Abstract
Staphylococcus aureus and Staphylococcus epidermidis often elaborate adherent biofilms, which contain the capsular polysaccharide-adhesin (PS/A) that mediates the initial cell adherence to biomaterials. Biofilm cells produce another antigen, termed polysaccharide intercellular adhesin (PIA), which is composed of a ∼28 kDa soluble linear β(1-6)-linked N-acetylglucosamine. We developed a new method to purify PS/A from S. aureus MN8m, a strain hyperproducing PS/A. Using multiple analytical techniques, we determined that the chemical structure of PS/A is also β(1-6)-N-acetylglucosamine (PNAG). We were unable to find N-succinylglucosamine residues in any of our preparations in contrast to previously reported findings (D. McKenney, K. Pouliot, Y. Wang, V. Murthy, M. Ulrich, G. Doring, J. C. Lee, D. A Goldmann, and G. B. Pier, Science 284:1523-1527, 1999). PNAG was produced with a wide range of molecular masses that could be divided into three major fractions with average molecular masses of 460 kDa (PNAG-I), 100 kDa (PNAG-II), and 21 kDa (PNAG-III). The purified antigens were not soluble at neutral pH unless first dissolved in 5 M HCl and then neutralized with 5 M NaOH. PNAG-I was very immunogenic in rabbits, but the responses of individual animals were variable. Immunization of mice with various doses (100, 50, or 10 μg) of PNAG-I, -II, and -III demonstrated that only PNAG-I was able to elicit an immunoglobulin G (IgG) immune response with the highest titers obtained with 100-μg dose. When we purified a small fraction of PNAG with a molecular mass of ∼780 kDa (PNAG-780) from PNAG-I, significantly higher IgG titers than those in mice immunized with the same doses of PNAG-I were obtained, suggesting the importance of the molecular mass of PNAG in the antibody response. These results further clarify the chemical structure of PS/A and help to differentiate it from PIA on the basis of immunogenicity, molecular size, and solubility.
Staphylococcus aureus is one of the most frequently isolated bacterial pathogens and infection is associated with high levels of morbidity and mortality. It is a common cause of both hospital and community-acquired infections and along with Staphylococcus epidermidis are the organisms most frequently isolated from medical implant-related infections (9, 19, 23). Both species colonize medical devices by forming adherent biofilms, which are believed to make the organisms more resistant to antibiotics and host defenses (24, 31). Numerous components of the extracellular biofilm layer have been identified, including the capsular polysaccharide-adhesin (PS/A) that mediates cell adhesion to biomaterials (20, 22, 27, 28, 30) and protects bacterial cells from opsonophagocytosis (13, 30). PS/A is a virulence factor for S. epidermidis infections in animal models of endocarditis (28). Initial chemical analysis of S. epidermidis PS/A revealed a composition of 54% hexoses, 20% aminosugars, and 10% uronic acids and as the specific sugars 22% galactose, 15% glucosamine, and 5% galactosamine (30). More recently, the composition of highly purified PS/A from S. epidermidis RP62A and M187 and S. aureus MN8m grown in a chemically defined medium (CDM) was reevaluated. These latter studies proposed that PS/A was a high-molecular-weight polymer of β(1-6)-linked glucosaminyl residues substituted on the amino group with succinate and acetate groups (20, 21). However, a complete chemical and structural analysis of PS/A has not yet been reported.
Another polysaccharide component of the S. epidermidis and S. aureus biofilm matrix is a structurally related homoglycan of ca. 28 kDa in size that has been described as a β(1-6)-linked N-acetylglucosamine polymer containing ca. 80% acetate substituents and small amounts of O-linked succinate and phosphate (16). This material is referred to as polysaccharide intercellular adhesin (PIA). PIA and PS/A are closely related chemically and immunologically and both are synthesized by the protein products of the icaADBC locus (8, 20). PIA had been proposed to be functionally distinct from PS/A, with PS/A mediating initial adherence to solid surfaces and PIA mediating accumulation of cells into biofilms (16, 17), although data evaluating PS/A's function indicate that it can mediate both processes (20). PIA also mediates hemagglutination of erythrocytes by S. epidermidis strains and has been found to be an important virulence factor in the pathogenesis of S. epidermidis biomaterial-associated infections (7, 18, 25, 26).
For this report we conducted a full chemical characterization of S. aureus PS/A and investigated some of its immunochemical properties. Using multiple analytical techniques we were able to demonstrate that the true chemical structure of PS/A is poly-β(1-6)-N-acetylglucosamine (PNAG) and not poly-β(1-6)-N-succinylglucosamine as previously reported (20). Our results found that PS/A and PIA are chemically related, but they can be differentiated based on several properties. These two polymers differ in terms of their molecular size, their biophysical properties, the degree of N acetylation, the degree of O succinylation, and immunogenicity. It also became clear that high-molecular-weight isoforms of PNAG are highly immunogenic when injected into mice and rabbits and that anti-PNAG antibodies can mediate the opsonophagocytic killing of various staphylococcal strains.
MATERIALS AND METHODS
Bacterial strains.
The S. aureus strain used in this study to purify antigen was MN8m, a constitutive, high-level producer of PS/A that has been described previously (21). S. aureus strains Reynolds, Newman, and 5827 were used in opsonophagocytic assays, as was S. epidermidis strain M187 (20). S. aureus strain MN8 with an interrupted ica locus was derived as described by Sarah Cramton and Fritz Goetz, Tubingen, Germany (4), and used to adsorb sera used in the phagocytosis assays.
Purification of PS/A.
PS/A was prepared from 16-liter cultures of a CDM based upon RPMI 1640 AUTO-MOD, a preparation of RPMI modified to allow sterilization by autoclaving (Sigma, St. Louis, Mo.). The CDM was supplemented with additional amino acids, vitamins, and nucleotides to adjust their concentration to those found in other CDM (11). The medium was also supplemented with sucrose and glucose to a final concentration of 1%.
