Aldosterone is a major regulator of extracellular fluid volume and the principal determinant of potassium metabolism 1,2,3,4,5. These effects are mediated by the binding of aldosterone to the mineralocorticoid receptor in target tissues, primarily the kidney. Volume is regulated through a direct effect on the collecting duct, where aldosterone promotes sodium retention and potassium excretion. The reabsorption of sodium ions produces a fall in the transmembrane potential, thus enhancing the flow of positive ions (such as potassium) out of the cell into the lumen. The reabsorbed sodium ions are transported out of the tubular epithellium into the renal interstitial fluid and from there into the renal capillary circulation.
Three primary mechanisms control aldosterone release—the renin-angiotensin system, potassium, and adrenocorticotropic hormone. The renin-angiotensin system controls extracellular fluid volume via regulation of aldosterone secretion. In effect, the renin-angiotensin system keeps the circulating blood volume constant by causing aldosterone-induced sodium retention during volume deficiency and by decreasing aldosterone-dependent sodium retention when volume is ample.
In recent years there has been a radical shift in our view of aldosterone's effects on the heart, the vasculature and the kidney6,7,8,9. Aldosterone's endocrine properties have taken on a broader perspective, involving non-classic actions in non-epithelial cells found in non-classic target tissues6, 10,11,12,13,14,15. The traditional concept, that aldosterone is synthesized only in the adrenal glomerulosa cell and acts almost exclusively on the kidney to modify sodium and potassium homoeostasis, needs to be expanded. There is increasing evidence that aldosterone can have an effect on vascular remodelling and collagen formation, and a non-genomic action to modify endothelial function. Among the most intriguing effects of aldosterone are its impact on fibrosis and activity associated with a cell surface receptor in certain target tissues, including endothelial cells 6, 7, 16,17,18,19. These actions contribute substantially to the pathophysiology of congestive heart failure (CHF), as well as progressive renal dysfunction. This new information has increased interest in the development of an antagonist to block aldosterone's effect, not just because of its diuretic action but primarily because of its potential cardiovascular and renal protective effects.
In this review I consider the broad spectrum of non-genomic effects of aldosterone. It is becoming increasingly evident that these effects, occurring independently of haemodynamic factors, contribute to enhanced cardiovascular risk manifested by congestive heart failure and progressive renal disease. I also discuss the clinical trials with selective aldosterone receptor antagonists that are currently underway in patients with left ventricular hypertrophy, essential hypertension and systolic hypertension, and in those who have experienced myocardial infarction. Such trials will enhance our understanding of the role of aldosterone in the pathophysiology of cardiovascular disease. Also, selective aldosterone receptor antagonism holds promise for a reduction in cardiovascular and renal disease morbidity and mortality, and for enhancement of patient wellbeing.
TRADITIONAL CONCEPT
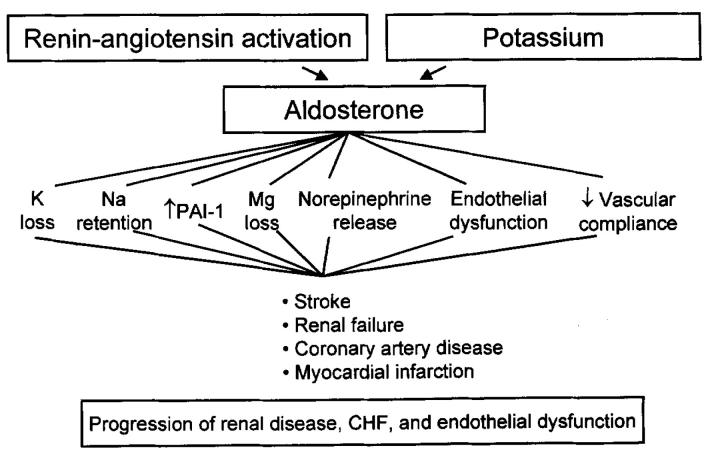
In its capacity as a mineralocorticoid hormone, aldosterone has receptor-ligand endocrine actions on epithelial cell sodium and potassium exchange in target tissues such as the kidneys, colon, and salivary and sweat glands1,2,3. It has a vital function during dietary sodium deprivation or salt and water loss. Through its effect on the distal renal tubular cell, aldosterone is involved in the regulation of sodium and body water homoeostasis, and consequently participates in the regulation of blood pressure4. Figure 1 summarizes the deleterious effects of activation of the renin-angiotensin—aldosterone system with a resultant increase in circulating aldosterone. As a consequence of renal sodium retention and enhanced potassium excretion, aldosterone increases the likelihood of stroke, coronary artery disease, myocardial infarction and sudden cardiac death. Its contribution to the pathophysiological origins of such salt-avid states as congestive heart failure, cirrhosis and nephrotic syndrome is well established. In addition, the newly discovered nonclassic actions contribute to the pathophyisology of cardiac fibrosis and cardiovascular dysfunction6,20 as well as progressive renal dysfunction.
