Studies published over the past three years have tracked the incidence and course of human immunodeficiency virus (HIV) infection in relation to cardiac illness in both children and adults1 (Table 1). This recent work shows that subclinical echocardiographic abnormalities independently predict adverse outcomes and identify high-risk groups who can then be targeted for early intervention and therapy2.
Table 1.
HIV-associated cardiovascular abnormalities
| Type | Possible aetiologies and associations | Incidence |
|---|---|---|
| Dilated cardiomyopathy | HIV, Toxoplasma gondii, coxsackievirus group B, Epstein—Barr virus, cytomegalvirus, adenovirus, autoimmune response to infection | Estimated 15.9 patients/1000 asymptomatic HIV-infected persons (Ref. 4) |
| Cocaine, possibly nucleoside analogues, IL-2, doxorubicin, interferon | ||
| Nutritional deficiency/wasting; selenium, B12, carnitine | ||
| Thyroid hormone, growth hormone, adrenal insufficiency; hyperinsulinaemia | ||
| TNF-α, nitric oxide, TGF-β, endothelin-1 | ||
| Hypothermia/hyperthermia, autonomic insufficiency | ||
| Encephalopathy | ||
| Acquired immunodeficiency, HIV viral load, length of immunosuppression | ||
| Coronary heart disease/arterial hypertension | Protease-inhibitor-induced metabolic and coagulative disorders | |
| HIV-induced endothelial dysfunction | ||
| Erythropoietin-induced increase of haematocrit and blood viscosity | ||
| Vasculitis | ||
| Pericardial effusion | Staphylococcus, Streptococcus, Proteus, Nocardia, Pseudomonas, Klebsiella, enterococcus, Listeria | 11% per year in AIDS (Ref. 27) Spontaneous resolution in up to 42% (Ref. 27) |
| Mycobacterium tuberculosis, M. avium intercellulare, M. kansasii | ||
| HIV, Herpes simplex virus, Herpes simplex virus type 2, cytomegalovirus | ||
| Cryptococcus, Toxoplasma, Histoplasma | ||
| Kaposi's sarcoma, | ||
| Capillary leak/wasting/malnutrition | ||
| Hypothyroidism | ||
| Prolonged immunodeficiency | ||
| Isolated right ventricular and pulmonary disease | Recurrent bronchopulmonary infections, pulmonary arteritis, microvascular pulmonary emboli due to thrombus or drug injection | |
| Primary pulmonary hypertension | Plexogenic pulmonary arteriopathy; mediator release from endothelium | 0.5% (Ref. 24) |
The introduction of highly active antiretroviral therapy (HAART) regimens has greatly modified the course of HIV disease, with longer survival and better quality of life; however, early data from those treated raise concerns about a possible increase in both peripheral and coronary arterial diseases. In global terms HAART is available only to a minority of HIV-infected individuals, and studies before the advent of HAART remain applicable. UNAIDS estimates that 36.1 million people were living with HIV infection at the end of the year 20003. If 9-10% of patients develop symptomatic heart failure over 2-5 years4, then 3 million cases of HIV-related heart failure will present in that time period5. In this review article, I discuss the principal HIV-associated cardiovascular manifestations, with an emphasis on new knowledge about prevalence, pathogenesis and treatment.
DILATED CARDIOMYOPATHY
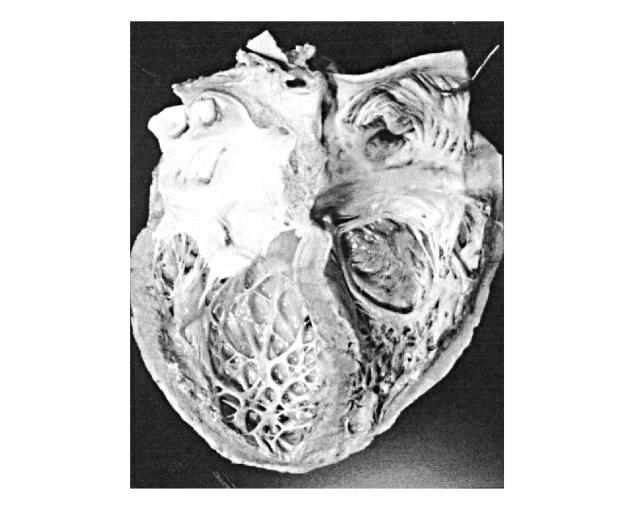
HIV disease is an important cause of dilated cardiomyopathy4 (Figure 1), with a prevalence reported as 3.6% among cardiomyopathy patients, increasing as patients with HIV infection live longer1. Patients with HIV-infection and dilated cardiomyopathy have a much worse prognosis than those with idiopathic dilated cardiomyopathy, hazard ratio of death 4.05,6. The importance of cardiac dysfunction is reflected by median survival to AIDS-related death—101 days in patients with left ventricular dysfunction and 472 days in patients with a normal heart by echocardiography at similar infection stage5.