Cultures were inoculated with a single colony of S. aureus MN8m from a tryptic soy agar plate grown at 37°C. Batch cultures were incubated at 37°C for 96 h, while being continuously stirred and flushed with oxygen at a rate of 2 liters/min. The pH was maintained at 7.0 throughout the growth period by the addition of 10 N NaOH via a pH titrator. Bacteria were sedimented at 9,000 × g for 30 min, and the supernatant was concentrated to an ∼500-ml via tangential-flow filtration (10,000-molecular-weight cutoff) and precipitated by the addition of 2 volumes of ethanol. After overnight dialysis against distilled water, the antigen was insoluble and was suspended in 50 ml of phosphate-buffered saline (PBS) to be digested with lysozyme (0.5 mg) and lysostaphin (0.5 mg) for 16 h at 37°C. Antigen suspensions were further treated with nucleases (0.5 mg) at 37°C for 16 h, followed by overnight incubation with proteinase K (5 mg) at 37°C. After dialysis and lyophilization, dried extracts were dissolved in 5 M HCl and the pH was adjusted to 2 with 5 N NaOH. Aliquots (20 ml) of this solution were size fractionated on a 5-by-88-cm column packed with Sephacryl S-300 (Pharmacia, Piscataway, N.J.) by using 0.1 N HCl-0.15 M NaCl buffer with the eluted polysaccharide identified by optical absorption at 206 nm. Fractions corresponding to the various peaks were separately pooled, dialyzed against water, and lyophilized.
Colorimetric assays.
Hexosamine was determined by the method described by Smith and Gilkerson (14) with N-acetylglucosamine as the standard. Hexose was quantified by the phenol-sulfuric acid method with glucose as the standard (5). Pentoses were quantified by the ferric-orcinol assay by using xylose as the standard (3). Ketoses were determined by the phenol-boric acid-sulfuric method with fructose as the standard (3). Phosphate content was determined by the method of Lowry (15) with NaH2PO4 as the standard. Proteins were estimated with the Bradford assay (2) with bovine serum albumin as the standard. The amount of free amino groups on the polysaccharide samples was determined by the trinitrobenzene sulfonic acid (TNBS) method with glucosamine as the standard (10).
GLC.
Succinate was quantified by gas-liquid chromatography (GLC) after the treatment of the antigens with 0.2 M NaOH at 65°C for 2 h. Hydrolyzed samples were acidified with concentrated sulfuric acid and derivatized overnight to methyl esters in boron-trifluoride methanol at 56°C. GLC was carried out on a Shimadzu 14A chromatograph (Shimadzu, Kyoto, Japan) by using a Supelcovax 10 column (0.55 mm by 30 m; Supelco, Bellefonte, Pa.). The temperature of injection was 225°C, and samples were run isothermically at 155°C by using N2 as carrier gas (30 ml/min).
1H-NMR spectroscopy.
1H-nuclear magnetic resonance (NMR) spectra were recorded on a Varian VXR500 spectrophotometer in D2O at 25°C. A total of 4 mg of S. aureus polysaccharide samples/ml were dissolved in 5 M DCl and neutralized with an equal volume of 5 M NaOD. The resulting polysaccharide solutions were exchanged into D2O by five cycles of concentration and dilution by using a Centriprep-10 cartridge (Amicon, Beverly, Mass.). The chemical shifts were given on the δ scale relative to 3-(trimethylsilyl)-propionic-2,2,3,3-d4. In the case of maltose and cellobiose NMR spectra were taken after samples were dissolved in D2O. The measurement conditions were as follows: a spectral window of 8,000.0 Hz, a pulse angle of 48.6°, an acquisition time of 1.89 s, and 32 scans with a delay of 1 s between scans.
High-performance-anion-exchange chromatography (HPAEC).
Fifty micrograms of each polysaccharide were dissolved in 2 ml of 3 M trifluoroacetic acid (TFA) and hydrolyzed at 100°C for 18 h. Hydrolyzed samples were dried under nitrogen at 50°C, resuspended in 10 ml of filtered deionized water and dried and resuspended for two more cycles to remove volatile TFA. Dried samples were suspended in 1 ml of deionized water and injected into a Dionex HPLC system (Dionex, Sunnyvale, Calif.). Samples were chromatographed on a CarboPac PA1 column (4 by 250 mm; Dionex) equilibrated with 20 mM NaOH at a flow rate of 1 ml/min. Monosaccharides were detected with a Pulsed Amperometric Detector with a gold electrode and applied potential of 0.05 V. After the elution of monosaccharide peaks, the electrode was regenerated with 9 ml of 200 mM NaOH and reequilibrated in 20 mM NaOH for each sample run. A group B Streptococcus capsular polysaccharide with a known structure containing N-acetylglucosamine, galactosamine, and glucose was prepared in the same manner to identify peaks corresponding to these monsaccharides.
Determination of PNAG molecular mass.
The molecular mass of PNAG-I, -II, and -III was determined with a Superose 6HR 10/30 column (Pharmacia, Piscataway, N.J) equilibrated with of a 0.2 M glycine HCl-0.15 M NaCl (pH 4) buffer. Samples were loaded and eluted at flow rate of 0.5 ml/min, and polysaccharides were detected by the hexosamine assay of Smith and Gilkerson (14). Using dextran standards of various molecular mass a linear relationship was obtained between the logarithm of the molecular mass and elution volume of the standards.
ELISA.
Immunolon 1 plates (Dynatech Laboratories, Chantilly, Va.) were coated at 37°C for 2 h with PNAG (4 μg/well) suspended in 0.04 M phosphate buffer (pH 7.4). The plates were rinsed three times with PBS, blocked with 5% skim milk for 1 h at 37°C, and rinsed again three times with PBS. Antibody samples were then diluted either twofold (for mouse antisera) or fourfold (for rabbit antisera) in 5% skim milk with 0.05% Tween 20, incubated for 1 h at 37°C, washed again, and incubated with appropriate alkaline phosphatase-conjugated secondary antibody (Sigma) for 1 h at 37°C. After washes, plates were incubated with p-nitrophenylphosphate substrate, and the optical density at 405 nm (OD405) was determined by an enzyme-linked immunosorbent assay (ELISA) plate reader (BioTek Instruments, Winoski, Ill.) after a 1-h incubation. Titers were calculated by plotting the data as the serum dilution versus the OD405 value and then identifying the values that lay on the linear portion of this curve. Background readings were automatically subtracted. The linear values were used to generate a formula for the line by using regression analysis, and the formula solved for the intersection of the plotted line with zero on the y axis. Thus, the titer was the serum dilution giving a final OD405 value of 0 under the conditions of the assay (i.e., endpoint titer).