Figure 1.
Multiple mechanisms whereby aldosterone promotes cardiovascular and renal dysfunction PAI-1=plasminogen activator inhibitor-1; CHF=congestive heart failure
Several lines of evidence now point to a major role of aldosterone in promoting progressive renal dysfunction. Observational studies in patients with primary aldosteronism (PA) suggest a pathogenetic role for hyperaldosteronism per se in the pathogenesis of renal disease21,22. Originally it was postulated that hypertensive patients with low levels of plasma renin activity have fewer cardiovascular complications than those with normal or raised plasma renin activity. However, in recent studies cardiovascular complications were found in 14-35% of the patients with PA. Moreover, in patients with PA, the prevalence and degree of proteinuria was reported to be greater than in patients with essential hypertension21,22.
CARDIOVASCULAR EFFECTS
Several reports have reviewed the adverse effects of aldosterone in the endothelial, smooth muscle, and adventitial layers of the blood vessel. The innermost layer of the blood vessel is the endothelium, which produces nitric oxide (NO). Last year, Farquharson and Struthers20 demonstrated, in an indirect manner, that aldosterone could play a major role in producing endothelial dysfunction in chronic heart failure. They reported that aldosterone blockade with spironolactone virtually doubled NO bioactivity and improved endothelial function in patients with chronic heart failure who were already receiving angiotensin-converting enzyme (ACE) inhibitor therapy. Recently these investigators conducted an elegant study directly demonstrating that aldosterone infusion in hypertensive patients can induce endothelial dysfunction, thereby substantiating this important effect (Farquharson CAF, Struthers AD, Unpublished).
The magnitude of the effect of aldosterone blockade seemed much greater than had been found for ACE inhibitors in comparable studies. Improvement of endothelial function may be highly beneficial in that endothelial dysfunction is closely linked to cardiovascular events.
On this basis one can readily marshal a compelling argument for a role of aldosterone receptor antagonism in several groups of patients other than those with severe heart failure due to systolic left ventricular dysfunction—including patients with essential hypertension6. Aldosterone receptor antagonists have been shown to prevent the progression of left ventricular hypertrophy, which is recognized as predisposing to sudden cardiac death. Recent data indicate that aldosterone blocks the uptake of norepinephrine (noradrenaline) into the myocardium, and that aldosterone receptor antagonists improve the uptake of norepinephrine, heart rate variability and baroreceptor function. Collectively, these findings make a strong theoretical case for inclusion of aldosterone antagonism in the antihypertensive regimen to prevent the cardiac sequelae of hypertension.
The Randomized Aldactone Evaluation Study (RALES)7 examined the effect of spironolactone on overall morbidity and mortality in patients with severe heart failure treated with standard therapy with an ACE inhibitor, a loop diuretic and digoxin, combined with either a non-haemodynamic dose of spironolactone (25 mg/day) or placebo. This seminal trial was discontinued early by the data safety monitoring board after a mean follow-up of 24 months because of the benefits: spironolactone-treated patients had a 30% lower risk of death from all causes than the placebo group. This reduction in mortality was largely attributed to fewer deaths from progressive heart failure and sudden cardiac death. The RALES investigators attributed the beneficial actions of spironolactone to the drug's favourable effects on myocardial and vascular fibrosis and its ability to increase myocardial uptake of norepinephrine, in addition to its anticipated ability to prevent sodium retention and potassium loss7.
