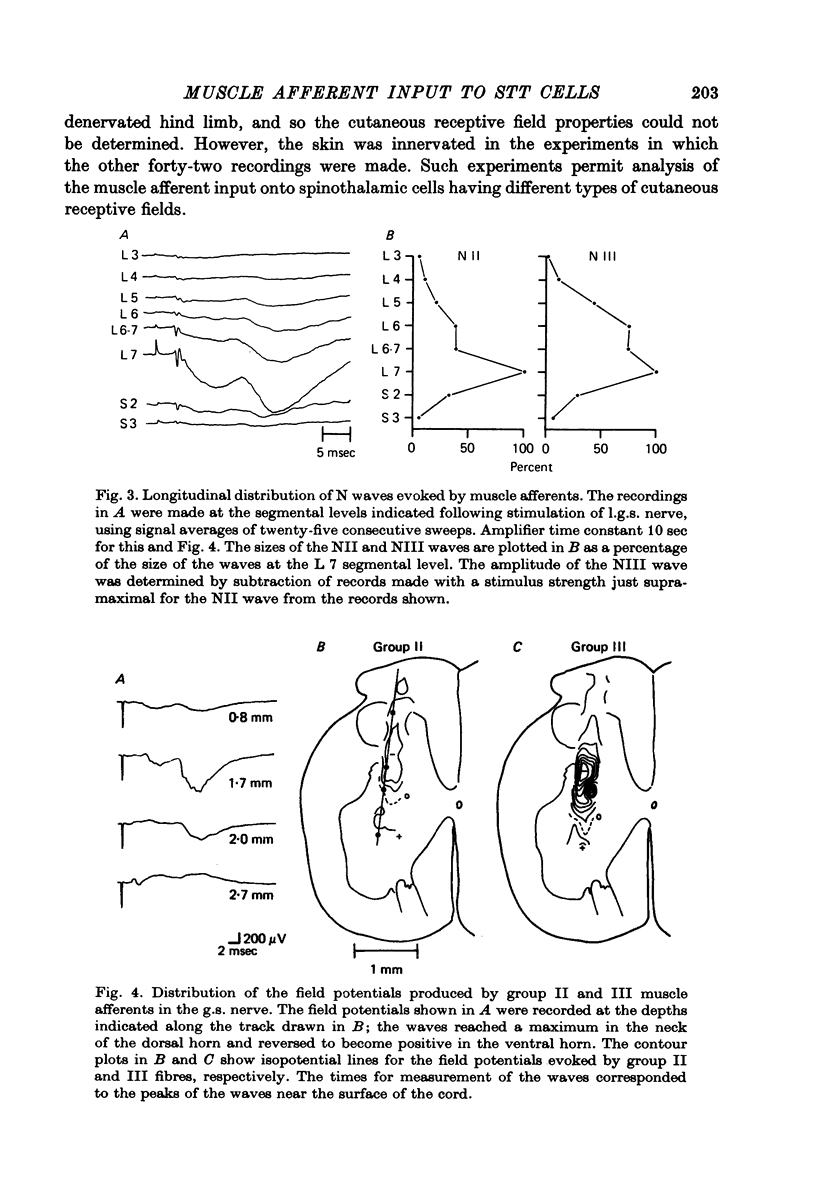
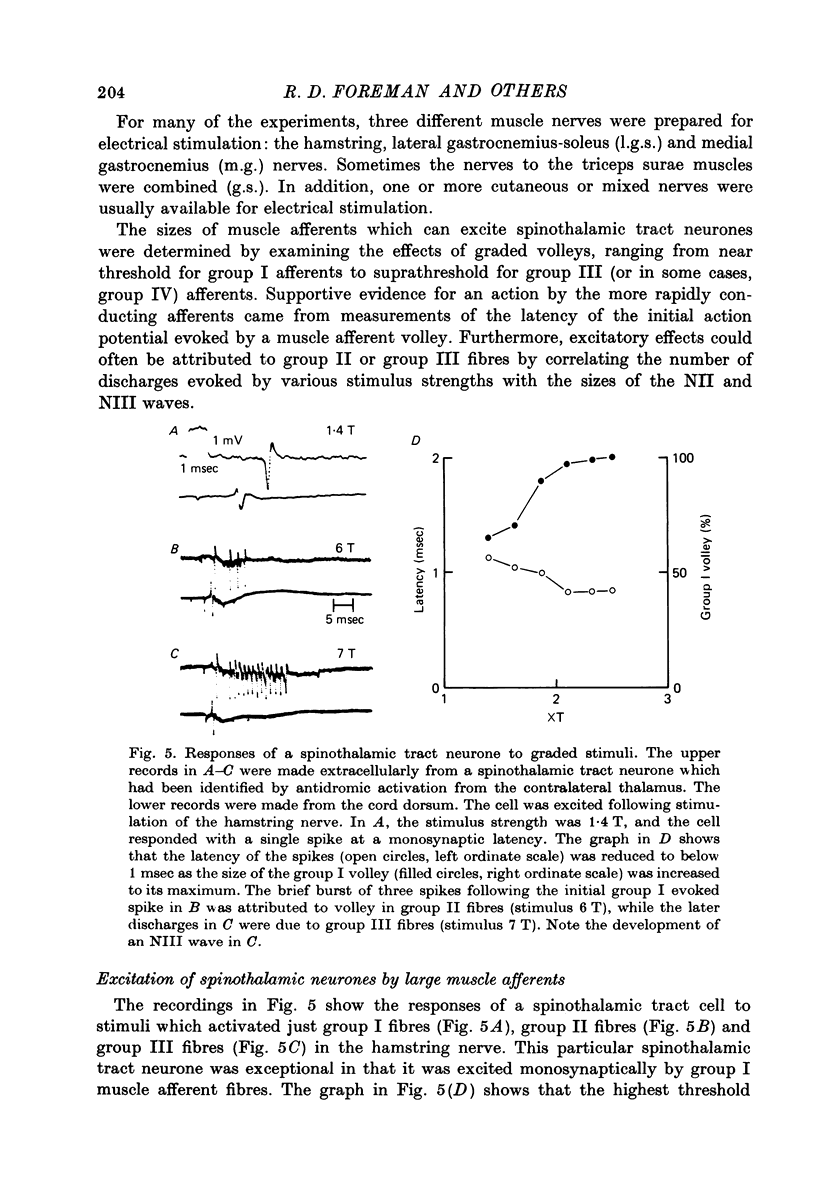
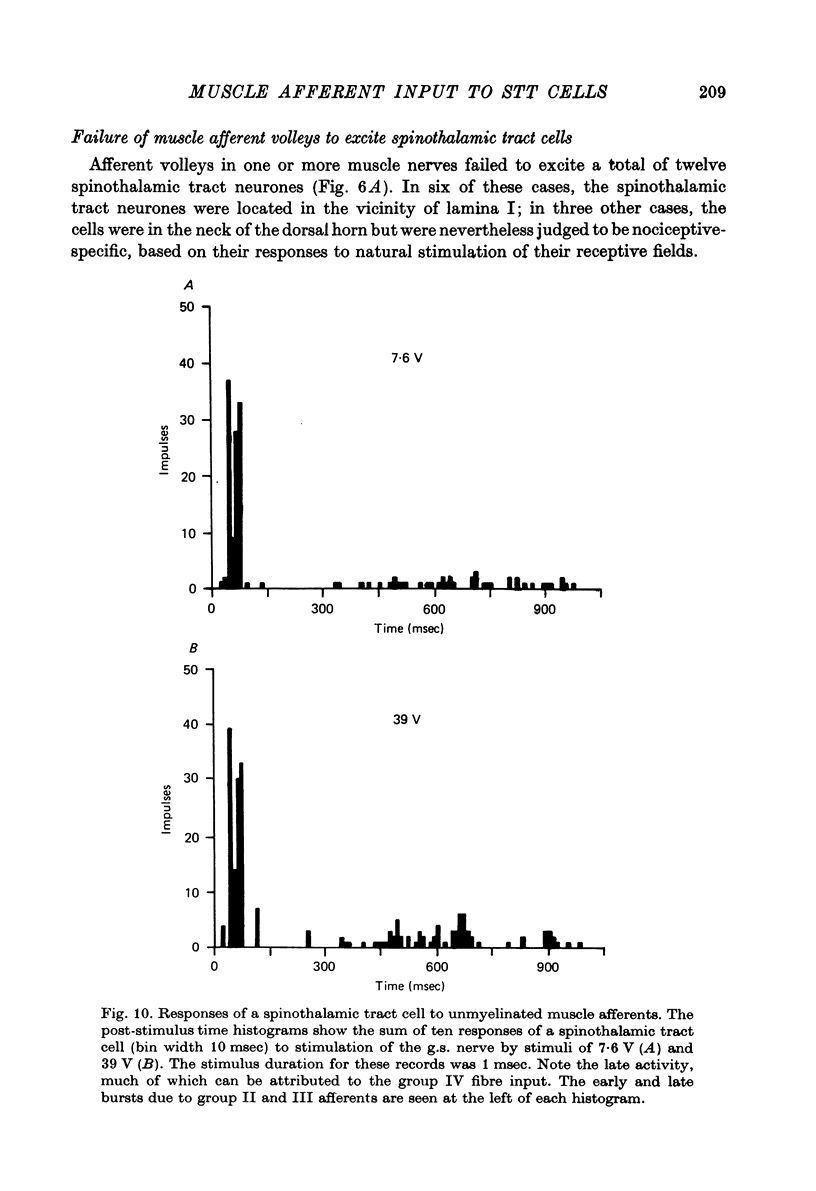
1. In anesthetized monkeys, stimulation of muscle afferents results in a sequence of cord dorsum potentials. These include a group I volley followed by several negative potentials called here the NI, NII and NIII waves. 2. Evidence based on the effects of graded stimulus strengths, measurements of latencies, and the results of anodal blockade of large muscle afferents indicate that the NI, NII and NIII waves are evoked, respectively, by group I, II and III muscle afferents. 3. The NII and NIII waves appear to be confined to the lumbosacral enlargement when evoked by hind limb muscle afferents. However, the group I volley and NI wave can be detected at least as far rostrally as L 3. 4. The NII and NIII waves were mapped in the depth of the cord. The maxima for these waves were found in the neck of the dorsal horn. The waves reversed to become positive in the ventral horn. 5. Using graded electrical stimulation of muscle nerves it was possible to demonstrate that a few spinothalamic tract neurones could be activated monosynaptically by group I volleys; other spinothalamic cells may have been activated polysynaptically by group I volleys. The lack of any substantial excitation of spinothalamic neurones by intra-arterial injections of succinylcholine suggests that these group I actions may have been due to group Ib afferents from Golgi tendon organs. 6. The most potent excitation of spinothalamic tract cells was due to the action of middle sized and small muscle afferents. Evidence was obtained for an excitatory action of group II, III and IV afferents. There was a good correlation between the effects of graded stimulation in evoking discharges in separate bursts associated with the arrival of volleys in group II and group III afferents and the generation of the NII and NIII waves. 