Antiserum.
Antibodies to purified PNAG-I were raised in New Zealand White rabbits by subcutaneous immunization with two 100-μg doses of polysaccharide emulsified for the first dose in complete Freund adjuvant and for the second dose in incomplete Freund adjuvant, followed 1 week later by three intravenous injections of antigen in saline spaced 3 days apart. Rabbits were bled every 2 weeks and sera tested by ELISA.
Immunogenicity of PNAG fractions in mice.
Groups of five mice (Swiss-Webster; female, 5 to 7 weeks of age) were immunized intraperitoneally, 1 week apart, with 100, 50, or 10 μg of PNAG-I, -II, or -III suspended in saline and bled 1, 2, and 5 weeks after the third immunization. For experiments with PNAG-780 kDa immunization doses were 10, 1, or 0.1 μg spaced 1 week apart, and the mice were bled 1, 3, and 5 weeks after the last immunization.
Opsonophagocytic assays.
Polymorphonuclear neutrophils were prepared from fresh human blood collected from healthy adult volunteers. A total of 25 ml was mixed with an equal volume of dextran-heparin-sulfate buffer (20 g of Dextran 500/liter, 65.6 g of heparin sulfate/liter, 9 g of sodium chloride/liter) and incubated at 37°C for 1 h. The upper layer containing the leukocytes was collected, and hypotonic lysis of the remaining erythrocytes was accomplished by resuspension in 1% NH4Cl. Subsequent wash steps were performed with RPMI with 15% fetal bovine serum (HyClone). After trypan blue staining, the viable polymorphonuclear neutrophil count was adjusted to 4 × 106 neutrophils per ml. The complement source (baby rabbit complement; Accurate Chemical and Scientific, Westbury, N.Y.) was adsorbed with S. aureus MN8m to remove antibodies that could react with the target strain. An isogenic mutant of S. aureus MN8m lacking the intact ica locus (20) was used to adsorb pre- and postimmune sera to provide specificity of the reaction for the PNAG antigen. After overnight growth in tryptic soy broth, bacterial cells were centrifuged, the pellet resuspended in 1 ml of a 1:15 dilution of complement or a 1:10 dilution of test serum and incubated at 4°C for 1 h for adsorption. All suspensions were again centrifuged and filter sterilized prior to use, and the sera further diluted for evaluation in the opsonophagocytic assay.
The opsonophagocytic assay was performed with 100 μl of leukocytes, 100 μl of bacteria (adjusted to 2 × 107/ml RPMI with 15% fetal calf serum spectrophotometrically and confirmed by viable counts), 100 μl of the test serum dilution and 100 μl of the complement source. The reaction mixture was incubated on a rotor rack at 37°C for 90 min; samples were taken at time zero and after 90 min. Each tube was sonicated for 5 s at 4 W (Sonic Dismembrator) and then diluted in tryptic soy broth containing 0.5% Tween and plated onto tryptic soy agar plates. Tubes lacking any serum and tubes with normal rabbit serum were used as controls. The percentage of killing was calculated by first dividing the mean CFU determined in the tubes with bacteria, complement, leukocytes, and sera with the mean CFU determined in tubes with bacteria, complement, and a 1:10 dilution of immune serum but lacking leukocytes. This ratio was multiplied by 100, and the quotients were subtracted from 100 to calculate the percentage of bacteria killed. The assay was repeated two to three times and mean and standard error of the mean (SEM) values for the percentage killing at each dilution determined.
RESULTS
Purification and chemical characterization of PS/A.
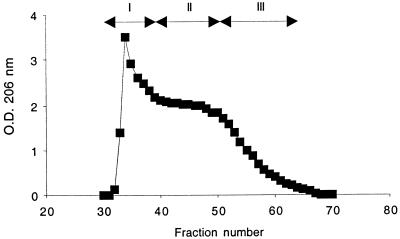
In this study we purified large quantities of PS/A from S. aureus MN8m, a strain that overproduces this polysaccharide (21). PS/A was extracted from supernatant cultures rather than from bacterial cells as previously described (20, 21), digested with enzymes, and further purified by gel filtration on Sephacryl S-300. PS/A eluted beginning at the void volume, followed by a long, slowly declining profile, eventually descending to baseline readings (Fig. 1). Fractions were pooled (Fig. 1) and designated PNAG-I, PNAG-II, and PNAG-III. When the colorimetric assay of Smith and Gilkerson was used to analyze these three major fractions, we found these samples to be very rich in hexosamine (82 to 99%) (Table 1). Further chemical analysis showed that the levels of both phosphate and ketose were low in all three PNAG samples, whereas hexoses, pentoses, uronic acids, and the protein concentration were below the level of detection (Table 1). The absence of hexoses, as well as the low levels of phosphate (<0.2%), found in all three polysaccharide fractions argues against any significant contamination of these antigen preparations with teichoic acids.
FIG. 1.
Fractionation of S. aureus MN8m crude extract by size exclusion chromatography. Column chromatography was performed by using Sephacryl S-300 as described in Materials and Methods. The material of fractions 32 to 39, 40 to 50, and 51 to 65 were pooled and are referred to as PNAG-I, PNAG-II, and PNAG-III, respectively.
TABLE 1.