THE ROLE OF ALDOSTERONE IN MEDIATING PROGRESSIVE RENAL DISEASE
By analogy with its effects on the cardiovascular system, aldosterone also has adverse effects on the kidney. I have previously reviewed the rapidly emerging evidence for aldosterone as a mediator of progressive renal disease9. Although the role of angiotensin II in mediating progressive renal disease has been documented extensively23,24,25,26,27, recent evidence also implicates aldosterone (independent of the renin-angiotensin system) as an important pathogenetic factor.
Hyperaldosteronism and adrenal hypertrophy are common findings in the remnant-kidney model, with plasma levels of aldosterone approximately tenfold normal28. Clinical studies27 have also demonstrated a relation between augmented levels of aldosterone and renal deterioration.
Several experimental models are consistent with the concept that aldosterone can mediate renal injury. In a study by Quan et al.29, hypertension, proteinuria, and structural renal injury were less prevalent in rats that underwent subtotal nephrectomy with adrenalectomy than in rats that had partial nephrectomy but intact adrenal glands. The adrenalectomized rats received large doses of replacement glucocorticoid but not aldosterone. In the deoxycorticosterone acetate salt-hypertensive rat model, exogenous administration of mineralocorticoids induced lesions of malignant nephrosclerosis and stroke30. The development of this type of lesion is probably due to the intrinsic action of mineralocorticoids, since these rats show low plasma renin activity and respond poorly to ACE inhibitor therapy. Several lines of experimental evidence confirm that selective blockade of aldosterone, independent of renin-angiotensin blockade, reduces proteinuria and nephrosclerosis in the spontaneously hypertensive strokeprone rat (SHRSP) model and controls proteinuria and glomerulosclerosis in the subtotally nephrectomized (remnant kidney) rat model.
Although many groups have reported that ACE inhibition retards progression of renal disease, this intervention does not differentiate between the contributions of renin-angiotensin and aldosterone. To evaluate the possible contribution of aldosterone per se, Rocha et al.8,31 conducted a series of experiments in SHRSP that succeeded in dissociating the relative contributions of aldosterone and the renin-angiotensin system. Initially, they implanted time-release pellets of spironolactone or placebo pellets in saline-drinking (1% NaCl) SHRSP ingesting a ‘stroke-prone rodent’ diet31. This diet induces severe hypertension and a thrombotic microangiopathy of the sort observed in malignant nephrosclerosis. Blood pressure and urinary protein excretion were assessed for 3-4 weeks. Mineralocorticoid receptor blockade with spironolactone greatly attenuated urinary protein excretion (150 mg/day for the placebo group, 39 mg/day for the spironolactone group, P<0.0001). About 12 weeks later, proteinuria was still 39 mg/day in the spironolactone group compared with 136 mg/day in the placebo-implanted animals (P<0.0001). There were fewer nephrosclerotic (P<0.01) and cere-brovascular (P<0.001) lesions in the spironolactone group than in the placebo group. Notably, systolic blood pressure did not differ between the two groups at any time during the study.
In a subsequent study, Rocha et al.8 evaluated whether an aldosterone infusion would reverse the renal-protective effects of captopril therapy in SHRSP. The study divided SHRSP into five groups—vehicle (control), captopril (50 mg/kg per day), aldosterone infusion (40 μg/kg per day), or captopril (50 mg/kg per day) with aldosterone infusion (at 20 and 40 μg/kg per day). Animals in the control and aldosterone infusion groups had marked proteinuria and comparable degrees of renal injury (21% and 29%, respectively). Captopril treatment reduced endogenous aldosterone levels, prevented the development of proteinuria, and prevented the development of glomerular and renal vascular lesions. However, subsequent aldosterone infusion reversed the ability of captopril to confer this protection. The aldosterone-infused captopril-treated rats had developed proteinuria, renal vascular lesions, and glomerular lesions despite ACE inhibitor treatment. It is noteworthy that systolic blood pressure in captopril-treated SHRSP receiving the aldosterone infusion did not differ from that in SHRSP treated with captopril alone. The fact that renal injury induced by aldosterone developed independently of an increase in blood pressure suggests a more direct tissue effect of aldosterone.