Figure 1.
Dilated cardiomyopathy in AIDS patient. The heart has a globular shape with rounded apex due to ventricular dilation. The left ventricular cavity is enlarged, with mild myocardial hypertrophy. There is diffuse endocardial fibrous thickening
Myocarditis is the best studied cause of dilated cardiomyopathy in HIV disease4. HIV virions infect myocardial cells in patchy distributions4,7 without a clear direct association between HIV and cardiac myocyte dysfunction4,7. How virus enters CD4-receptor-negative cells such as myocytes is unknown. Reservoir cells (e.g. dendritic cells) may play a pathogenic role in the interaction between HIV and the myocyte and in the activation of multifunctional cytokines (e.g. tumour necrosis factor-alpha [TNF-α], interleukin-1 [IL-1], interleukin-6, interleukin-10) that contribute to progressive and late tissue damage6.
Myocarditis and dilated cardiomyopathy are associated with local cytokine production; thus, circulating levels tend to be uninformative6. Viral infection in the context of a nonspecific stimulator of monokines such as IL-1 or TNF-α is much more likely to lead to myocarditis and myocyte damage than viral infection alone. TNF-α produces a negative inotropic effect, by altering intracellular calcium homoeostasis and possibly by inducing nitric oxide synthesis, which likewise reduces myocyte contractility6,8. The intensity of both TNF-α and inducible nitric oxide synthase staining has been reported much higher in myocardial biopsy samples from patients with HIV-associated cardiomyopathy—specifically in those with a myocardial viral infection independently of antiretroviral treatment—than in those with idiopathic dilated cardiomyopathy.
When children with HIV-infection have received monthly immunoglobulin infusions they have shown improvement in left ventricular dysfunction, an increase in left ventricular wall thickness, and a reduction in peak left ventricular wall stress; these observations suggest that both impaired myocardial growth and left ventricular dysfunction are immunologically mediated9. The apparent efficacy of immunoglobulin therapy may be due to inhibition of cardiac autoantibodies (e.g. anti-α-myosin), the prevalence of which is increased in HIV-infected patients with dilated cardiomyopathy10, by competing for Fc receptors; alternatively, immunoglobulins might dampen the secretion or effects of cytokines and cellular growth factors11. Further study is needed to evaluate the efficacy of immunomodulatory therapy in adults and children with declining left ventricular function. In the published series there is no evidence of benefit to HIV-associated cardiomyopathy from HAART. However, the better control of cytomegalovirus infection, which is an important cause of cardiomyopathy in HIV disease, is one way in which HAART might lessen the incidence and improve the clinical course of HIV-associated heart disease. Encephalopathy influences negatively the clinical course of dilated cardiomyopathy in HIV disease8. Several studies have indicated that patients with encephalopathy are more likely to die of congestive heart failure than are patients without encephalopathy, hazard ratio after multivariate analysis 3.48. Immune modulating factors and cytokines have both cardiotoxic and neurotoxic actions and may play a crucial role in the development and progression of dilated cardiomyopathy and encephalopathy, perhaps explaining the association between the two. Proinflammatory cytokines activate inducible NO synthase (iNOS), thus stimulating production of NO, a sequence of events that may contribute to the association between dilated cardiomyopathy and encephalopathy in HIV disease8. HIV can persist in reservoir cells in the myocardium and the cerebral cortex even after antiretroviral treatment8. The reservoir cells may hold HIV-1 on their surfaces for long periods, and chronic release of cytotoxic cytokines (e.g. TNF-α, interleukin-6, endothelin-1) could contribute to progressive and late tissue damage in both systems independently of HAART regimens8.
CORONARY HEART DISEASE AND HYPERTENSION
A possible reason for accelerated coronary artery disease in HIV-infected patients is stimulation of local monocyte-macrophages by the virus. A wide range of inflammatory vascular diseases (e.g. polyarteritis nodosa, Henoch—Schönlein purpura, drug-induced hypersensitivity vasculitis) occur in HIV-infected individuals12. Vascular inflammation appears multifactorial and may result from HIV-induced immunological abnormalities and exposure to xenoantigens such as HIV-1 itself, other infectious agents and drugs12.