Chemical and physical characterization of PNAG peaks I, II, and III purified from S. aureus MN8m
| Component or parameter | Fraction |
||
|---|---|---|---|
| PNAG-I | PNAG-II | PNAG-III | |
| Mean % content ± SEMa | |||
| Hexosamine | 82 ± 0.21 | 99 ± 0.28 | 90 ± 0.33 |
| Hexose | LLDb | LLD | LLD |
| Pentose | LLD | LLD | LLD |
| Ketose | 0.44 ± 0.0074 | 0.47 ± 0.080 | 0.39 ± 0.017 |
| Uronic acids | LLD | LLD | LLD |
| Protein | LLD | LLD | LLD |
| Phosphorus | 0.19 ± 0.020 | 0.027 ± 0.0040 | 0.07 ± 0.0053 |
| O-succinate | 2.41 ± 0 | 2.85 ± 0.22 | 2.78 ± 0.035 |
| Mean no. of free amino groups ± SEMc | 7.9 ± 0.11 | 2.8 ± 0.31 | 4.6 ± 0.29 |
| Degree of N acetylationd | 95 | 100 | 100 |
| Mean molecular mass (kDa) | 460 | 100 | 21 |
Percentage of component in 100 μg of sample. This value represents the mean of at least three measurements shown, along with the standard deviation.
LLD, less than the Lower Limit of Detection in the assay.
Number of free amino groups per 100 molecules of glucosamine.
Number of N-acetyl residues per 100 molecules of glucosamine. These values were estimated by 1H-NMR spectroscopy.
A minor component of PIA, termed polysaccharide-II, contains O-linked succinate residues. Succinate was reported to be present at a molar ratio of 0.06 to total glucosamine (16). We determined the amount of O-succinate residues in the three PNAG samples by GLC after samples were hydrolyzed for 2 h under mild alkaline conditions. Succinate was detected in all three samples at concentrations that ranged between 2.41 and 2.85% (Table 1). Thus, the levels of O-linked succinate in PNAG were higher than those reported for the polysaccharide-II of PIA.
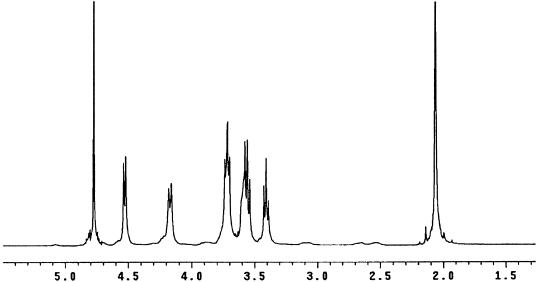
In the 1H-NMR spectrum of PNAG-I in deuterated water (D2O) (Fig. 2), the resonance at ca. 2.062 ppm can be easily assigned to the N-acetyl protons with no other acetyl protons such as O-acetyl protons detected. We estimated the degree of N acetylation from the ratio of the integral intensity of the N-acetyl groups to the sum of integral intensities of the H-1 (δ 4.5 ppm), H-2, H-3, H-4, H-5, H-6a (δ = 3.3 to 4 ppm) and H-6b (δ = 4.17 ppm) groups. The results from these calculations showed 95% of the glucosamine residues in PNAG-I were substituted with acetate residues. NMR spectra of PNAG-II and PNAG-III were almost identical to that of PNAG-I (data not shown), and the degree of N acetylation was 100% in both of the smaller polysaccharides. The degree of N acetylation that was estimated from the number of free amino groups identified in the 1H-NMR spectrum confirmed the high level of substitution with acetate. Further analysis of the level of the free amino groups in all three of the PS/A samples with the TNBS reagent revealed the presence of 2.8 to 7.9% free amino groups in the different antigen preparations (Table 1). Overall, the degree of N acetylation obtained by the TNBS assay correlated well with those calculated by 1H-NMR spectroscopy.
FIG. 2.
1H-NMR spectrum (500 MHz) of PNAG-I in D2O.
1H-NMR analysis of PNAG did not reveal the presence of N-linked succinate signals as previously described (20). We searched for succinate by 1H-NMR spectroscopy after PNAG was purified from S. aureus and S. epidermidis cultures grown under various growth conditions (CDM-rich media; 24 to 96 h of incubation, pH 4.5 to 7) and multiple purification methods. Our 1H-NMR results did not reveal N-succinate under any of the growth conditions or purification methods used and demonstrated that those signals previously identified as N-succinate could be attributed to an artifact caused by the use of strong acids to hydrolyze (4 M TFA, 2 h for 95°C) and solubilize PNAG prior to analysis by 1H-NMR (20).
The 1H-NMR spectra of PNAG-I, PNAG-II, and PNAG-III were used to identify the anomeric configuration of the glycosidic linkage of the polysaccharides. For such measurements two reference disaccharides—maltose [glucose-α(1-4)-glucose] and cellobiose [glucose-β(1-4)-glucose]—were used for comparison. The typical anomeric proton multiplicity, chemical shift, and coupling constants of α and β sugars are shown in Table 2. The H-1 of maltose and cellobiose involved in the glycosidic linkage had chemical shifts and coupling constants as expected for an α and β configuration, respectively (Table 2). 1H-NMR analysis of PNAG-I, PNAG-II, and PNAG-III under the same conditions used for these disaccharides showed that both the chemical shift and the coupling constant values were consistent with a β linkage between the glucosamine residues (Table 2).
TABLE 2.
Proton chemical shifts and coupling patterns for α and β sugars, standards, and PS/A samples
| Compound | Chemical shift (δ ppm) | Multiplicitya | Coupling constant J (Hz) |
|---|---|---|---|
| α-Sugars | 5.5-5.0 | d | 3.5 |
| β-Sugars | 4.4-5.0 | d | 8.0 |
| Maltose | 5.4 | d | 3.6 |
| Cellobiose | 4.5 | d | 7.6 |
| PS/A-I | 4.6 | d | 7.6 |
| PS/A-II | 4.6 | d | 7.4 |
| PS/A-III | 4.6 | d | 7.6 |
d, doublet.
