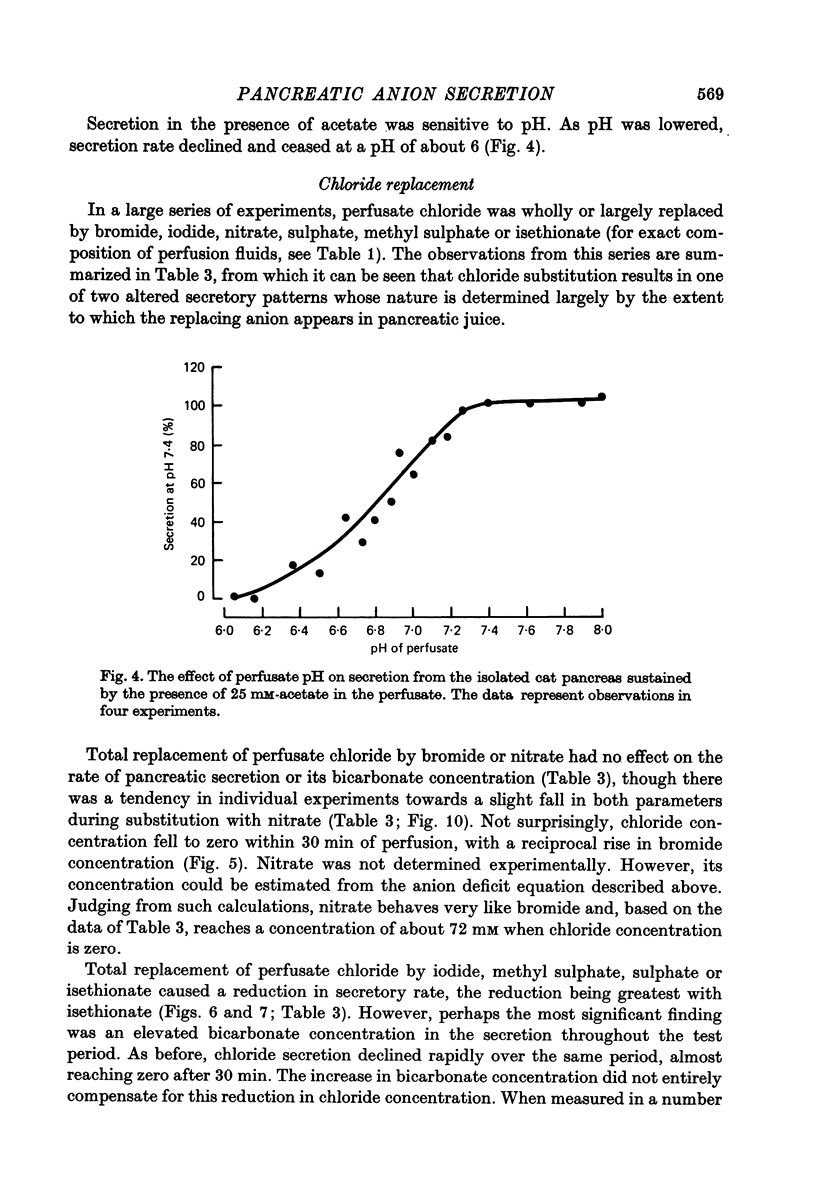
Abstract
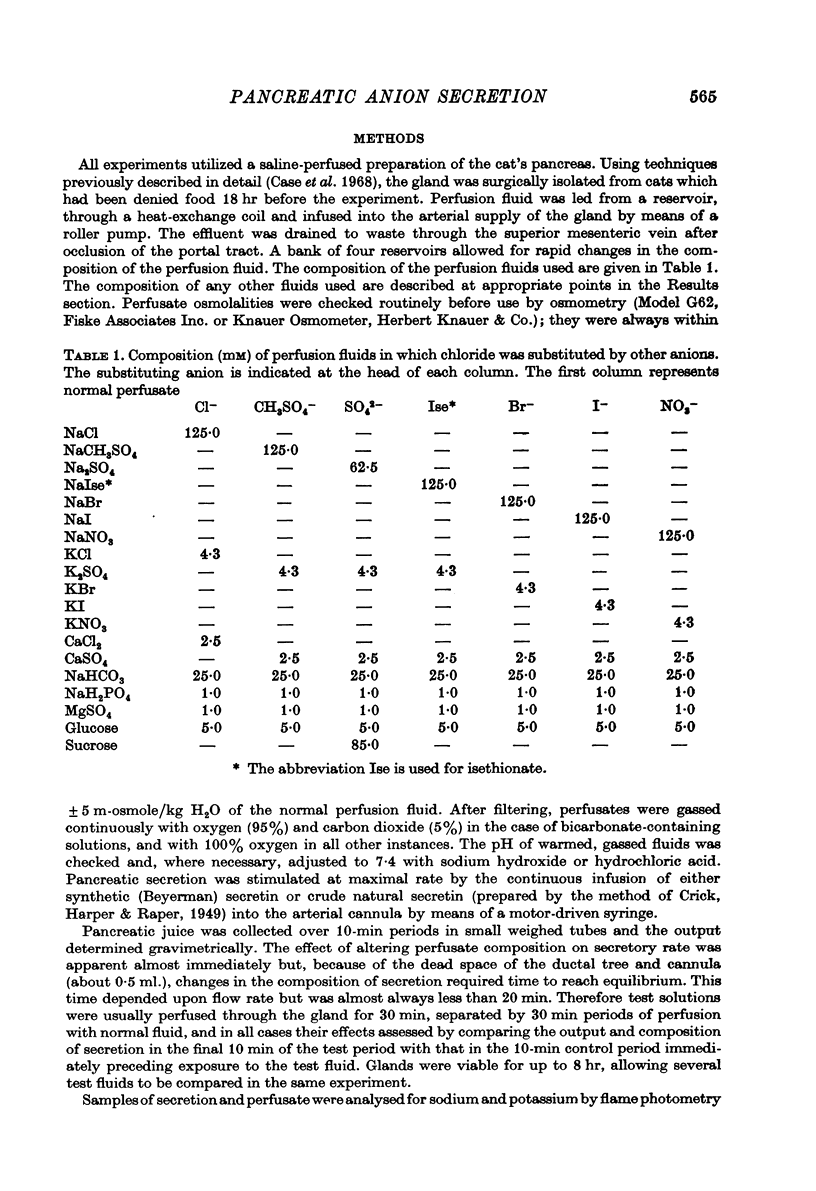
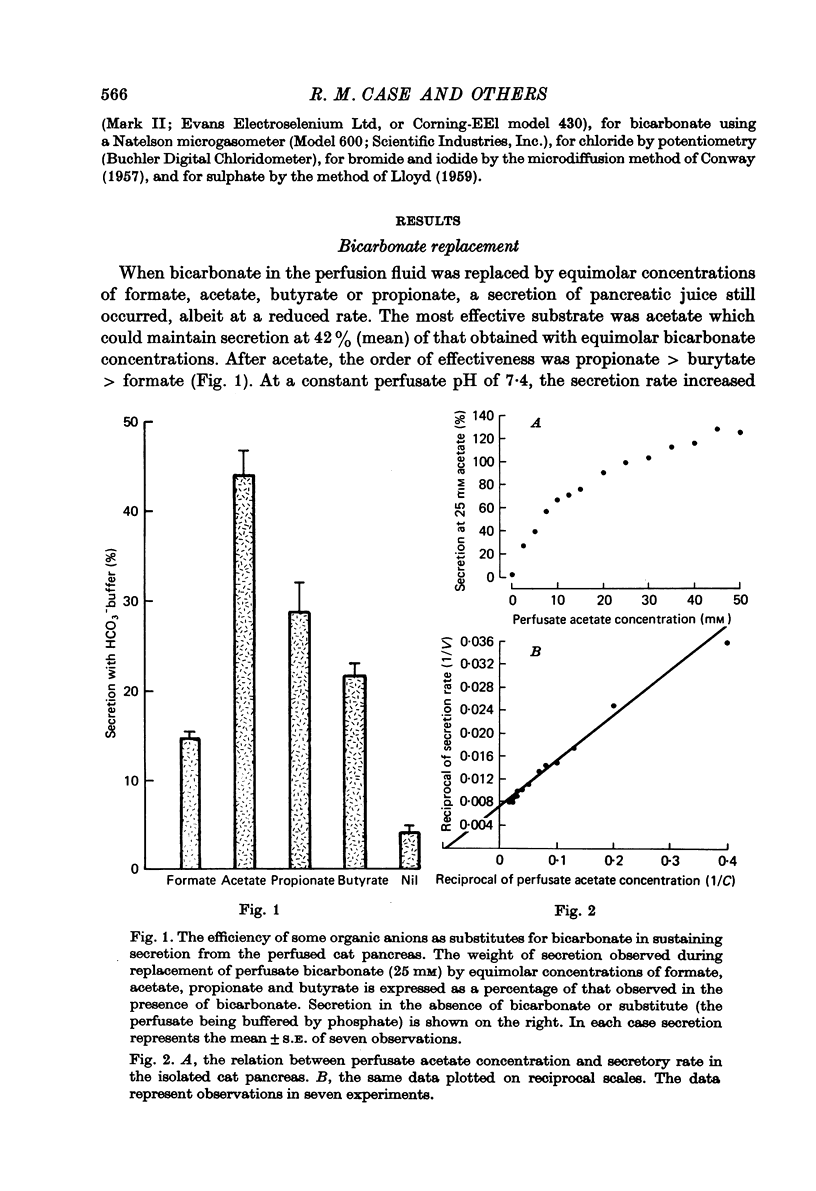
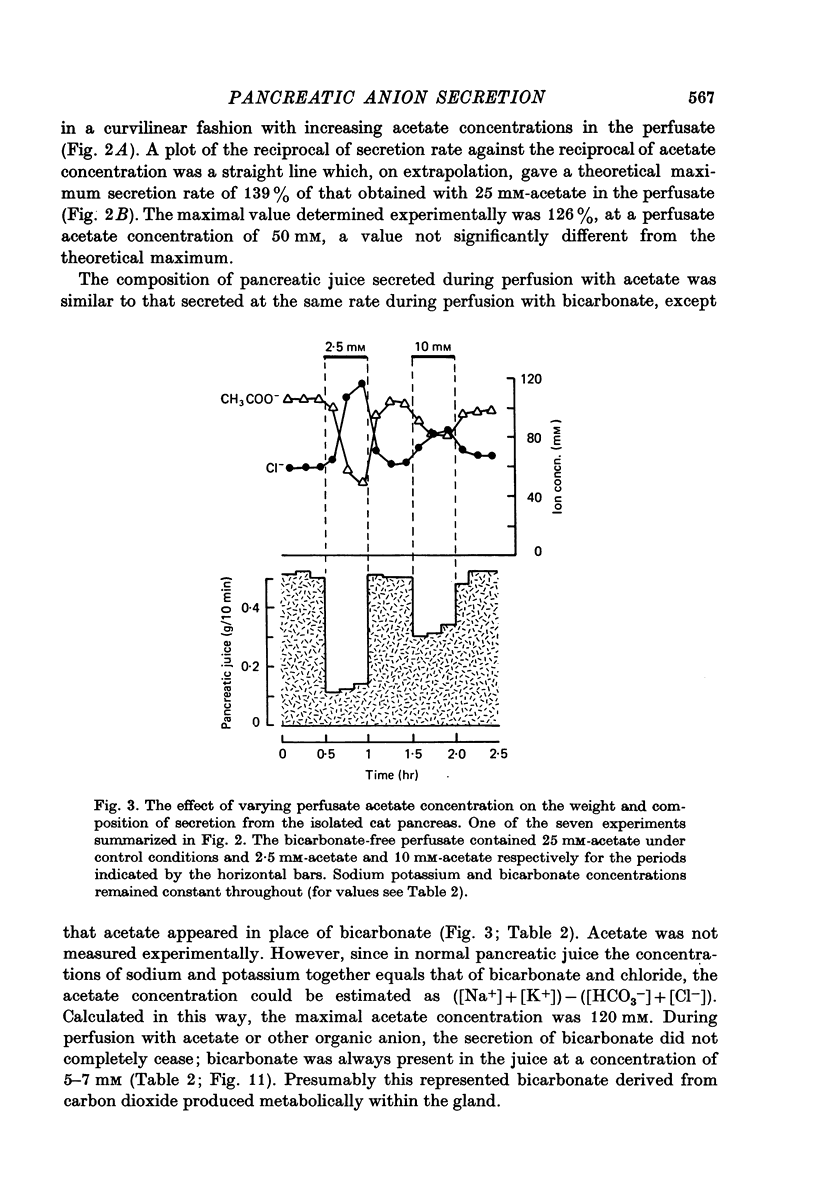
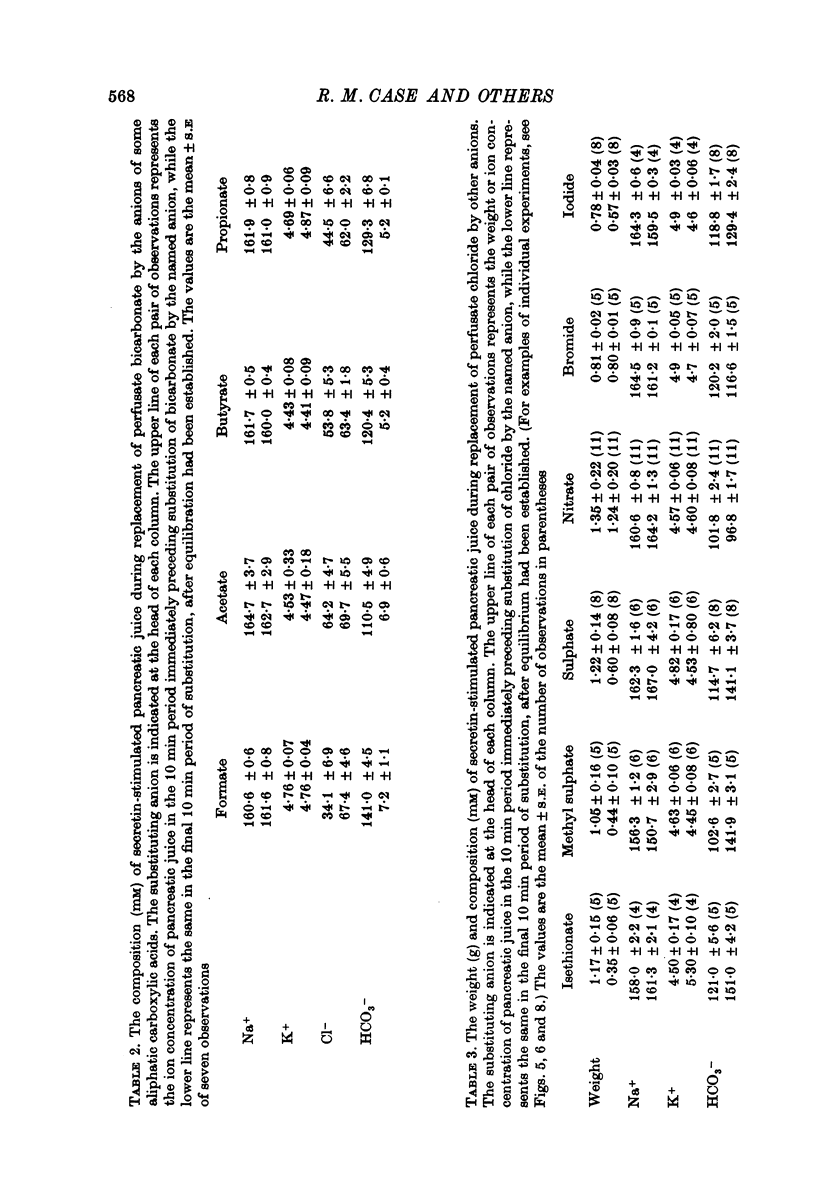
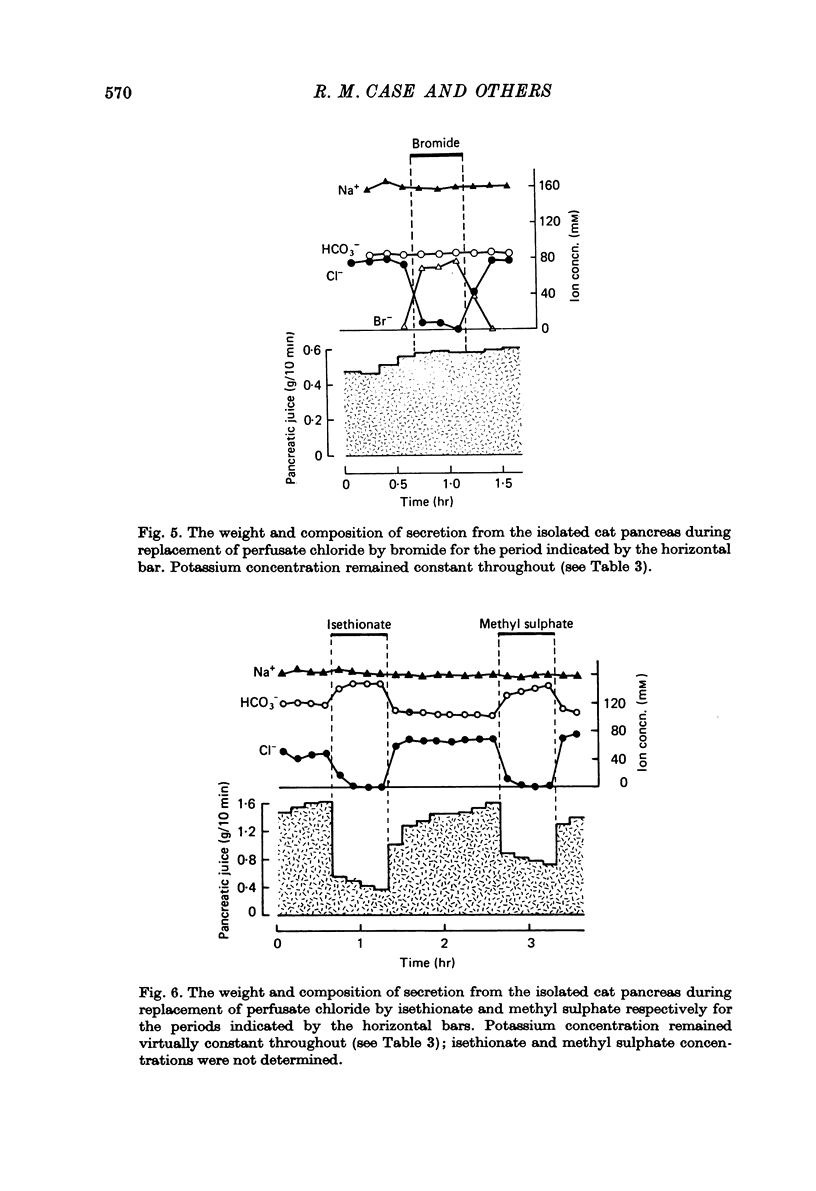
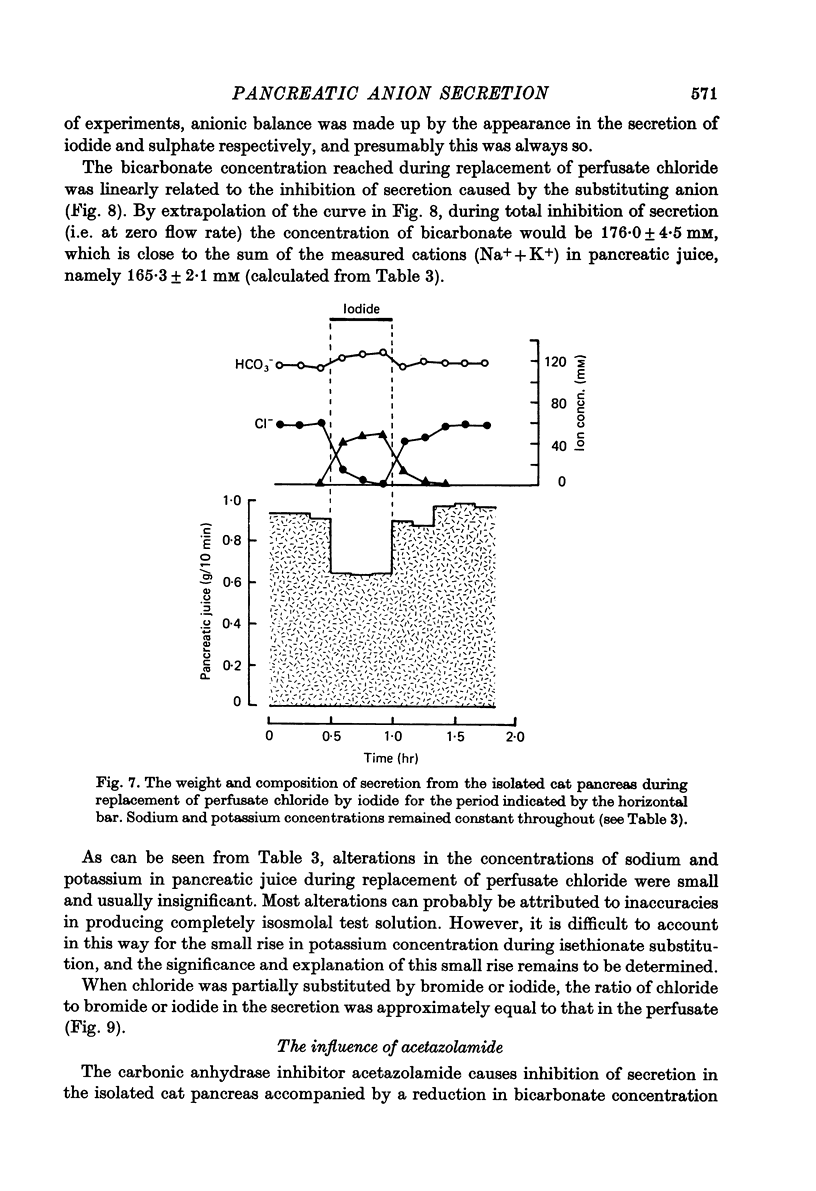
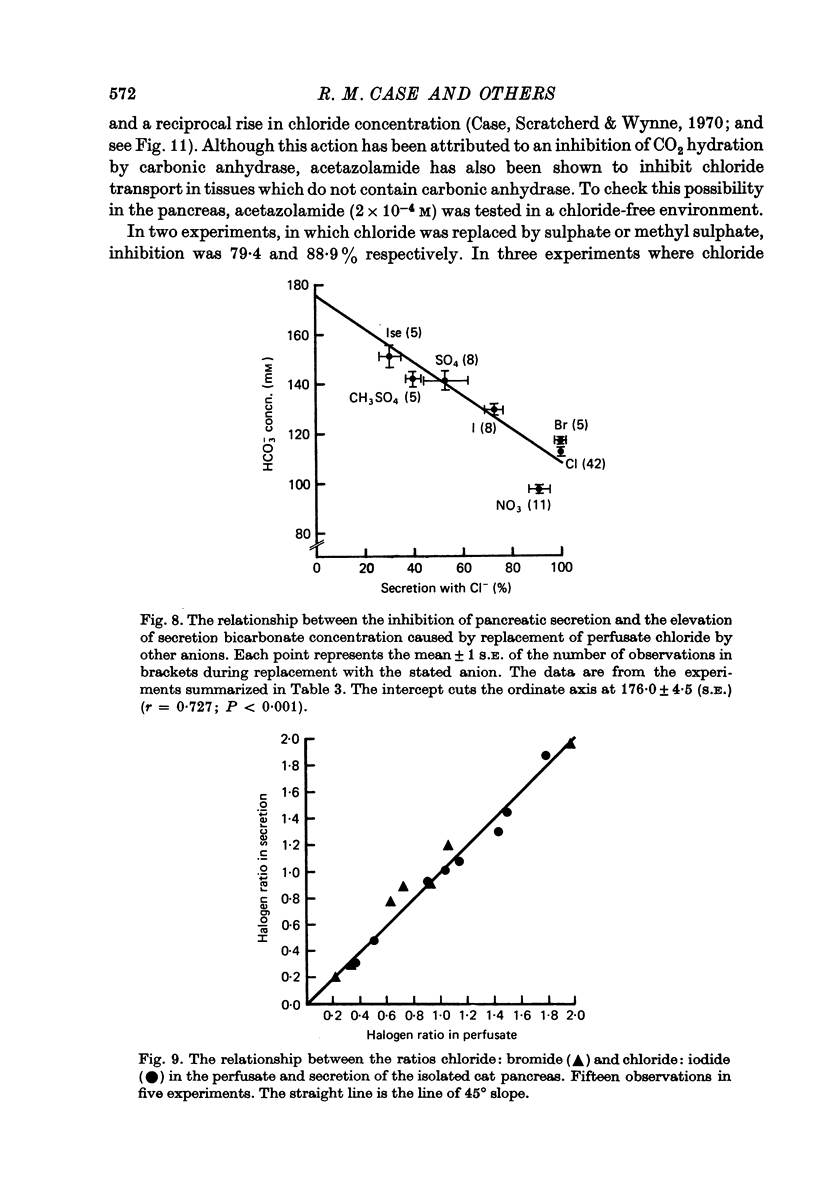
1. The effect of replacing extracellular bicarbonate and chloride by other anions on the volume and