Endothelial dysfunction injury, pivotal to the development of cardiovascular and inflammatory disease, has been described in HIV infection13. There is evidence of endothelial activation, in the form of soluble adhesion molecules and procoagulant proteins13. Virus may gain entry to endothelial cells via CD4 antigen or galactosyl-ceramide receptors. Other possible mechanisms of entry include chemokine receptors. Nevertheless, endothelial activation may also occur via secretion of cytokines in response to mononuclear or adventitial cell activation by virus, or else by the effects of the secreted HIV-associated proteins gp 120 (envelope glycoprotein) and tat (transactivator of viral replication) on endothelium. Enhanced adhesiveness of endothelial cells, endothelial cell proliferation and apoptosis as well as activation of cytokine secretion have all been demonstrated. Selected inflammatory cytokines and viral proteins have been found synergistic in inducing endothelial injury. In HIV infection, dysfunctional or injured endothelial cells potentiate tissue injury, inflammation and remodelling, and accelerate the development of cardiovascular disease13.
Coronary artery disease is observed with increasing frequency among HIV patients receiving therapy with protease inhibitors (PI) in the ambit of HAART regimens1. Despite the clinical benefits of PI therapy, complications such as lipodystrophy, insulin resistance, and high levels of low-density lipoprotein cholesterol and triglyceride develop in up to 60% of patients treated with these regimens1. In 10-20% of patients the effects are severe, with unstable angina, myocardial infarction, and stroke developing even in young individuals14,15,16,17,18.
As regards lipodystrophy, similarities between HIV-associated fat redistribution and metabolic abnormalities with both inherited lipodystrophies and benign symmetric lipomatosis suggest the pathophysiological involvement of nuclear factors such as lamin A/C and drug-induced mitochondrial dysfunction19. Moreover, there is some evidence that cytokines and hormones impair fat and glucose homoeostasis in patients with HIV receiving HAART19. Three years after the first description of HIV-therapy-associated abnormal fat redistribution, there is still discussion about the case definition, diagnostic procedure and treatment options for both body shape changes and metabolic disturbances19.
In the evaluation of patients for HAART and in continued therapy, it is wise to look at traditional coronary risk profiles and to alter those that can be favourably modified. Diet and exercise should not be overlooked, because both can be effective without causing further side-effects20. Fibric acid derivatives and statins can lower HIV-associated hypercholesterolaemia and hypertriglyceridaemia, although further data are needed on interactions between statins and PI20,21. Lovastatin should be avoided in patients receiving drugs that might potentiate the skeletal muscle toxicity of this agent22. Hypoglycaemic agents may have some role in management of glucose abnormalities, although troglitazone cannot be recommended for fat abnormalities alone and metformin may cause lactic acidosis21. Perhaps a better understanding of PI effects on lipid and metabolic pathways will lead to a new generation of drug therapies without metabolic alterations.
HIV patients are at higher risk of becoming hypertensive than the general population, and hypertension develops at a younger age23. Predisposing factors include vasculitis in small, medium, and large vessels in the form of leukocytoclastic vasculitis, atherosclerosis secondary to HAART regimens, and aneurysms of the large vessels such as the carotid and femoral arteries and the abdominal aorta, with impairment of flow to the renal arteries23.
PULMONARY HYPERTENSION AND RIGHT VENTRICULAR DYSFUNCTION
The incidence of HIV-associated pulmonary hypertension is estimated to be 1/200, much higher than the 1/200 000 found in the general population1. Common reasons for pulmonary hypertension in HIV-infected patients are lung infections, venous thromboembolism and left ventricular dysfunction. Pulmonary hypertension found on screening echocardiography or right heart catheterization warrants an aggressive evaluation for treatable pulmonary infections24.
Primary pulmonary hypertension has been reported in HIV-infected patients without a history of thromboembolic disease, intravenous drug use, or pulmonary infections associated with HIV. One necropsy specimen and one biopsy specimen revealed precapillary muscular pulmonary artery and arteriole medial hypertrophy, fibroelastosis, and eccentric intimal fibrosis without direct viral infection of pulmonary artery cells24,25. This suggests mediator release from infected cells elsewhere and possibly cytokine mediated injury.
The pathogenesis of primary pulmonary hypertension is multifactorial and poorly understood. Primary pulmonary hypertension has been found in haemophilic patients receiving lyophylized factor VIII, in intravenous drug users and in patients with left ventricular dysfunction, obscuring any relationship with the human immunodeficiency virus1,24,25. It may be that HIV causes endothelial damage and mediator-related vasoconstriction through stimulation by the envelope gp 120, including direct release and effects of endothelin-1 (vasoconstrictor), interleukin-6 and TNF-α in the pulmonary arteries24,25.
HIV is frequently identified in alveolar macrophages24,25. These macrophages release TNF-α, oxide anions and proteolytic enzymes in response to infection. Lymphokines may also have a role in the aetiology of the endothelial proliferation seen in pulmonary hypertension, by promoting leukocyte adhesion to the endothelium26. Clinical symptoms and outcome of patients with right ventricular dysfunction are related to the degree of pulmonary hypertension, varying from a mild symptomless condition to severe cardiac impairment with cor pulmonale and death1,24. Activation of α1-receptors and genetic factors (increased frequency of HLA DR6 and DR52) have been also hypothesized for the pathogenesis of HIV-associated pulmonary hypertension1,26. The influence of HAART regimens on the clinical course of HIV-associated pulmonary hypertension is unknown.
