Abstract
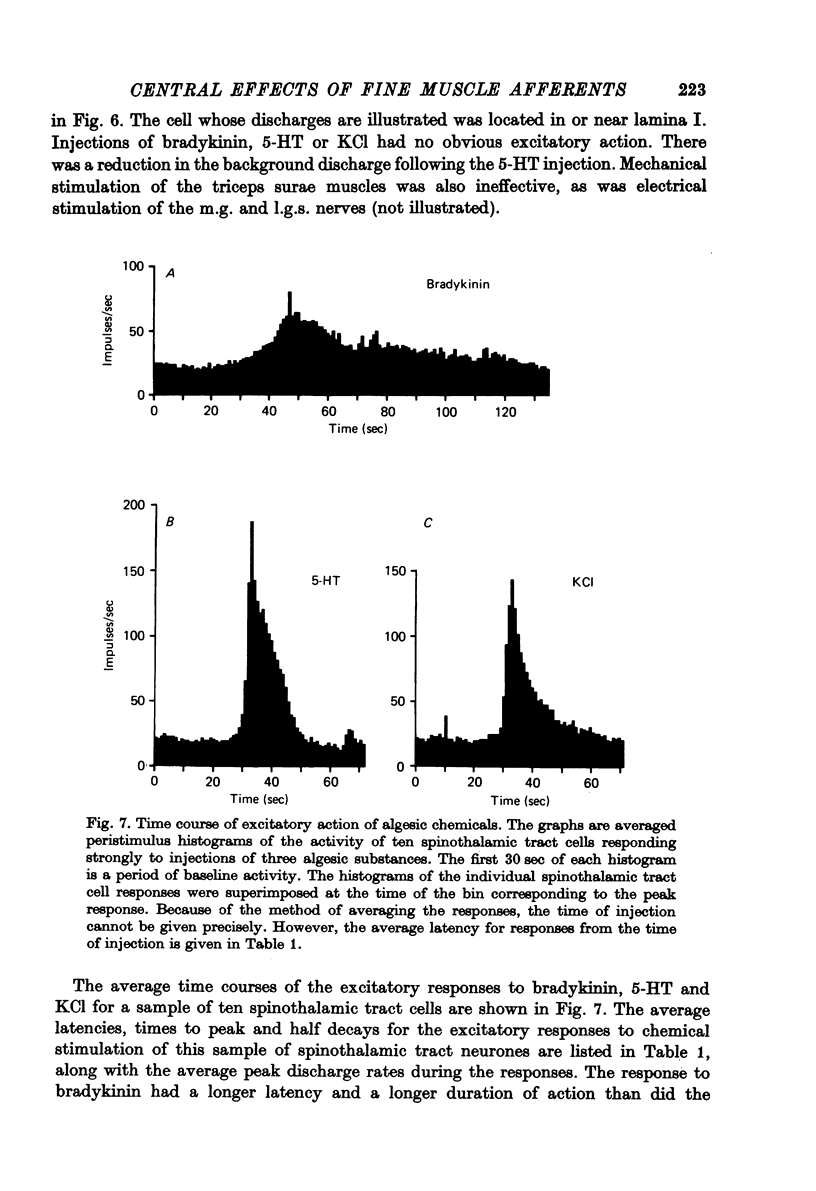
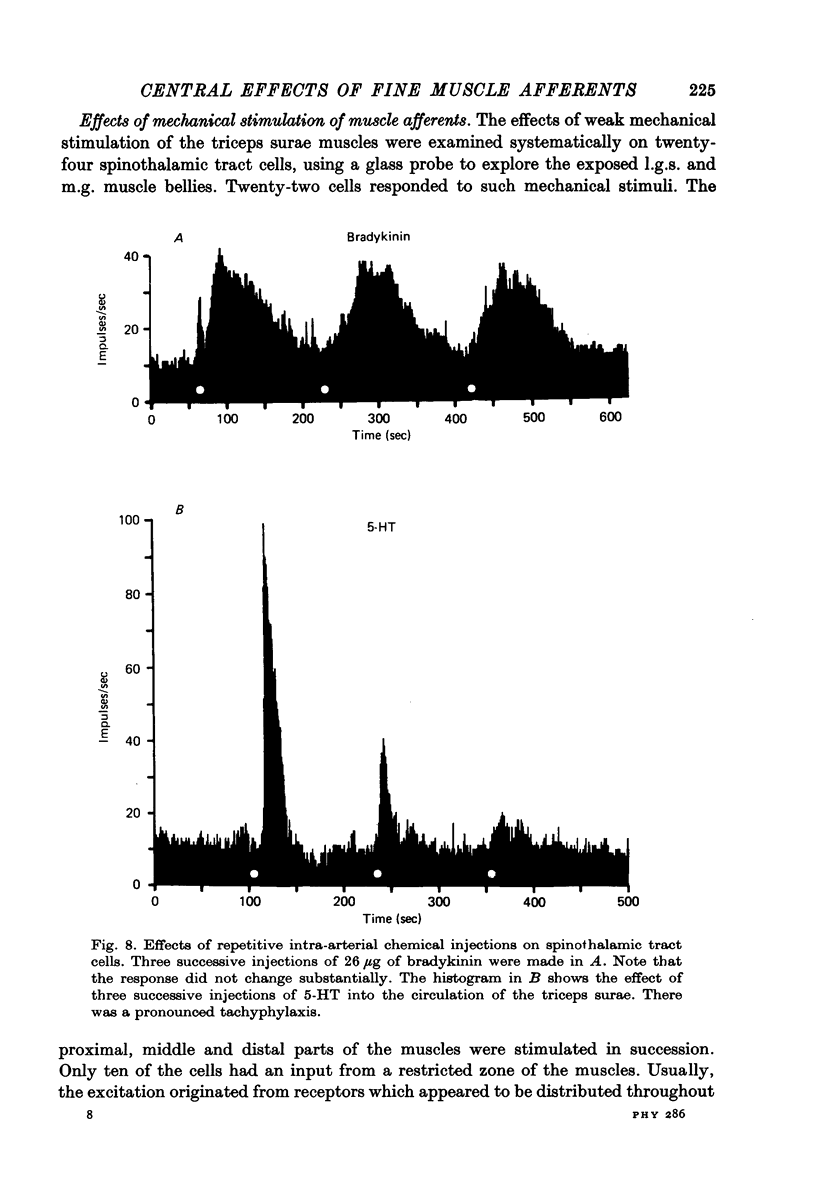
1. Injections of algesic chemicals were made into the arterial circulation of the triceps surae muscles in anaesthetized monkeys. 2. The responses of a sample of primary muscle afferents suggest that what is known about the activation of muscle afferents in the cat by algesic agents applies also to the monkey. One exception to this is the activation of many group I afferents by KCl in the monkey, but not in the cat. 3. Many spinothalamic tract cells were powerfully excited by the intra-arterial injection of algesic chemicals (bradykinin, 5-hydroxytryptamine (5-HT), KCl) in preparations in which the hind limb was denervated except for the nerves to the triceps surae muscles. The excitatory action of bradykinin had a slower time course than did that of 5-HT or KCl. 4. A number of the spinothalamic tract cells which failed to respond to chemical activation of muscle afferents were located in lamina I of the spinal cord. 5. Repeated injections of bradykinin produced similar responses, whereas the effects of 5-HT injections showed marked tachyphylaxis. 6. No evidence was obtained that activation of muscle spindle afferents by succinylcholine injections resulted in the excitation of spinothalamic tract neurones in the population sampled. 7. Injections of hypertonic NaCl into muscle or tendon produced a prolonged excitation of many spinothalamic tract cells. 8. It is concluded that a substantial proportion of primate spinothalamic tract cells receive a convergent input from cutaneous and muscle receptors. The muscle receptors involved appear to include primary afferents of group III and IV calibre. The possibility is suggested that such cells could play a role in the production of poorly localized pain.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beall J. E., Applebaum A. E., Foreman R. D., Willis W. D. Spinal cord potentials evoked by cutaneous afferents in the monkey. J Neurophysiol. 1977 Mar;40(2):199–211. doi: 10.1152/jn.1977.40.2.199. [DOI] [PubMed] [Google Scholar]
- Besson J. M., Conseiller C., Hamann K. F., Maillard M. C. Modifications of dorsal horn cell activities in the spinal cord, after intra-arterial injection of bradykinin. J Physiol. 1972 Feb;221(1):189–205. doi: 10.1113/jphysiol.1972.sp009748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Franz D. N. Responses of spinocervical tract neurones to natural stimulation of identified cutaneous receptors. Exp Brain Res. 1969;7(3):231–249. doi: 10.1007/BF00239031. [DOI] [PubMed] [Google Scholar]