7. Some spinothalamic neurones, including several located in lamina I, were unaffected by the muscle afferent volleys used. It is suggested that such neurones might help to signal well localized pain, whereas the cells which respond to a variety of cutaneous and muscle afferents might be involved in signalling poorly localized pain which is subject to referral.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albe-Fessard D., Levante A., Lamour Y. Origin of spino-thalamic tract in monkeys. Brain Res. 1974 Jan 18;65(3):503–509. doi: 10.1016/0006-8993(74)90238-8. [DOI] [PubMed] [Google Scholar]
- BROCK L. G., ECCLES J. C., RALL W. Experimental investigations on the afferent fibres in muscle nerves. Proc R Soc Lond B Biol Sci. 1951 Oct 30;138(893):453–475. doi: 10.1098/rspb.1951.0035. [DOI] [PubMed] [Google Scholar]
- Beall J. E., Applebaum A. E., Foreman R. D., Willis W. D. Spinal cord potentials evoked by cutaneous afferents in the monkey. J Neurophysiol. 1977 Mar;40(2):199–211. doi: 10.1152/jn.1977.40.2.199. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Hamann W. C., Martin H. F., 3rd Effects of activity in non-myelinated afferent fibres on the spinocervical tract. Brain Res. 1975 Nov 14;98(2):243–259. doi: 10.1016/0006-8993(75)90004-9. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Rudomin P., Vyklický L., Zajac F. E., 3rd Primary afferent depolarization and flexion reflexes produced by radiant heat stimulation of the skin. J Physiol. 1971 Feb;213(1):185–214. doi: 10.1113/jphysiol.1971.sp009376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., CURTIS D. R., LANDGREN S. Spinal cord potentials generated by impulses in muscle and cutaneous afferent fibres. J Neurophysiol. 1956 Sep;19(5):452–467. doi: 10.1152/jn.1956.19.5.452. [DOI] [PubMed] [Google Scholar]
- Cervero F., Iggo A., Ogawa H. Nociceptor-driven dorsal horn neurones in the lumbar spinal cord of the cat. Pain. 1976 Mar;2(1):5–24. doi: 10.1016/0304-3959(76)90042-7. [DOI] [PubMed] [Google Scholar]
- Cheney P. D., Preston J. B. Classification and response characteristics of muscle spindle afferents in the primate. J Neurophysiol. 1976 Jan;39(1):1–8. doi: 10.1152/jn.1976.39.1.1. [DOI] [PubMed] [Google Scholar]