PERICARDIAL EFFUSION
The incidence of pericardial effusion among those meeting criteria for AIDS is 11% per year27. The prevalence of symptomless pericardial effusion in AIDS patients is estimated at 22%27. HIV infection should be included in the differential diagnosis of unexplained pericardial effusion or tamponade. AIDS patients with pericardial effusion survive a median of 6 months—considerably less time than AIDS patients without effusion. Survival is independent of CD4 count and albumin levels27.
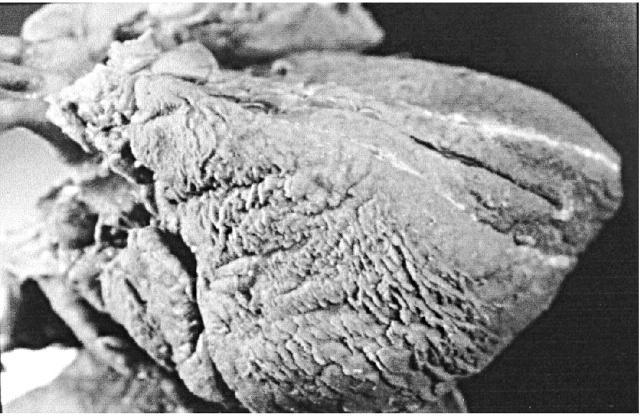
Pericardial effusion in HIV disease may be related to opportunistic infections (Figure 2) or to malignancy (Kaposi's sarcoma, non-Hodgkin lymphoma), but most often a clear aetiology is not found27. The effusion may be part of a generalized serous effusive process also involving pleural and peritoneal surfaces. This capillary leak syndrome is probably related to enhanced cytokine expression (e.g. TNF-α) in the later stages of HIV disease1,27. Pericardial effusion spontaneously resolves in up to 42% of patients1,27. Pericardiocentesis is currently recommended only for large or poorly tolerated effusions, for diagnostic evaluation of systemic illness or in the presence of cardiac tamponade1. Even if the effusion resolves over time1, these patients still have excess mortality. The effects of HAART therapy on pericardial effusion are largely unexplored.
Figure 2.
Fibrinous pericarditis in AIDS patient induced by M. avium intracellulare infection. Note focal thickening of epicardium suggesting simultaneous involvement of myocardium below
ENDOCARDIAL INVOLVEMENT
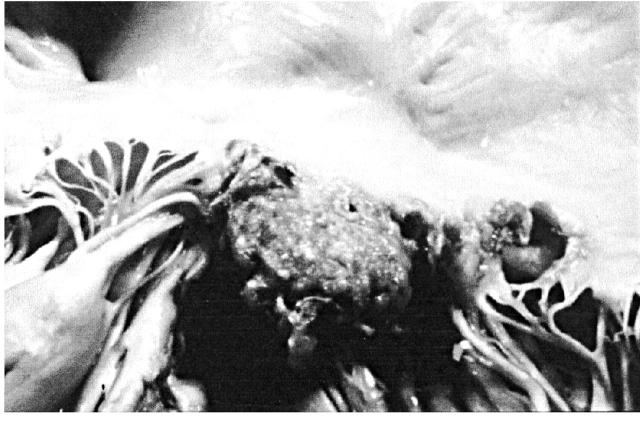
The prevalence of infective endocarditis in HIV-infected patients is similar to that in other patients of similar behaviour risk—i.e. intravenous drug use1. Estimates of endocardititis occurrence vary from 6.3% to 34% of HIV-infected patients who use intravenous drugs1,7. Right-sided valves are predominantly affected and the most frequent pathogens are Staphylococcus aureus, Candida albicans (Figure 3), Aspergillus fumigatus, Histoplasma capsulatum, and Cryptococcus neoformans1,7. Patients with and without HIV generally have similar presentation and survival rates from infective endocarditis. However, patients with late-stage HIV disease have higher mortality from infective endocarditis than do those with earlier disease1.
Figure 3.
Candida albicans endocarditis of tricuspid valve in drug abuser with AIDS (necropsy finding). A large polypoid thrombotic lesion is seen on the posterior leaflet
As the autoimmune response to bacterial endocarditis is often largely responsible for associated valvular destruction, HIV-infected patients may have an altered course. For example, HIV-infected patients have a higher risk of developing salmonella endocarditis than immunocompetent patients because they are more likely to develop salmonella bacteraemia during salmonella infection. However, they respond better to antibiotic therapy, and may be less likely to sustain valvular damage because of impaired immune function1.