composition of secretin-stimulated pancreatic juice has been analysed in the isolated, perfused cat pancreas. 2. The anions of some aliphatic carboxylic acids were able partially to substitute for bicarbonate in sustaining pancreatic secretion. The order of effectiveness was: acetate greater than proprionate greater than butyrate greater than formate. 3. The rate of secretion in the presence of 25 mM-acetate was 42% of that achieved with 25 mM-bicarbonate. The concentration of acetate in the secretion varied with flow rate, reaching a maximum of 120 mM at high flow rates and declining at lower flow rates, with reciprocal changes in chloride concentration. Bicarbonate was always present in the secretion at a concentration of 5--7 mM. 4. Inorganic anions were able totally or partially to substitute for chloride in sustaining secretion. In relation to chloride, their degree of effectiveness was: chloride = bromide = or greater than nitrate greater than iodide greater than sulphate greater than methyl sulphate greater than isethionate. Those anions which had no effect on secretion rate (i.e. bromide and nitrate) also had no effect on the bicarbonate concentration of the secretion and themselves appeared in the secretion in place of chloride. Those anions which inhibited secretion increased the bicarbonate concentration in the secretion in proportion to the degree of inhibition they caused (i.e. the increase was greatest with isethionate). 5. When perfusate chloride was only partially replaced by bromide or iodide the ratios of chloride: bromide and chloride: iodide in the secretion were approximately equal to those in the perfusate. 6. The carbonic anhydrase inhibitor acetazolamide reduced secretory rate and bicarbonate concentration when added to normal perfusion fluid or chloride-substituted fluids, but had no effect following replacement of perfusate bicarbonate by acetate. 7. These observations illustrate that an extracellular source of permeant anions is required for optimal pancreatic bicarbonate secretion to occur. This may indicate the participation of an anion exchange carrier in the transport events responsible for this secretory process.