The monosaccharide composition of PNAG-I, PNAG-II, and PNAG-III was determined by HPAEC analysis after samples were hydrolyzed in 3 M TFA for 18 h at 100°C. HPAEC data revealed glucosamine as the single sugar component in all three S. aureus PS/A fractions (data not shown). These results are consistent with our extensive 1H-NMR data from native and acid-hydrolyzed PNAG antigens and are in agreement with those reported by McKenney et al. (20) using S. epidermidis, as well as recombinant S. carnosus (pCN27) that contained a cloned icaADBC locus that expresses what was referred to as the PS/A antigen.
In any glucosamine polymer, periodate sensitivity would only be present if there was a 1-6 linkage between the monosaccharides. All other potential linkages would be periodate resistant, since no vicinal carbons containing free hydroxyl groups would be present. Periodate oxidation (0.2 M for 14 h at room temperature) of all three polysaccharide fractions led to an almost complete loss of their serological reactivity, indicating a 1-6 linkage between glucosamine residues.
Earlier studies (30) on the previously described PS/A antigen purified from either S. epidermidis RP62A, S. epidermidis M187 or S. carnosus (pCN27) have only given a partial characterization of its molecular mass. These reports indicated that a major fraction of this PS/A eluted in the void-volume fractions of a Sepharose 4B column and had a molecular mass higher than 100 kDa. We used a more accurate method to determine the molecular mass of S. aureus PNAG antigens by gel filtration on a Superose 6HR column calibrated with a series of molecular weight standards. The results showed average molecular masses of 460 kDa for PNAG-I, 100 kDa for PNAG-II, and 21 kDa for PNAG-III.
All three PNAG antigens are insoluble in water, organic solvents (such as acetone, pyridine, chloroform, or dimethyl sulfoxide), and commonly used buffers regardless of their pH. PNAG-II and PNAG-III became completely soluble in 5 M HCl or 5 M NaOH, whereas PNAG-I forms homogenous suspensions in these solvents. After neutralization, the antigens remained either soluble or as a homogenous suspension, but if they were again dialyzed they became insoluble and could only be resolubilized by initial dissolution in strong acid or base. Altogether, these results demonstrate that the chemical form of the previously characterized S. aureus and S. epidermidis PS/A antigens is PNAG and that the molecular weight appeared to be the major chemical difference among the three samples.
Immunological properties of PNAG.
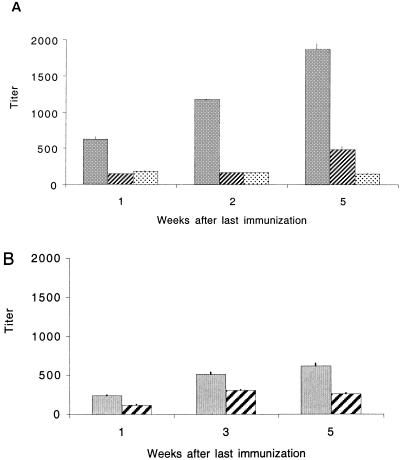
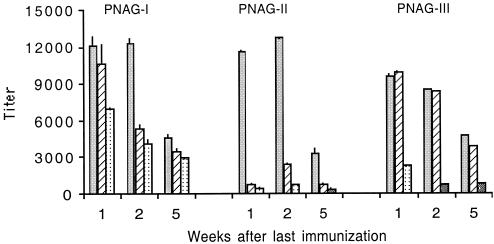
In four polyclonal immune rabbit antisera to PNAG-I titers by ELISA of ca. 20,000, 83,000, 231,000, and 1,100,000 were determined. These results show that PNAG-I was highly immunogenic, although individual rabbit responses were variable. Mean IgG titers of mice immunized with 100 μg of PNAG-I increased steadily after immunization (Fig. 3A), whereas PNAG-II and III failed to elicit a detectable IgG response at any dose (data not shown). All preimmune titers were <25. The fact that the highest-molecular-weight antigen, PNAG-I, was the only one capable of eliciting an IgG response in mice prompted us to investigate the role of molecular size in the immune response to this antigen. Therefore, we further purified PNAG-I on Sephacryl S-300 to obtain a small fraction of PNAG with an average molecular mass of 780 kDa (PNAG-780), as determined by Superose 6HR gel filtration (data not shown). Mice were immunized with 0.1, 1.0, or 10 μg of PNAG-780 and sera collected 1, 3, and 5 weeks after the last immunization. Figure 3B shows that mice immunized with 10 μg of PNAG-780 had a higher antibody titer (titers of 240 to 620) than mice immunized with the same dose of PNAG-I-460 (titers of 150 to 180). The mean titers at 1 week for 10 μg doses of PNAG-I-460 and PNAG-780 were 187 ± 15 and 237 ± 3, respectively (P < 0.001, two-sample t test), and at 5 weeks they were 151 ± 8 and 587 ± 27, respectively (P < 0.001, two-sample t test). These results suggest that size per se might play an important role in PNAG immunogenicity. We also tested by ELISA sera from individual mice in the groups immunized with either 100 μg of PNAG-I or 10 or 1 μg of PNAG-780 to assess variability in the individual animal's responses. Sera from individual animals immunized with 100 μg of PNAG-I (diluted 1:50) and 10 and 1 μg of PNAG-780 (diluted 1:100) gave OD450 values that ranged between 0 and 2.5, indicating high variability among individual mice in their response to PNAG. We deliberately used outbred Swiss-Webster mice to determine the range of titer to expect in an outbred population. All three PNAG antigens elicited high IgM titers in a dose-dependent fashion that in general declined during the weeks postimmunization (Fig. 4).
FIG. 3.
(A) Mean IgG titers of five mice immunized IP with 100 μg (▪), 50 μg (▨), and 10 μg (░⃞) of PNAG-I. (B) Mean IgG titers of five mice immunized with 10 μg (▪) or 1 μg (▨) of PNAG-780. The bars represent means, and error bars indicate the standard deviation. All preimmune titers were <25.
FIG. 4.
Mean IgM titers of five mice immunized with 100 μg (▪), 50 μg (▨), and 10 μg (░⃞) of PNAG-I, PNAG-II, and PNAG-III. Bars represent the means, and the error bars indicate the standard deviation.
Opsonophagocytosis of staphylococcal strains by serum raised to PNAG.