Non-bacterial thrombotic endocarditis, also known as marantic endocarditis, is most common in patients with HIV wasting syndrome1. It is characterized by friable endocardial vegetations, consisting of platelets within a fibrin mesh with few inflammatory cells. The lesions may involve any of the four valves but are more common on the left side. Systemic or pulmonary embolization can cause serious end-organ damage in spleen, kidneys and myocardium. Systemic embolization from marantic endocarditis is a rare cause of death in AIDS patients1.
DRUG CARDIOTOXICITY
An association between zidovudine and dilated cardiomyopathy has been reported in both adult and paediatric patients28,29. Studies performed on transgenic mice suggest that zidovudine is associated with diffuse destruction of cardiac mitochondrial ultrastructures and inhibition of mitochondrial DNA replication30. Lactic acidosis related to mitochondrial dysfunction may further contribute to myocardial cell dysfunction31. However, from a clinical point of view, in a study of selected HIV-infected children zidovudine neither worsened nor ameliorated cardiomyopathy32. Infants born to HIV-positive mothers were followed from birth to age 5 years with serial echocardiographic studies every 4-6 months. No association with acute or chronic abnormalities in left ventricular structure or function was found with perinatal exposure to zidovudine33. Other antiretroviral drugs, such as didanosine and zalcitabine, do not seem either to promote or to prevent dilated cardiomyopathy4.
In AIDS patients with Kaposi's sarcoma, reversible cardiac dysfunction was associated with prolonged high-dose therapy with interferon alpha1,34. High-dose interferon alpha treatment is not associated with myocardial dysfunction in other patient populations, so a synergistic effect with HIV infection has been proposed35.
Doxorubicin, used to treat AIDS-related Kaposi's sarcoma and non-Hodgkin lymphoma, has a dose-related effect on dilated cardiomyopathy36, as does foscarnet sodium when used to treat cytomegalovirus oesophagitis37. The prevalence of hypertension associated with erythropoietin therapy is 47%; the effect may be related to the increase in haematocrit and blood viscosity38.
Cardiac arrhythmias (ventricular tachycardia or ventricular fibrillation, torsade de pointes [related to prolongation of QTc interval], atrioventricular conduction abnormalities) have been described with the administration of amphotericin B39, ganciclovir40, co-trimoxazole41 and pentamidine42. Prolongation of QTc interval has been described also with the administration of macrolide antibacterials (erythromycin, clarithromycin)42. In HIV-infected patients cisapride, a prokinetic agent often used for the treatment of gastro-oesophageal reflux, may increase the risk of torsade de pointes when administered in combination with other drugs such as macrolide antibacterials, azole antifungal agents (fluconazole, itraconazole, ketoconazole), all PI (amprenavir, indinavir, nelfinavir, ritonavir, saquinavir), and drugs that have potential additive effects on the QT interval (adenosine, class Ia and III antiarrhythmic drugs)43.
NUTRITIONAL DEFICIENCIES
Nutritional deficiencies are common in HIV infection, particularly in late-stage disease, and may contribute to ventricular dysfunction independently of HAART regimens. Poor absorption and diarrhoea both lead to electrolyte imbalances and deficiencies in elemental nutrients. Deficiencies of trace elements have been associated directly or indirectly with cardiomyopathy44. For example, selenium deficiency increases the virulence of coxsackievirus to cardiac tissue45. Selenium replacement reverses cardiomyopathy and restores left ventricular function in nutritionally depleted patients44. Levels of vitamin B12, carnitine, growth hormone and thyroid hormone may also be altered in HIV disease; all have been associated with left ventricular dysfunction44. Electrolyte imbalances (e.g. hypokalaemia, hypocalcaemia, hypomagnesaemia), related to malnutrition or to chronic diarrhoea, may further contribute to induction of ventricular arrhythmias (torsade de pointes and ventricular fibrillation) by prolongation of the QTc interval.
CARDIAC INVOLVEMENT IN AIDS-RELATED TUMOURS
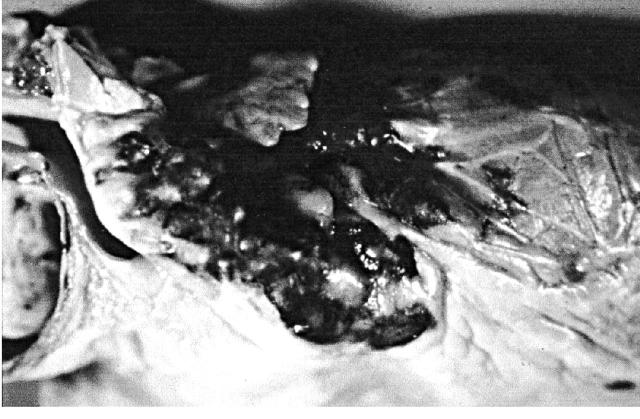
Cardiac Kaposi's sarcoma in AIDS may cause visceral and parietal pericardial lesions and, less frequently, myocardial lesions. The prevalence has ranged from 12% to 28% in retrospective necropsy studies in the pre-HAART era1,2,3,4,5,6,7 (Figure 4). Cardiac Kaposi's sarcoma is not usually obstructive or associated with clinical cardiac dysfunction, morbidity or mortality1.