Because the above data with spironolactone may be confounded by the modest affinity of spironolactone for other steroid receptors, Rocha and colleagues performed two experiments utilizing eplerenone, a selective aldoster-one receptor antagonist, to further evaluate the role of the mineralocorticoids in renal vascular lesion development32. In the first experiment, the impact of eplerenone on urinary protein excretion and blood pressure was compared with a control group following infusions of aldosterone or angiotensin II. Eplerenone prevented proteinuria (15 mg/day for eplerenone vs 92 mg/day for the control group, P<0.001) and renal lesions (2% for eplerenone vs 40% for the control group, P<0.0005). Because blood pressure did not differ significantly between the eplerenone and control groups (226 mm Hg vs 234 mm Hg), we must conclude that the protective effects of eplerenone were independent of any confounding effects of blood pressure. In the second experiment, five groups were studied—vehicle (control), captopril, captopril followed by an aldosterone infusion, captopril followed by an angiotensin II infusion, and the combination of captopril and eplerenone followed by an angiotensin II infusion. After 2 weeks, in the control, captopril plus aldosterone, and captopril plus angiotensin II groups vs the captopril group alone, proteinuria was 158, 121 and 96 mg/day vs 16 mg/day (P<0.001), the rate of glomerular lesions was 18%, 15% and 16% vs 0% (P<0.001) and the rate of renal vascular lesions was 24%, 26% and 17% vs 0% (P<0.001). Thus, both the aldosterone and the angiotensin II infusions reversed the renal-protective effects of captopril. However, when the captopril+angiotensin II group was compared with captopril+eplerenone+angiotensin II, the latter combination group manifested substantially less proteinuria (96 mg/day vs 28 mg/day; P<0.001), and fewer glomerular lesions (16% vs 4%; P<0.001) and renal vascular lesions (17% vs 4%; P<0.001). In contrast to the reversal of renal protection seen when angiotensin II was added to captopril treatment, the addition of eplerenone to the aforementioned regimen attenuated proteinuria and renal damage in SHRSP. Although mean systolic blood pressures were raised after 2 weeks of treatment, there were no statistically significant differences in systolic blood pressure among the five treatment groups. These pivotal data further support a major role of aldosterone per se, independent of the renin-angiotensin system, as a mediator of renal injury in saline-drinking SHRSP.
HOW DOES ALDOSTERONE PROMOTE FIBROSIS AND TARGET-ORGAN DISEASE?
Box 1 lists mechanisms that may account for the ability of aldosterone to promote fibrosis and target-organ dysfunction in the hypertensive patient. These include plasminogen activator inhibitor (PAI-1) expression and consequent alterations of vascular fibrinolysis33,34, stimulation of transforming growth factor β135, and stimulation of reactive oxygen species (ROS)36. Another attractive mechanism incorporates the well-established ability of aldosterone to potentiate the pressor effects of angiotensin II by upregulating angiotensin II receptors in vascular smooth muscle cells37. Aldosterone has been hypothesized to exert a direct cellular effect to induce fibrosis and hypertrophy in vascular smooth muscle cells and myocardial cells—effects that may previously have been inaccurately attributed solely to the systemic hypertension caused by mineralocorticoids. Several studies16,38 have linked mineralocorticoids with myocardial fibrosis via stimulation of collagen formation in myocardial cells.
BOX 1 Potential mechanisms whereby aldosterone mediates fibrosis and collagen formation (modified by permission from Ref. 9)
Upregulation of angiotensin II receptors
Potentiation of the pressor responses of angiotensin II
Increases in sodium influx in vascular smooth muscle cells
Inhibition of norepinephrine uptake in vascular smooth muscle cells and myocardial cells
Participation in vascular smooth muscle cell hypertrophy
Modulation of the effect of angiotensin II on plasminogen activator inhibitor-1 expression
Stimulation of transforming growth factor beta-1 synthesis
Generation of reactive oxygen species
Production of endothelial dysfunction
Circulating aldosterone may mediate vascular fibrosis by the direct interaction of this steroid hormone with high-affinity, low-capacity corticoid receptors that are located in the cytosol of vascular fibroblasts. When activated, the receptor loses its heat-shock protein, and its monomeric form reaches the cell nucleus, where it binds to DNA to initiate the expression of mRNA for synthesis of type 1 collagen (or other proteins)39.