- Christensen B. N., Perl E. R. Spinal neurons specifically excited by noxious or thermal stimuli: marginal zone of the dorsal horn. J Neurophysiol. 1970 Mar;33(2):293–307. doi: 10.1152/jn.1970.33.2.293. [DOI] [PubMed] [Google Scholar]
- Dilly P. N., Wall P. D., Webster K. E. Cells of origin of the spinothalamic tract in the cat and rat. Exp Neurol. 1968 Aug;21(4):550–562. doi: 10.1016/0014-4886(68)90072-1. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., LANDGREN S. Central pathway for direct inhibitory action of impulses in largest afferent nerve fibres to muscle. J Neurophysiol. 1956 Jan;19(1):75–98. doi: 10.1152/jn.1956.19.1.75. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Magni F., Willis W. D. Depolarization of central terminals of Group I afferent fibres from muscle. J Physiol. 1962 Jan;160(1):62–93. doi: 10.1113/jphysiol.1962.sp006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R. M., Phillips C. G., Chien-Ping W. Motor innervation, motor unit organization and afferent innervation of m. extensor digitorum communis of the baboon's forearm. J Physiol. 1968 Sep;198(1):179–192. doi: 10.1113/jphysiol.1968.sp008600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEHR H. U. ACTIVATION BY SUXAMETHONIUM OF PRIMARY AND SECONDARY ENDINGS OF THE SAME DE-EFFERENTED MUSCLE SPINDLE DURING STATIC STRETCH. J Physiol. 1965 May;178:98–110. doi: 10.1113/jphysiol.1965.sp007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman R. D., Applebaum A. E., Beall J. E., Trevino D. L., Willis W. D. Responses of primate spinothalamic tract neurons to electrical stimulation of hindlimb peripheral nerves. J Neurophysiol. 1975 Jan;38(1):132–145. doi: 10.1152/jn.1975.38.1.132. [DOI] [PubMed] [Google Scholar]
- Foreman R. D., Schmidt R. F., Willis W. D. Convergence of muscle and cutaneous input onto primate spinothalamic tract neurons. Brain Res. 1977 Apr 1;124(3):555–560. doi: 10.1016/0006-8993(77)90956-8. [DOI] [PubMed] [Google Scholar]