Figure 4.
Cardiac Kaposi's sarcoma in AIDS patient. The epicardial lesions appear as red purple coalescent plaques
Malignant lymphoma involving the heart is infrequent in AIDS1,7,46. Lymphomatous infiltration may be diffuse or may result in discrete isolated lesions, which are usually derived from the Burkitt or immunoblastic B cells. The lesions tend to be nodular or polypoid, and they predominantly involve the pericardium, with variable myocardial infiltration1,7. The prognosis of patients with HIV-associated cardiac lymphoma is generally poor, although clinical remission has been observed with combination chemotherapy1.
The better control of human herpes virus 8 and Epstein—Barr virus infection, which are involved in the development of HIV-associated tumours, may account for the reduction, since the advent of HAART, in overall incidence of cardiac involvement by both Kaposi's sarcoma and non-Hodgkin lymphomas.
CONCLUSIONS
As therapies improve, late complications of HIV infection are becoming more prevalent. Cardiac and pulmonary complications of HIV disease are generally late manifestations and may be due to prolonged immunosuppression and a complex interplay of mediator effects from opportunistic infections, viral infections, autoimmune response to viral infection, drug-related cardiotoxicity and nutritional deficiencies. Careful clinical evaluation is required for patients who receive antiretroviral treatment (with zidovudine and PI) or drugs with recognized cardiotoxic action (doxorubicin, interferon alpha, pentamidine), for patients whose CD4 count is below 400/μL, and for patients with HIV-associated encephalopathy. HIV-associated heart disease may be an important model for the mechanisms behind dilated cardiomyopathy. The study of immunological factors may provide useful information about testable therapies for HIV-associated cardiomyopathy. Such findings would also have important implications for non-HIV-related cardiovascular diseases. Since the role of infection and inflammation in many other cardiovascular diseases is beginning to be recognized, discovery of the molecular mechanisms of HIV-related heart disease might provide the basis for rational therapy in a broader range of patients5.
Acknowledgments
I thank Giulia D'Amati MD, Department of Experimental Medicine and Pathology, University ‘La Sapienza’, Rome, for providing the illustrations.
References
- 1.Rerkpattanapipat P, Wongpraparut N, Jacobs LE, Kotler MN. Cardiac manifestations of acquired immunodeficiency syndrome. Arch Intern Med 2000;160: 602-8 [DOI] [PubMed] [Google Scholar]
- 2.Lipshultz SE, Easley DA, Orav EJ, et al. Cardiac dysfunction and mortality in HIV-infected children. The Prospective P2C2 HIV Multicenter Study. Circulation 2000;102: 1542-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Temesgen Z. Overview of HIV infection. Ann Allergy Asthma Immunol 1999;83: 1-5 [DOI] [PubMed] [Google Scholar]
- 4.Barbaro G, Di Lorenzo G, Grisorio B, Barbarini G, for the Gruppo Italiano per lo Studio Cardiologico dei pazienti affetti da AIDS investigators. Incidence of dilated cardiomyopathy and detection of HIV in myocardial cells of HIV positive patients. N Engl J Med 1998; 339: 1093-9 [DOI] [PubMed] [Google Scholar]
- 5.Lipshultz SE. Dilated cardiomyopathy in HIV-infected patients [Editorial]. N Engl J Med 1998;339: 1153-5 [DOI] [PubMed] [Google Scholar]
- 6.Barbaro G, Di Lorenzo G, Soldini M, et al. The intensity of myocardial expression of inducible nitric oxide synthase influences the clinical course of human immunodeficiency virus-associated cardiomyopathy. Circulation 1999;100: 633-9 [DOI] [PubMed] [Google Scholar]
- 7.Barbaro G, Di Lorenzo G, Grisorio B, Barbarini G, and Gruppo Italiano per lo Studio Cardiologico dei pazienti affetti da AIDS investigators. Cardiac involvement in the acquired immunodeficiency syndrome. A multicenter clinical-pathological study. AIDS Res Hum Retroviruses 1998; 14: 1071-7 [DOI] [PubMed] [Google Scholar]