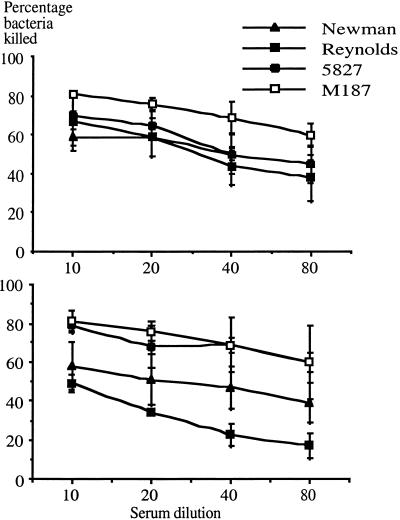
We investigated the ability of antibodies raised to PNAG in two rabbits to mediate killing by human polymorphonuclear leukocytes in the opsonophagocytic assay using four different clinical staphylococcal strains. For these experiments we used one strain of S. epidermidis (M187) that constitutively expresses PNAG and three S. aureus strains (Newman, Reynolds, and 5827) that produce variable levels of this surface polysaccharide in vitro. These four clinical isolates all produce considerably lower levels of PNAG than the constituve, high-level producer of PS/A, S. aureus MN8m. Incubation of bacteria with human peripheral blood leukocytes, a complement source and PNAG-immune rabbit sera at a dilution of 1:10, resulted in ca. 50 to 75% killing of the bacteria (Fig. 5). Progressive dilution of the sera resulted in concomitant reductions in the number of bacteria killed in the assay (18 to 45% killing at1:80 dilution). Preimmune sera did not yield any opsonic killing >15% at a dilution of 1:10. These results show that phagocytosis and bacterial killing of various staphylococcal strains can be mediated by a population of antibodies to PNAG contained in immune sera.
FIG. 5.
Opsonophagocytosis of S. epidermidis strain indicated in the legend by immune serum from rabbit 1 (upper graph) or rabbit 2 (lower graph). Each point represents the mean of two to three assays, and the error bars indicate the SEM. Killing by preimmune sera at a dilution of 1:10 was always <15%.
DISCUSSION
The availability of S. aureus strain MN8m, a high-level producer of staphylococcal PNAG, was critical to purification of the previously described staphylococcal PS/A and to achieve high yields of this surface polysaccharide. Strain MN8m is a derivative of strain MN8, a prototypical producer of toxic shock syndrome toxin 1 (1). Strain MN8m was isolated from a fermenter culture of strain MN8 and, upon subculture on solid medium, had a highly mucoid colony appearance. Overproduction of cell-free PNAG by this strain permitted use of culture supernates to purify the antigen. PNAG from strain MN8m, like previously described preparations designated PS/A (20), was insoluble at neutral pH after precipitation from the culture supernatant with ethanol and remained insoluble until dissolved in 5 M HCl or 5 M NaOH. The typical yields obtained obtained by earlier methods (0.5 to 2 mg/liter of culture) (20) were substantially increased (up to 75 mg/liter of culture) by using S. aureus strain MN8m and the purification protocol described above. Finally, the analysis of PNAG recovered from the overproducing strain indicated that it is chemically and structurally identical to similar preparations obtained from wild-type strains, indicating there has been no alteration to the molecule as a result of the high level of synthesis.
In protocols previously published for purifying PS/A it was found necessary to treat crude extracts with 24% (vol/vol) hydrofluoric acid (HF) at 4°C for 48 h (20) to remove charged contaminants such as teichoic acids. When we used HF to remove contaminants from the PNAG antigens isolated from strain MN8m, there was some degradation of the overall molecular size of the antigen (initially observed by C. Abeygunawardana, J. Joyce, and G. Mark III), although the degree of acetylation was maintained at ca. 95% as determined by NMR spectroscopy. We found, however, that if PNAG-containing extracts were first suspended in 5 M HCl and then the pH brought up to 2 for chromatography in a 0.1 N HCl and 0.15 M NaCl buffer on a Sephacryl S-300 gel, we were able to obtain a significant yield of polymers of PNAG with a mean molecular mass of 460 kDa. This material also had minimal contamination with phosphate (<0.2%), which is indicative of teichoic acid contamination. The dissolution of the PNAG in 5 M HCl prior to size chromatography and use of acidic chromatography buffers appeared to help in removing phosphate-containing contaminants that eluted long after all of the PNAG polymers.
Using various colorimetric assays we were able to determine that all PNAG antigens were mainly composed of hexosamine and contained minimal or negligible amounts of other potential contaminants such as protein, hexoses, pentoses, ketoses, or uronic acids. These results were confirmed by HPAEC, which revealed glucosamine as the only sugar component in all three PNAG fractions, and were in agreement with our NMR data from native and acid-hydrolyzed antigens, as well as the data derived by others (J. Joyce, unpublished data). Although the hexosamine content of all three PNAG fractions was high, PNAG-I had a slightly lower amount of glucosamine (ca. 82% of total sample). In order to quantify the hexosamine concentration of PNAG with the Smith and Gilkerson assay, antigens were first dissolved in 5 M HCl, diluted to 0.5 M HCl, and hydrolyzed under this mild acidic conditions at 100°C for 2 h. While PNAG-II and PNAG-III remained completely soluble in 0.5 M HCl, PNAG-I was only partially soluble in this solvent, which might explain the incomplete reactivity of this antigen in the assay. A similar observation was made by McKenney et al. (20) when they used this colorimetic assay to analyze high-molecular-mass PS/A extracted from S. epidermidis and S. carnosus (pCN27). Although they reported a much poorer reactivity of PS/A in the Smith and Gilkerson assay (<1% aminosugars) than the PNAG antigens, they found it necessary to hydrolyze PS/A under much stronger acidic conditions in order to solubilize this antigen and to demonstrate by GLC-MS and NMR that glucosamine was its main component.