- Bryan R. N., Coulter J. D., Willis W. D. Cells of origin of the spinocervical tract in the monkey. Exp Neurol. 1974 Mar;42(3):574–586. doi: 10.1016/0014-4886(74)90080-6. [DOI] [PubMed] [Google Scholar]
- Cervero F., Iggo A., Molony V. Responses of spinocervical tract neurones to noxious stimulation of the skin. J Physiol. 1977 May;267(2):537–558. doi: 10.1113/jphysiol.1977.sp011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B. N., Perl E. R. Spinal neurons specifically excited by noxious or thermal stimuli: marginal zone of the dorsal horn. J Neurophysiol. 1970 Mar;33(2):293–307. doi: 10.1152/jn.1970.33.2.293. [DOI] [PubMed] [Google Scholar]
- Coote J. H., Hilton S. M., Perez-Gonzalez J. F. The reflex nature of the pressor response to muscular exercise. J Physiol. 1971 Jul;215(3):789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fock S., Mense S. Excitatory effects of 5-hydroxytryptamine, histamine and potassium ions on muscular group IV afferent units: a comparison with bradykinin. Brain Res. 1976 Apr 9;105(3):459–469. doi: 10.1016/0006-8993(76)90593-x. [DOI] [PubMed] [Google Scholar]
- Foreman R. D., Kenshalo D. R., Jr, Schmidt R. F., Willis W. D. Field potentials and excitation of primate spinothalamic neurones in response to volleys in muscle afferents. J Physiol. 1979 Jan;286:197–213. doi: 10.1113/jphysiol.1979.sp012614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz M., Mense S. Muscle receptors with group IV afferent fibres responding to application of bradykinin. Brain Res. 1975 Jul 18;92(3):369–383. doi: 10.1016/0006-8993(75)90323-6. [DOI] [PubMed] [Google Scholar]
- GRANIT R., SKOGLUND S., THESLEFF S. Activation of muscle spindles by succinylcholine and decamethonium, the effects of curare. Acta Physiol Scand. 1953;28(2-3):134–151. doi: 10.1111/j.1748-1716.1953.tb00964.x. [DOI] [PubMed] [Google Scholar]
- Guilbaud G., Benelli G., Besson J. M. Responses of thoracic dorsal horn interneurons to cutaneous stimulation and to the administration of algogenic substances into the mesenteric artery in the spinal cat. Brain Res. 1977 Apr 1;124(3):437–448. doi: 10.1016/0006-8993(77)90945-3. [DOI] [PubMed] [Google Scholar]
- Hancock M. B., Foreman R. D., Willis W. D. Convergence of visceral and cutaneous input onto spinothalamic tract cells in the thoracic spinal cord of the cat. Exp Neurol. 1975 May;47(2):240–248. doi: 10.1016/0014-4886(75)90253-8. [DOI] [PubMed] [Google Scholar]