- 8.Barbaro G, Di Lorenzo G, Soldini M, et al. Clinical course of cardiomyopathy in HIV-infected patients with or without encephalopathy related to the myocardial expression of TNF-α and iNOS. AIDS 2000;14: 827-38 [DOI] [PubMed] [Google Scholar]
- 9.Lipshultz SE, Orav EJ, Sanders SP, Colan SD. Immunoglobulins and left ventricular structure and function in paediatrics HIV infection. Circulation 1995;92: 2220-5 [DOI] [PubMed] [Google Scholar]
- 10.Currie PF, Goldman JH, Caforio AL, et al. Cardiac autoimmunity in HIV related heart muscle disease. Heart 1998;79: 599-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gullestad L, Aass H, Fjeld JG, et al. Immunomodulating therapy with intravenous immunoglobulin in patients with chronic heart failure. Circulation 2001;103: 220-5 [DOI] [PubMed] [Google Scholar]
- 12.Gherardi R, Belec L, Mhiri C, et al. The spectrum of vasculitis in human immunodeficiency virus-infected patients. A clinicopathologic evaluation. Arthritis Rheum 1993;36: 1164-74 [DOI] [PubMed] [Google Scholar]
- 13.Chi D, Henry J, Kelly J, Thorpe R, Smith JK, Krishnaswamy G. The effects of HIV infection on endothelial function. Endothelium 2000; 7: 223-42 [DOI] [PubMed] [Google Scholar]
- 14.Sullivan AK, Nelson MR, Moyle GJ, Newell AM, Feher MD, Gazzard BG. Coronary artery disease occurring with protease inhibitor therapy. Int J STD AIDS 1998;9: 711-12 [DOI] [PubMed] [Google Scholar]
- 15.Henry K, Melrow H, Huebsch J, et al. Severe coronary heart disease with protease inhibitors [Letter]. Lancet 1998;351: 1328. [DOI] [PubMed] [Google Scholar]
- 16.Behrens G, Schmidt H, Meyer D, Stoll M, Schmidt RE. Vascular complications associated with use of HIV protease inhibitors [Letter]. Lancet 1998;351: 1958. [DOI] [PubMed] [Google Scholar]
- 17.Jutte A, Schwenk A, Franzen C, et al. Increasing morbidity from myocardial infarction during HIV protease inhibitor treatment? [Letter] AIDS 1999;13: 1796-7 [DOI] [PubMed] [Google Scholar]
- 18.Flynn TE, Bricker LA. Myocardial infarction in HIV-infected men receiving protease inhibitors [Letters]. Ann Intern Med 1999;131: 548. [DOI] [PubMed] [Google Scholar]
- 19.Beherns GM, Stoll M, Schmidt RE. Lipodystrophy syndrome in HIV infection: what is it, what causes it and how can it be managed? Drug Saf 2000;23: 57-76 [DOI] [PubMed] [Google Scholar]
- 20.Dube MP, Sprecher D, Henry WK, et al. Preliminary guidelines for the evaluation and management of dyslipidemia in adults infected with human immunodeficiency virus and receiving antiretroviral therapy: recommendations of the Adult AIDS Clinical Trial Group Cardiovascular Disease Focus Group. Clin Infect Dis 2000;31: 1216-24 [DOI] [PubMed] [Google Scholar]
- 21.Currier JS. How to manage metabolic complications of HIV therapy: what to do while we wait for answers. AIDS Read 2000;10: 162-9 [PubMed] [Google Scholar]
- 22.Penzak SR, Chuck SK, Stajich GV. Safety and efficacy of HMG-CoA reductase inhibitors for treatment of hyperlipidemia in patients with HIV infection. Pharmacotherapy 2000;20: 1066-71 [DOI] [PubMed] [Google Scholar]
- 23.Aoun S, Ramos E. Hypertension in the HIV-infected patient. Curr Hypertens Rep 2000;2: 478-81 [DOI] [PubMed] [Google Scholar]
- 24.Pellicelli A, Barbaro G, Palmieri F, et al. Primary pulmonary hypertension in HIV disease: a systematic review. Angiology 2001; 52: 31-41 [DOI] [PubMed] [Google Scholar]
- 25.Pellicelli AM, Palmieri F, D'Ambrosio C, et al. Role of human immunodeficiency virus in primary pulmonary hypertension: case reports. Angiology 1998;49: 1005-11 [DOI] [PubMed] [Google Scholar]
- 26.Mesa RA, Edell ES, Dunn WF, Edwards WD. Human immunodeficiency virus infection and pulmonary hypertension. Mayo Clin Proc 1998;73: 37-44 [DOI] [PubMed] [Google Scholar]