- Foreman R. D., Schmidt R. F., Willis W. D. Effects of mechanical and chemical stimulation of fine muscle afferents upon primate spinothalamic tract cells. J Physiol. 1979 Jan;286:215–231. doi: 10.1113/jphysiol.1979.sp012615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz D. N., Iggo A. Dorsal root potentials and ventral root reflexes evoked by nonmyelinated fibers. Science. 1968 Dec 6;162(3858):1140–1142. doi: 10.1126/science.162.3858.1140. [DOI] [PubMed] [Google Scholar]
- Fu T. C., Santini M., Schomburg E. D. Characteristics and distribution of spinal focal synaptic potentials generated by group II muscle afferents. Acta Physiol Scand. 1974 Jul;91(3):298–313. doi: 10.1111/j.1748-1716.1974.tb05686.x. [DOI] [PubMed] [Google Scholar]
- Fukushima K., Kato M. Spinal interneurons responding to group II muscle afferent fibers in the cat. Brain Res. 1975 Jun 13;90(2):307–312. doi: 10.1016/0006-8993(75)90311-x. [DOI] [PubMed] [Google Scholar]
- GRANIT R., SKOGLUND S., THESLEFF S. Activation of muscle spindles by succinylcholine and decamethonium, the effects of curare. Acta Physiol Scand. 1953;28(2-3):134–151. doi: 10.1111/j.1748-1716.1953.tb00964.x. [DOI] [PubMed] [Google Scholar]
- HUNT C. C. Relation of function to diameter in afferent fibers of muscle nerves. J Gen Physiol. 1954 Sep 20;38(1):117–131. doi: 10.1085/jgp.38.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa T., Perl E. R. Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn functional organization. J Comp Neurol. 1978 Feb 1;177(3):417–434. doi: 10.1002/cne.901770305. [DOI] [PubMed] [Google Scholar]
- MORIN F., SCHWARTZ H. G., O'LEARY J. L. Experimental study of the spinothalamic and related tracts. Acta Psychiatr Neurol Scand. 1951;26(3-4):371–396. doi: 10.1111/j.1600-0447.1951.tb09681.x. [DOI] [PubMed] [Google Scholar]