Full text
PDF

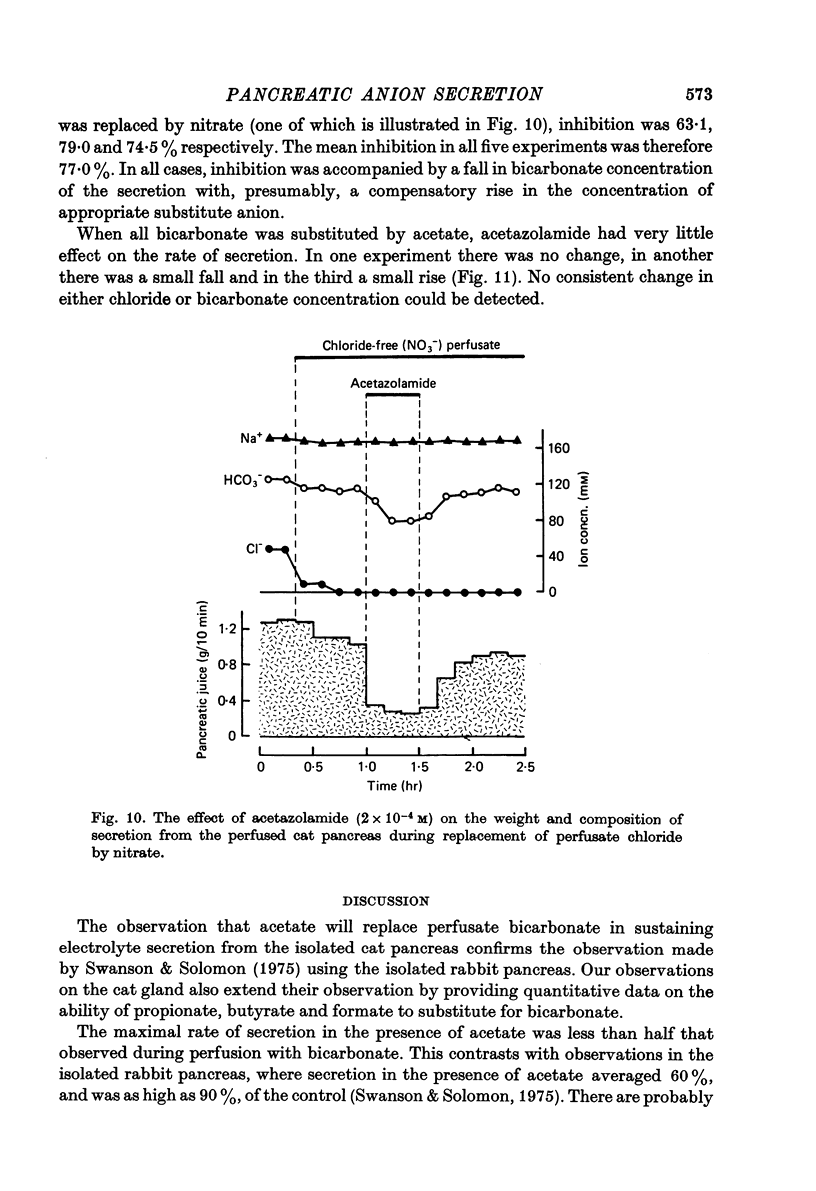
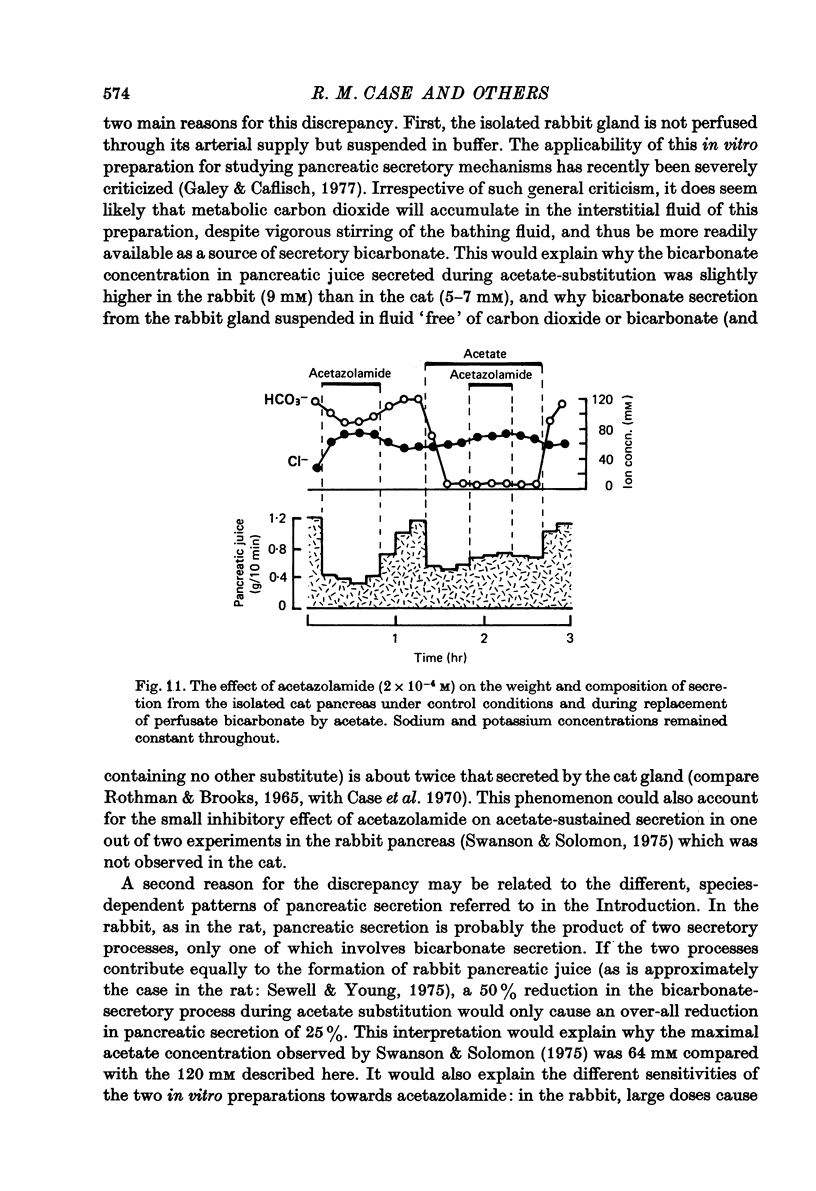


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRICK J., HARPER A. A., RAPER H. S. On the preparation of secretin and pancreozymin. J Physiol. 1949 Dec;110(3-4):367–376. doi: 10.1113/jphysiol.1949.sp004445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Harper A. A., Scratcherd T. The secretion of electrolytes and enzymes by the pancreas of the anaesthetized cat. J Physiol. 1969 Apr;201(2):335–348. doi: 10.1113/jphysiol.1969.sp008759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Harper A. A., Scratcherd T. Water and electrolyte secretion by the perfused pancreas of the cat. J Physiol. 1968 May;196(1):133–149. doi: 10.1113/jphysiol.1968.sp008499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Scratcherd T., Wynne R. D. The origin and secretion of pancreatic juice bicarbonate. J Physiol. 1970 Sep;210(1):1–15. doi: 10.1113/jphysiol.1970.sp009193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galey W. R., Caflisch C. R. The sources of sodium in rabbit pancreatic juice (39870). Proc Soc Exp Biol Med. 1977 Oct;156(1):35–39. doi: 10.3181/00379727-156-39870. [DOI] [PubMed] [Google Scholar]
- Kitahara S., Fox K. R., Hogben C. A. Depression of chloride transport by carbonic anhydrase inhibitors in the absence of carbonic anhydrase. Nature. 1967 May 20;214(5090):836–837. doi: 10.1038/214836a0. [DOI] [PubMed] [Google Scholar]