Further structural analysis demonstrated that the three fractions of PNAG polymers were all chemically composed N-acetyl-β(1-6)-glucosamine, which confirmed an initial finding by Abeygunawardana et al. (unpublished data) about the composition of this polymer. The degree of acetylation of the PNAG antigens calculated both by NMR spectroscopy and the TNBS assay were consistently very high (92 to 97%), but no N-linked succinate was detected in any of the preparations. This study therefore corrects previous reports (20, 21) which proposed that succinate was substituting between 65 to 100% of the amino groups of the previously characterized PS/A. Peaks corresponding to what appeared to be succinate were clearly present in the NMR spectra of the previously described PS/A antigens (21), but their presence was due to generation of breakdown products of glucosamine due to the use of strong hydrolysis conditions that were needed to solubilize the high-molecular-weight polysaccharide for NMR analysis. Details of the basis for this misidentification have been submitted for publication.
PNAG and the analogous antigen, PIA, share a common β(1-6)-polyglucosamine backbone but differ, among other things, in their molecular mass. PIA had an average molecular mass of 28 kDa and therefore is unequivocally smaller than PNAG-I and PNAG-II. One likely explanation for this difference in molecular mass might be found in the protocol used by Mack et al. (16) to purify PIA. PIA was extracted from S. epidermidis cells by sonication in PBS. This was followed by two consecutive centrifugation steps used to remove cell debris and clarify the extracts (16, 17). We believe that any high molecular mass, PNAG-I- or PNAG-II-like polymers present in these extracts would have been readily removed by such centrifugation steps due to the insolubility of the larger-size PNAG polymers. In addition, other steps in the protocol used by Mack et al. (16) likely selected for the soluble fractions of PIA, and in this way it might have enriched the preparations with the smaller molecular mass polymers.
Mack et al. (16) reported levels of O-linked succinate of 0.01 to 0.06 (molar ratios to total glucosamine) for the polysaccharide I and II of PIA, respectively. The values of O-linked succinic acid residues found in this work are ca. 100 times higher than those reported for the polysaccharide II of PIA, and therefore this represents yet another chemical difference between PNAG and PIA. However, whether this difference would be maintained among PNAG isolated from other strains or under other growth conditions is not known.
Capsular polysaccharides are generally considered to be T-independent type 2 (TI-2) antigens, since they can stimulate specific antibody production in the absence of T-lymphocytes. High-molecular-mass polysaccharides containing many repeating epitopes are able to cross-link multiple polysaccharide-specific surface immunoglobulin receptors on B cells, leading to cellular proliferation and differentiation. The TI-2 antigen response leads to the generation of plasma cells that secrete specific antibodies, predominantly IgM, and lower amounts of IgG in mice and in humans. PNAG elicited responses in adult mice that showed the typical characteristic of a TI-2 antigen. IgM antibody titers were initially high in mice immunized with all three PNAG antigens and exhibited a dose-dependent response but rapidly declined within 5 weeks of the last immunization. On the other hand only the highest-molecular-mass PNAG antigen, PNAG-I elicited IgG antibodies, and the titer increased over the 5-week period of assessment. Furthermore, when a small amount of a very high-molecular-mass fraction of PNAG-I was isolated to obtain PNAG-780 and used to immunize mice, we obtained high IgG titers with lower amounts of PNAG-780 compared to the same dose of the PNAG-I fraction. Thus, the larger sized polymers are more efficient at generating an IgG response than their lower-molecular-weight counterparts, and IgG antibodies are usually more protective against infection.
IgG responses were also obtained in rabbits immunized with PNAG-I plus adjuvants. In both mice and rabbits there was a high level of variability in the IgG response to PNAG-I, indicating that in outbred populations one might expect PNAG-I to have a variable ability to induce IgG antibodies. This variability may also be the result of prior environmentally introduced infection of some of these animals with staphylococci. Use of other means to enhance the production of IgG antibodies, such as by preparing conjugate vaccines, are currently being pursued. The IgG antibodies elicited in the rabbits were able to mediate opsonic killing, a key feature of protective antibodies against bacterial pathogens (6, 12, 21). Previous work with PS/A isolated from S. epidermidis indicated an association of IgG opsonic antibodies with protective immunity against bacteremia (13) and endocarditis (29). In addition, opsonic rabbit IgG antibodies raised to S. aureus PS/A that is identical in structure to the antigen described in this study were broadly protective against a variety of S. aureus strains in a mouse renal infection model (21). Thus, the ability of the PNAG-I to elicit IgG antibodies will likely be a key feature needed to produce PNAG-based vaccines for further evaluation.
Acknowledgments
This work was supported by NIH grant AI 46706 and by a fellowship grant to Tomás Maira-Litrán from the Ministerio de Ciencia y Tecnología (Spain).
We thank Barbara Reinap, Channing Laboratory, for assistance with the monosaccharide analysis of PNAG and Sarah E. Cramton and Fritz Goetz of the University of Tuebingen, Germany, for providing S. aureus MN8 Δica.
Editor: E. I. Tuomanen
REFERENCES
- 1.Blomster-Hautamaa, D. A., and P. M. Schlievert. 1988. Preparation of toxic shock syndrome toxin-1. Methods Enzymol. 165:37-43. [DOI] [PubMed] [Google Scholar]
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Chaplin, M. F. 1986. Monosaccharides, p. 1-36. In M. F. Chaplin and J. F. Kennedy (ed.), Carbohydrate analysis: a practical approach. IRL Press, Oxford, England.