- Mendell L. M. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966 Nov;16(3):316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol. 1977 May;267(1):75–88. doi: 10.1113/jphysiol.1977.sp011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S., Schmidt R. F. Activation of group IV afferent units from muscle by algesic agents. Brain Res. 1974 Jun 7;72(2):305–310. doi: 10.1016/0006-8993(74)90870-1. [DOI] [PubMed] [Google Scholar]
- PAINTAL A. S. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol. 1960 Jul;152:250–270. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAINTAL A. S. Participation by pressure-pain receptors of mammalian muscles in the flexion reflex. J Physiol. 1961 May;156:498–514. doi: 10.1113/jphysiol.1961.sp006689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeranz B., Wall P. D., Weber W. V. Cord cells responding to fine myelinated afferents from viscera, muscle and skin. J Physiol. 1968 Dec;199(3):511–532. doi: 10.1113/jphysiol.1968.sp008666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington C. S. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol. 1910 Apr 26;40(1-2):28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevino D. L., Coulter J. D., Willis W. D. Location of cells of origin of spinothalamic tract in lumbar enlargement of the monkey. J Neurophysiol. 1973 Jul;36(4):750–761. doi: 10.1152/jn.1973.36.4.750. [DOI] [PubMed] [Google Scholar]
- WALL P. D. Cord cells responding to touch, damage, and temperature of skin. J Neurophysiol. 1960 Mar;23:197–210. doi: 10.1152/jn.1960.23.2.197. [DOI] [PubMed] [Google Scholar]
- Wall P. D. The laminar organization of dorsal horn and effects of descending impulses. J Physiol. 1967 Feb;188(3):403–423. doi: 10.1113/jphysiol.1967.sp008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis W. D., Trevino D. L., Coulter J. D., Maunz R. A. Responses of primate spinothalamic tract neurons to natural stimulation of hindlimb. J Neurophysiol. 1974 Mar;37(2):358–372. doi: 10.1152/jn.1974.37.2.358. [DOI] [PubMed] [Google Scholar]
- YOSS R. E. Studies of the spinal cord. Part 3. Pathways for deep pain within the spinal cord and brain. Neurology. 1953 Mar;3(3):163–175. doi: 10.1212/wnl.3.3.163. [DOI] [PubMed] [Google Scholar]