Another mechanism whereby aldosterone may promote fibrosis in several target organs, including the kidney, centres on its effects on the plasminogen activator system (see Box 1). The effect of the renin-angiotensin—aldosterone system (RAAS) on the plasminogen activator system serves as one of the major endogenous defence mechanisms against intravascular thrombosis and is also important in vascular and tissue remodelling. Vascular fibrinolytic balance is to a large extent determined by the competing effects of plasminogen activators and PAI-1, both of which are locally synthesized in the blood vessel wall, the vascular endothelium in particular. Brown and colleagues33,34 have hypothesized that a major component of the vascular toxicity brought on by activation of the RAAS is derived from the deleterious effects of angiotensin on fibrinolytic balance. There is a growing body of evidence—including data at molecular/cellular level, experimental work in animals, and clinical studies—indicating that ACE is strategically located to regulate vascular fibrinolytic balance.
Brown and colleagues33 have proposed that aldosterone contributes to the regulation of PAI-1 expression. Furthermore, recent data33,34 indicate that plasma PAI-1 levels strongly correlate with serum aldosterone in salt-depleted healthy subjects and that aldosterone enhances the induction of PAI-1 expression in smooth muscle cells in vitro. In-vivo data indicate that aldosterone activity, as assessed by serum aldosterone levels, correlates with PAI-1 antigen levels, thus favouring an interaction between aldosterone and the fibrinolytic system40. Probably angiotensin and aldosterone act in concert to concomitantly regulate vascular fibrinolysis and tissue remodelling. Consequently, studies should be initiated to investigate the effects of specific aldosterone antagonists on PAI-1 production and the effects of such interventions on resultant cardiovascular and renal dysfunction.
In light of this theoretical construct, randomized clinical studies have been initiated to delineate the potential renal-protective effects of aldosterone receptor blockade in patients with hypertension of diverse aetiology.
LIMITATIONS OF NON-SELECTIVE ALDOSTERONE RECEPTOR BLOCKADE
Although spironolactone is an effective antialdosterone agent, its widespread use in patients is limited by its undesirable side-effects. At standard doses it can cause impotence and gynaecomastia in men and menstrual disturbances in women. These adverse effects, due to the binding of spironolactone to progesterone and androgen receptors, are an important cause of drug discontinuation. In a study involving 43 patients treated with long-term spironolactone for mineralocorticoid excess syndromes, 13 patients (30%) were switched to alternative therapy because of gynaecomastia (6/20 men) and menstrual disturbances or breast pain (7/23 women)41. The RALES trial7 reported a 10% incidence of gynaecomastia or breast pain in males (patients in this trial received 25-50 mg spironolactone per day). This incidence was significantly higher than with placebo (10% vs 1%, P<0.001) and caused significantly more patients to discontinue treatment (2% vs 0.2%, P=0.006).
Although troublesome, these side-effects are reversible and dose-related. At doses of 50 mg/day or less, the incidence of gynaecomastia is 6.9% but it rises to 52% with doses greater than 150 mg/day42. Moreover, the onset of this adverse effect is faster at higher doses43. Studies in women taking spironolactone for dermatological disorders have again shown the dose relationship of these sexual side-effects, with doses substantially beyond 100 mg/day more frequently associated with menstrual disturbances and breast enlargement44,45.
SELECTIVE ALDOSTERONE RECEPTOR ANTAGONISTS (SARAs)
With SARAs these sexual side-effects are less frequent; thus, patients adhere more closely to antialdosterone therapy46. Preliminary data on one SARA, eplerenone, are promising for the effective blockade of aldosterone—and its harmful effects—without the sexual disturbances of spironolactone46,47,48,49. This agent is currently in phase III trials for the treatment of hypertension and heart failure. Eplerenone has a much lower affinity for androgen and progesterone receptors than spironolactone, but has twice the potency with respect to antimineralocorticoid activity47. As mentioned previously, animal models have shown the ability of aldosterone antagonists, including eplerenone, to prevent the occurrence of proteinuria and renal lesions in saline-drinking SHRSP8,31,32. Furthermore, in the rat model of ascending aortic stenosis, investigators examined the effect of eplerenone versus no drug on left ventricular (LV) hypertrophy in the presence of severe pressure overload. Despite having similar increases in LV pressure to untreated animals, rats treated with eplerenone had significantly lower heart weight, LV end-diastolic pressure, and LV mass (P<0.05 for all comparisons)48.