- LLOYD A. G. Studies on sulphatases. 24. The use of barium chloranilate in the determination of enzymically liberated sulphate. Biochem J. 1959 May;72(1):133–136. doi: 10.1042/bj0720133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren T. H., Rayburn C. S., Liddell N. E. Inhibition by anions of human red cell carbonic anhydrase B: physiological and biochemical implications. Science. 1976 Feb 6;191(4226):469–472. doi: 10.1126/science.813299. [DOI] [PubMed] [Google Scholar]
- Ridderstap A. S. The additive effect of acetazolamide and ouabain on pancreatic secretion in vitro. Pflugers Arch. 1969;311(3):199–204. doi: 10.1007/BF00590524. [DOI] [PubMed] [Google Scholar]
- Rothman S. S., Brooks F. P. Pancreatic secretion in vitro in "Cl-free," "Co-2-free," and low-Na+environment. Am J Physiol. 1965 Oct;209(4):790–796. doi: 10.1152/ajplegacy.1965.209.4.790. [DOI] [PubMed] [Google Scholar]
- Schulz I. Influence of bicarbonate-CO 2 - and glycodiazine buffer on the secretion of the isolated cat's pancreas. Pflugers Arch. 1971;329(4):283–306. doi: 10.1007/BF00588001. [DOI] [PubMed] [Google Scholar]
- Scratcherd T., Case R. M. The secretion of electrolytes by the pancreas. Am J Clin Nutr. 1973 Mar;26(3):326–339. doi: 10.1093/ajcn/26.3.326. [DOI] [PubMed] [Google Scholar]
- Sewell W. A., Young J. A. Secretion of electrolytes by the pancreas of the anaestetized rat. J Physiol. 1975 Nov;252(2):379–396. doi: 10.1113/jphysiol.1975.sp011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson C. H., Solomon A. K. Micropuncture analysis of the cellular mechanisms of electrolyte secretion by the in vitro rabbit pancreas. J Gen Physiol. 1975 Jan;65(1):22–45. doi: 10.1085/jgp.65.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Anion selectivity in biological systems. Physiol Rev. 1977 Jan;57(1):109–156. doi: 10.1152/physrev.1977.57.1.109. [DOI] [PubMed] [Google Scholar]