- 27.Heidenreich PA, Eisenberg MJ, Kee LL, et al. Pericardial effusion in AIDS. Incidence and survival. Circulation 1995;92: 3229-34 [DOI] [PubMed] [Google Scholar]
- 28.Herskowitz A, Willoughby SB, Baughman KL, Schulman SP, Bartlett JD. Cardiomyopathy associated with anti-retroviral therapy in patients with HIV infection: a report of six cases. Ann Intern Med 1992; 116: 311-13 [DOI] [PubMed] [Google Scholar]
- 29.Domanski MJ, Sloas MM, Follman DA, et al. Effect of zidovudine and didanosine treatment on heart function in children infected with human immunodeficiency virus. Paediatrics 1995;127: 137-46 [DOI] [PubMed] [Google Scholar]
- 30.Lewis W, Grupp IL, Grupp G, et al. Cardiac dysfunction in the HIV-1 transgenic mouse treated with zidovudine. Lab Invest 2000;80: 187-97 [DOI] [PubMed] [Google Scholar]
- 31.Lewis W. Cardiomyopathy in AIDS: a pathophysiological perspective. Prog Cardiovasc Dis 2000;43: 151-70 [DOI] [PubMed] [Google Scholar]
- 32.Lipshultz SE, Orav EJ, Sanders SP, Hale HR, McIntosh K, Colan SD. Cardiac structure and function in children with human immunodeficiency virus infection treated with zidovudine. N Engl J Med 1992;327: 1260-5 [DOI] [PubMed] [Google Scholar]
- 33.Lipshultz SE, Easley KA, Orav EJ, et al. Absence of cardiac toxicity of zidovudine in infants. N Engl J Med 2000;343: 759-66 [DOI] [PubMed] [Google Scholar]
- 34.Sonnenblick EH, Rosin A. Cardiotoxicity of interferon: a review of 44 cases. Chest 1991;99: 557-61 [DOI] [PubMed] [Google Scholar]
- 35.Deyton L, Walker R, Kovacs J, et al. Reversible cardiac dysfunction associated with interferon alpha therapy in AIDS patients with Kaposi's sarcoma. N Engl J Med 1989;321: 1246-9 [DOI] [PubMed] [Google Scholar]
- 36.Bristow MR, Mason JW, Billingham ME, Daniels JR. Doxorubicin cardiomyopathy: evaluation by phonocardiography, endomyocardial biopsy and cardiac catheterization. Ann Intern Med 1978;88: 168-75 [DOI] [PubMed] [Google Scholar]
- 37.Brown DL, Sather S, Cheitlin MD. Reversible cardiac dysfunction associated with foscarnet therapy for cytomegalovirus esophagitis in an AIDS patient. Am Heart J 1993;125: 1439-41 [DOI] [PubMed] [Google Scholar]
- 38.Raine AE. Hypertension, blood viscosity and cardiovascular morbidity in renal failure: implication of erythropoietin therapy. Lancet 1998; 1: 97-100 [DOI] [PubMed] [Google Scholar]
- 39.Arsura EL, Ismail Y, Freeman S, Karunakav AR. Amphotericin B-induced dilated cardiomyopathy. Am J Med 1994;97: 560-2 [DOI] [PubMed] [Google Scholar]
- 40.Cohen AJ, Weiser B, Afzal Q, Fuhrer J. Ventricular tachycardia in two patients with AIDS receiving ganciclovir (DHPG). AIDS 1990;4: 807-9 [DOI] [PubMed] [Google Scholar]
- 41.Lopez JA, Harold JG, Rosenthal MC, Oseran DS, Schapira JN, Peter T. QT prolongation and torsade de pointes after administration of trimethoprim—sulfamethoxaole. Am J Cardiol 1987;59: 376-7 [DOI] [PubMed] [Google Scholar]
- 42.Giancaspro G, Barbaro G. Disturbi del ritmo nella malattia da HIV/AIDS. In: Barbaro G, Pellicelli AM, Barbarini G, eds. Cardiologia nell'AIDS. Milan: Excerpta Medica, 200: 267-75
- 43.Michalets EL, Williams CR. Drug interactions with cisapride: clinical manifestations. Clin Pharmacokinet 2000;39: 49-75 [DOI] [PubMed] [Google Scholar]
- 44.Hoffman M, Lipshultz SE, Miller TL. Malnutrition and cardiac abnormalities in the HIV-infected patients. In: Miller TL, Gorbach S, eds. Nutritional Aspects of HIV Infection. London: Arnold, 1999: 33-9
- 45.Beck MA, Kolbeck PC, Shi Q, Rohr LH, Morris VC, Levander OA. Increased virulence of a human enterovirus (coxackievirus B3) in selenium-deficient mice. J Infect Dis 1994;170: 351-7 [DOI] [PubMed] [Google Scholar]
- 46.Yunis NA, Stone VE. Cardiac manifestations of HIV/AIDS: a review of disease spectrum and clinical management. J AIDS Hum Retrovirol 1998; 18: 145-54 [DOI] [PubMed] [Google Scholar]