- Kniffki K. D., Mense S., Schmidt R. F. The spinocervical tract as a possible pathway for muscular nociception. J Physiol (Paris) 1977 Sep;73(3):359–366. [PubMed] [Google Scholar]
- Kumazawa T., Mizumura K. Thin-fibre receptors responding to mechanical, chemical, and thermal stimulation in the skeletal muscle of the dog. J Physiol. 1977 Dec;273(1):179–194. doi: 10.1113/jphysiol.1977.sp012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levante A., Lamour Y., Guilbaud G., Besson J. M. Spinothalamic cell activity in the monkey during intense nociceptive stimulation: intra-arterial injection of bradykinin into the limbs. Brain Res. 1975 May 9;88(3):560–564. doi: 10.1016/0006-8993(75)90671-x. [DOI] [PubMed] [Google Scholar]
- McCloskey D. I., Mitchell J. H. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972 Jul;224(1):173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell L. M. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966 Nov;16(3):316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol. 1977 May;267(1):75–88. doi: 10.1113/jphysiol.1977.sp011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S., Schmidt R. F. Activation of group IV afferent units from muscle by algesic agents. Brain Res. 1974 Jun 7;72(2):305–310. doi: 10.1016/0006-8993(74)90870-1. [DOI] [PubMed] [Google Scholar]
- PAINTAL A. S. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol. 1960 Jul;152:250–270. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeranz B., Wall P. D., Weber W. V. Cord cells responding to fine myelinated afferents from viscera, muscle and skin. J Physiol. 1968 Dec;199(3):511–532. doi: 10.1113/jphysiol.1968.sp008666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevino D. L., Coulter J. D., Willis W. D. Location of cells of origin of spinothalamic tract in lumbar enlargement of the monkey. J Neurophysiol. 1973 Jul;36(4):750–761. doi: 10.1152/jn.1973.36.4.750. [DOI] [PubMed] [Google Scholar]
- Trevino D. L., Maunz R. A., Bryan R. N., Willis W. D. Location of cells of origin of the spinothalamic tract in the lumbar enlargement of cat. Exp Neurol. 1972 Jan;34(1):64–77. doi: 10.1016/0014-4886(72)90188-4. [DOI] [PubMed] [Google Scholar]
- Willis W. D., Trevino D. L., Coulter J. D., Maunz R. A. Responses of primate spinothalamic tract neurons to natural stimulation of hindlimb. J Neurophysiol. 1974 Mar;37(2):358–372. doi: 10.1152/jn.1974.37.2.358. [DOI] [PubMed] [Google Scholar]