Early clinical studies46 support the concept that eplerenone is effective in hypertension without antiandrogenic adverse effects. In patients with mild-to-moderate hypertension, eplerenone yielded clinically meaningful blood pressure reductions which were sustained over the 24-hour dosing period. The incidence of adverse effects with eplerenone was similar to that of placebo, with no reports of gynaecomastia46. In patients with New York Heart Association (NYHA) class II to IV heart failure, both eplerenone and spironolactone produced significant decreases in brain natriuretic peptide and increased urinary aldosterone and plasma renin compared with placebo. Consistent with the lower affinity of eplerenone for androgen and progesterone receptors seen in animal studies, male patients receiving spironolactone had a higher plasma testosterone (P≤0.02) than those receiving eplerenone49.
THE FUTURE
In view of the positive results of the RALES Study7, a large randomized prospective study has been initiated to investigate the effects of selective aldosterone receptor blockade on cardiovascular mortality and morbidity in patients with heart failure due to systolic dysfunction complicating acute myocardial infarction—Eplerenone's Neurohormonal Efficacy and Survival Study (EPHESUS)49. It is hoped that this study, and parallel work in patients with hypertension and progressive renal dysfunction, will help delineate the role of SARAs as target protective agents in the hypertensive patient.
Acknowledgments
Parts of this review are adapted from an earlier paper9. This is a US Government work.
References
- 1.Foster RH, MacFarlane CH, Bustamante MO. Recent progress in understanding aldosterone secretion. Gen Pharmacol 1997;28: 647-51 [DOI] [PubMed] [Google Scholar]
- 2.Fuller PJ. Aldosterone's effects and mechanism of action. Curr Opin Endocrinol Diabetes 1997;4: 218-24 [Google Scholar]
- 3.Luetscher JA Jr, Neher JP, Wettstein A. Isolation of crystalline aldosterone from the urine of a nephrotic patient. Experientia 1954;10: 456-8 [DOI] [PubMed] [Google Scholar]
- 4.Edelman IS, Fimognari GM. On the biochemical mechanism of action of aldosterone. Rec Prog Horm Res 1968;24: 1-44 [DOI] [PubMed] [Google Scholar]
- 5.Rossier BC. Mechanisms of action of mineralocorticoid hormones. Endocr Res 1989;15: 206-26 [DOI] [PubMed] [Google Scholar]
- 6.Duprez D, De Buyzere M, Rietzchel ER, Clement DL. Aldosterone and vascular damage. Curr Hypertens Rep 2000;2: 327-34 [DOI] [PubMed] [Google Scholar]
- 7.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341: 709-17 [DOI] [PubMed] [Google Scholar]
- 8.Rocha R, Chander PN, Zuckerman A, Stier CT. Role of aldosterone in renal vascular injury in stroke-prone hypertensive rats. Hypertension 1999;33: 232-7 [DOI] [PubMed] [Google Scholar]
- 9.Epstein M. Aldosterone and the hypertensive kidney: its emerging role as a mediator of progressive renal dysfunction: a paradigm shift. J Hypertension 2001;19: 829-42 [DOI] [PubMed] [Google Scholar]
- 10.Wehling M. Non-genomic actions of steroid hormones. Trends Endocrinol Metab 1994;5: 347-53 [DOI] [PubMed] [Google Scholar]
- 11.Wehling M, Spes CH, Win N, et al. Rapid cardiovascular action of aldosterone in man. J Clin Endocrinol Metab 1998;83: 3517-22 [DOI] [PubMed] [Google Scholar]
- 12.Christ M, Douwes K, Eisen C, et al. Rapid non-genomic effects of aldosterone on sodium transport in rat vascular smooth muscle cells: involvement of the Na+/H+ antiport. Hypertension 1995;25: 117-23 [DOI] [PubMed] [Google Scholar]