- 4.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Gotz. 1999. The intercellular adhesin (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Reabers, and F. Smith. 1956. Colorimetric method for the determination of sugars and related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 6.Fattom, A., J. Sarwar, A. Ortiz, and R. Naso. 1996. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect. Immun. 64:1659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fey, P. D., J. S. Ulphani, F. Gotz, C. Heilmann, D. Mack, and M. E. Rupp. 1999. Characterization of the relationship between polysaccharide intercellular adhesin and hemagglutination in Staphylococcus epidermidis. J. Infect. Dis. 179:1561-1564. [DOI] [PubMed] [Google Scholar]
- 8.Gerke, C., A. Kraft, R. Sussmuth, O. Schweitzer, and F. Gotz. 1998. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. J. Biol. Chem. 273:18586-18593. [DOI] [PubMed] [Google Scholar]
- 9.Goldmann, D. A., and G. B. Pier. 1993. Pathogenesis of infections related to intravascular catheterization. Clin. Microbiol. Rev. 6:176-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habeeb, A. F. S. A. 1966. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal. Biochem. 14:328-336. [DOI] [PubMed] [Google Scholar]
- 11.Hussain, M., J. G. M. Hastings, and P. J. White. 1991. A chemically defined medium for slime production by coagulase-negative staphylococci. J. Med. Microbiol. 34:143-147. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, S. E., L. Rubin, S. Romero-Steiner, J. K. Dykes, L. B. Pais, A. Rizvi, E. Ades, and G. M. Carlone. 1999. Correlation of opsonophagocytosis and passive protection assays using human anticapsular antibodies in an infant mouse model of bacteremia for Streptococcus pneumoniae. J. Infect. Dis. 180:133-140. [DOI] [PubMed] [Google Scholar]
- 13.Kojima, Y., M. Tojo, D. A. Goldmann, T. D. Tosteson, and G. B. Pier. 1990. Antibody to the capsular polysaccharide/adhesin protects rabbits against catheter-related bacteremia due to coagulase-negative staphylococci. J. Infect. Dis. 162:435-441. [DOI] [PubMed] [Google Scholar]
- 14.Lane-Smith, R., and E. Gilkerson. 1979. Quantitation of glycosaminoglycan hexosamine using 3-methyl-2-benzothiazolone hydrazone hydrochloride. Anal. Biochem. 98:478-480. [DOI] [PubMed] [Google Scholar]
- 15.Lowry, O. H., N. R. Roberts, K. Y. Leiner, M. L. Wu, and L. Farr. 1954. The quantitative histochemistry of brain. I. Chemical methods. J. Biol. Chem. 207:1-17. [PubMed] [Google Scholar]
- 16.Mack, D., W. Fischer, A. Krokotsch, K. Leopold, R. Hartmann, H. Egge, and R. Laufs. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mack, D., M. Nedelmann, A. Krokotsch, A. Schwarzkopf, J. Heesemann, and R. Laufs. 1994. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect. Immun. 62:3244-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mack, D., J. Riedewald, H. Rohde, T. Magnus, H. H. Feucht, H. A. Elsner, R. Laufs, and M. E. Rupp. 1999. Essential functional role of the polysaccharide intercellular adhesin of Staphylococcus epidermidis in hemagglutination. Infect. Immun. 67:1004-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin, M. A., M. A. Pfaller, and R. P. Wenzel. 1989. Coagulase-negative staphylococcal bacteremia. Mortality and hospital stay. Ann. Intern. Med. 110:9-16. [DOI] [PubMed] [Google Scholar]
- 20.McKenney, D., J. Hubner, E. Muller, Y. Wang, D. A. Goldmann, and G. B. Pier. 1998. The ica locus of Staphylococcus epidermidis encodes production of the capsular polysaccharide/adhesin. Infect. Immun. 66:4711-4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKenney, D., K. Pouliot, Y. Wang, V. Murthy, M. Ulrich, G. Doring, J. C. Lee, D. A Goldmann, and G. B. Pier. 1999. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 284:1523-1527. [DOI] [PubMed] [Google Scholar]
- 22.Muller, E., J. Hubner, N. Gutierrez, S. Takeda, D. A. Goldmann, and G. B. Pier. 1993. Isolation and characterization of transposon mutants of Staphylococcus epidermidis deficient in capsular polysaccharide/adhesin and slime. Infect. Immun. 61:551-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaller, M. A., and L. A. Herwaldt. 1988. Laboratory, clinical, and epidemiological aspects of coagulase-negative staphylococci. Clin. Microbiol. Rev. 1:281-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Proctor, R. A. 2000. Toward an understanding of biomaterial infections: a complex interplay between the host and bacteria. J. Lab. Clin. Med. 135:14-15. [DOI] [PubMed] [Google Scholar]
- 25.Rupp, M. E., and G. L. Archer. 1992. Hemagglutination and adherence to plastic by Staphylococcus epidermidis. Infect. Immun. 60:4322-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rupp, M. E., N. Sloot, H. G. Meyer, J. Han, and S. Gatermann. 1995. Characterization of the hemagglutinin of Staphylococcus epidermidis. J. Infect. Dis. 172:1509-1518. [DOI] [PubMed] [Google Scholar]
- 27.Shiro, H., G. Meluleni, A. Groll, E. Muller, T. D. Tosteson, D. A. Goldmann, and G. B. Pier. 1995. The pathogenic role of Staphylococcus epidermidis capsular polysaccharide/adhesin in a low-inoculum rabbit model of prosthetic valve endocarditis. Circulation 92:2715-2722. [DOI] [PubMed] [Google Scholar]
- 28.Shiro, H., E. Muller, N. Gutierrez, S. Boisot, M. Grout. T. D. Tosteson, D. A. Goldmann, and G. B. Pier. 1994. Transposon mutants of Staphylococcus epidermidis deficient in elaboration of capsular polysaccharide/adhesin and slime are avirulent in a rabbit model of endocarditis. J. Infect. Dis. 169:1042-1049. [DOI] [PubMed] [Google Scholar]
- 29.Takeda, S., G. B. Pier, Y. Kojima, M. Tojo, E. Muller, T. Tosteson, and D. A. Goldmann. 1991. Protection against endocarditis due to Staphylococcus epidermidis by immunization with capsular polysaccharide/adhesin. Circulation 84:2539-2546. [DOI] [PubMed] [Google Scholar]
- 30.Tojo, M., N. Yamashita, D. A. Goldmann, and G. B. Pier. 1988. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J. Infect. Dis. 157:713-722. [DOI] [PubMed] [Google Scholar]
- 31.von Eiff, C., C. Heilmann, and G. Peters. 1999. New aspects in the molecular basis of polymer-associated infections due to staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. 18:843-846. [DOI] [PubMed] [Google Scholar]