- 13.Wehling M, Neylon CB, Fullerton M, et al. Non-genomic effects of aldosterone on intracellular calcium in vascular smooth muscle cells. Circ Res 1995;76: 973-9 [DOI] [PubMed] [Google Scholar]
- 14.Wehling M. Looking beyond the dogma of genomic steroid action: insights and facts of the 1990s. J Mol Med 1995;73: 439-47 [DOI] [PubMed] [Google Scholar]
- 15.Ebata S, Muto S, Okada K, et al. Aldosterone activates Na+/H+ exchange in vascular smooth muscle cells by non-genomic and genomic mechanisms. Kidney Int 1999;56: 1400-12 [DOI] [PubMed] [Google Scholar]
- 16.Young M, Fullerton M, Dilley R, Funder J. Mineralocorticoids, hypertension and cardiac fibrosis. J Clin Invest 1994;93: 2578-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonvalet JP, Alfaidy N, Farman N, Lombes M. Aldosterone: intracellular receptors in human heart. Eur Heart J 1995;16(suppl N): 92-7 [DOI] [PubMed] [Google Scholar]
- 18.Kornel L. Colocalization of 11β-hydroxysteroid dehydrogenase and mineralocorticoid receptors in cultured vascular smooth muscle cells. Am J Hypertens 1994;7: 100-3 [DOI] [PubMed] [Google Scholar]
- 19.Lombes M, Oblin ME, Gasc JM, Baulieu EE, Farman N, Bonvalet JP. Immunohistochemical and biochemical evidence for a cardiovascular mineralocorticoid receptor. Circ Res 1992;71: 503-10 [DOI] [PubMed] [Google Scholar]
- 20.Farquharson CAJ, Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation 2000;101: 594-7 [DOI] [PubMed] [Google Scholar]
- 21.Conn JW, Knopf RF, Nesbit RM. Clinical characteristics of primary aldosteronism from an analysis of 145 cases. Am J Surg 1964;107: 159-72 [DOI] [PubMed] [Google Scholar]
- 22.Nishimura M, Uzu T, Fujii T, et al. Cardiovascular complications in patients with primary aldosteronism. Am J Kidney Dis 1999;33: 261-6 [DOI] [PubMed] [Google Scholar]
- 23.Meyer TW, Anderson S, Rennke HG, Brenner BM. Reversing glomerular hypertension stabilizes established glomerular injury. Kidney Int 1987;31: 752-9 [DOI] [PubMed] [Google Scholar]
- 24.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993;329: 1456-62 [DOI] [PubMed] [Google Scholar]
- 25.Remuzzi G, Ruggenenti P, Benigni A. Understanding the nature of renal disease progression. Kidney Int 1997;51: 2-15 [DOI] [PubMed] [Google Scholar]
- 26.The GISEN Study Group. Randomized placebo-controlled trial of effect of ramipril on decline in GFR and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group. Lancet 1997;349: 1857-63 [PubMed] [Google Scholar]
- 27.Ruggenenti P, Perna A, Gherardi G, Gasdpari F, Benini R, Remuzzi G. Renal function and requirement for dialysis in chronic nephropathy patients on long-term ramipril: REIN follow-up trial. Gruppo Italiano di Studi Epidemiolgici in Nefrologia (GISEN). Ramipril Efficacy in Nephropathy. Lancet 1998;352: 1252-6 [DOI] [PubMed] [Google Scholar]
- 28.Greene E, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest 1996;98: 1063-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quan ZY, Walser M, Hill GS. Adrenalectomy ameliorates ablative nephropathy in the rat independently of corticosterone maintenance level. Kidney Int 1992;41: 326-33 [DOI] [PubMed] [Google Scholar]
- 30.Gavras H, Brunner HR, Laragh JH, et al. Malignant hypertension resulting from deoxycortisone acetate and salt excess: role of renin and sodium in vascular changes. Circ Res 1975;36: 300-9 [DOI] [PubMed] [Google Scholar]
- 31.Rocha R, Chander PN, Khanna K, Zuckerman A, Stier CT. Mineralocorticoid blockade reduces vascular injury in stroke-prone hypertensive rats. Hypertension 1998;31: 451-8 [DOI] [PubMed] [Google Scholar]
- 32.Rocha R, Chander PN, Zuckerman A, Stier CR Jr. Role of mineralocorticoids in renal injury in stroke-prone hypertensive rats [Abstract]. Hypertension 1998;32: 598. [DOI] [PubMed] [Google Scholar]
- 33.Brown NJ, Kim KS, Chen YQ, et al. Synergistic effect of adrenal steroids and angiotensin II on plasminogen activator inhibitor-1 production. J Clin Endocrinol Metab 2000;85: 336-44 [DOI] [PubMed] [Google Scholar]
- 34.Brown NJ, Nakamura S, Ma L, et al. Aldosterone modulates plasminogen activator inhibitor-1 and glomerulosclerosis in vivo. Kidney Int 2000;58: 1219-27 [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Zhang J, Zhang JQ, Ramires FJ. Local angiotensin II and transforming growth factor-beta 1 in renal fibrosis of rats. Hypertension 2000;35: 1078-84 [DOI] [PubMed] [Google Scholar]
- 36.Stier C Jr., Zuckerman A, Harashima H, Chander P. Antioxidants reduce aldosterone-induced renal vascular injury in stroke-prone spontaneously hypertensive rats [Abstract P4.03] Proceedings of the International Society of Hypertension, Chicago, 23 August, 2000
- 37.Schiffrin EL, Franks DJ, Gutkowska J. Effect of aldosterone on vascular angiotensin II receptors in the rat. Can J Physiol Pharmacol 1985;63: 1522-7 [DOI] [PubMed] [Google Scholar]
- 38.Brilla CG, Matsubara LS, Weber KT. Antifibrotic effects of spironolactone in preventing myocardial fibrosis in systemic arterial hypertension. Am J Cardiol 1993;71: 12A-16A [DOI] [PubMed] [Google Scholar]
- 39.Weber KT, Anversa P, Armstrong PW, et al. Remodeling and reparation of the cardiovascular system. J Am Coll Cardiol 1992;20: 3-16 [DOI] [PubMed] [Google Scholar]
- 40.Brown NJ, Agirbasli MA. Williams GH, Litchfield WR, Vaughan DE. Effect of activation and inhibition of the renin-angiotensin system on plasma PAI-I. Hypertension 1998;32: 965-71 [DOI] [PubMed] [Google Scholar]
- 41.Mantero F, Opocher G, Rocco S, Carpene G, Armanini D. Long-term treatment of mineralocorticoid excess syndromes. Steroids 1995;60: 81-6 [DOI] [PubMed] [Google Scholar]
- 42.Jeunemaitre X, Chatellier G, Kreft-Jais C, et al. Efficacy and tolerance of spironolactone in essential hypertension. Am J Cardiol 1987;60: 820-5 [DOI] [PubMed] [Google Scholar]
- 43.de Gasparo M, Whitebread SE, Preiswerk G, Jeunemaitre X, Corvol P, Menard J. Antialdosterones: incidence and prevention of sexual side effects. J Steroid Biochem 1989;32: 223-7 [DOI] [PubMed] [Google Scholar]
- 44.Helfer EL, Miller JL, Rose LI. Side-effects of spironolactone therapy in the hirsute woman. J Clin Endocrin Metab 1988;66: 208-11 [DOI] [PubMed] [Google Scholar]
- 45.Hughes BR, Cunliffe WJ. Tolerance of spironolactone. Br J Dermatol 1988;118: 687-91 [DOI] [PubMed] [Google Scholar]
- 46.Epstein M, Alexander JC, Roniker B. Efficacy and safety of eplerenone, a novel and selective aldosterone receptor antagonist (SARA), in patients with mild to moderate hypertension [abstract]. Hypertension 1999;33: 1075 [Google Scholar]
- 47.Rabasseda X, Silvestre J, Castañer J. Eplerenone. Drugs Future 1999;24: 488-501 [Google Scholar]
- 48.Hasan F, Weinberg EO, Delyani J, et al. Aldosterone and cardiac hypertrophy: effects of eplerenone on LV function and hypertrophic remodeling in LV pressure overload [Abstract]. Circulation 1999;100(suppl I): 1565 [Google Scholar]
- 49.Pitt B, Williams G, Remme W, et al. The EPHESUS Trial: eplerenone in patients with heart failure due to systolic dysfunction complicating acute myocardial infarction (Eplerenone's Neurohormonal Efficacy and Survival Study) (In press) [DOI] [PubMed]