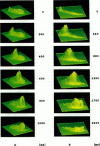
Abstract
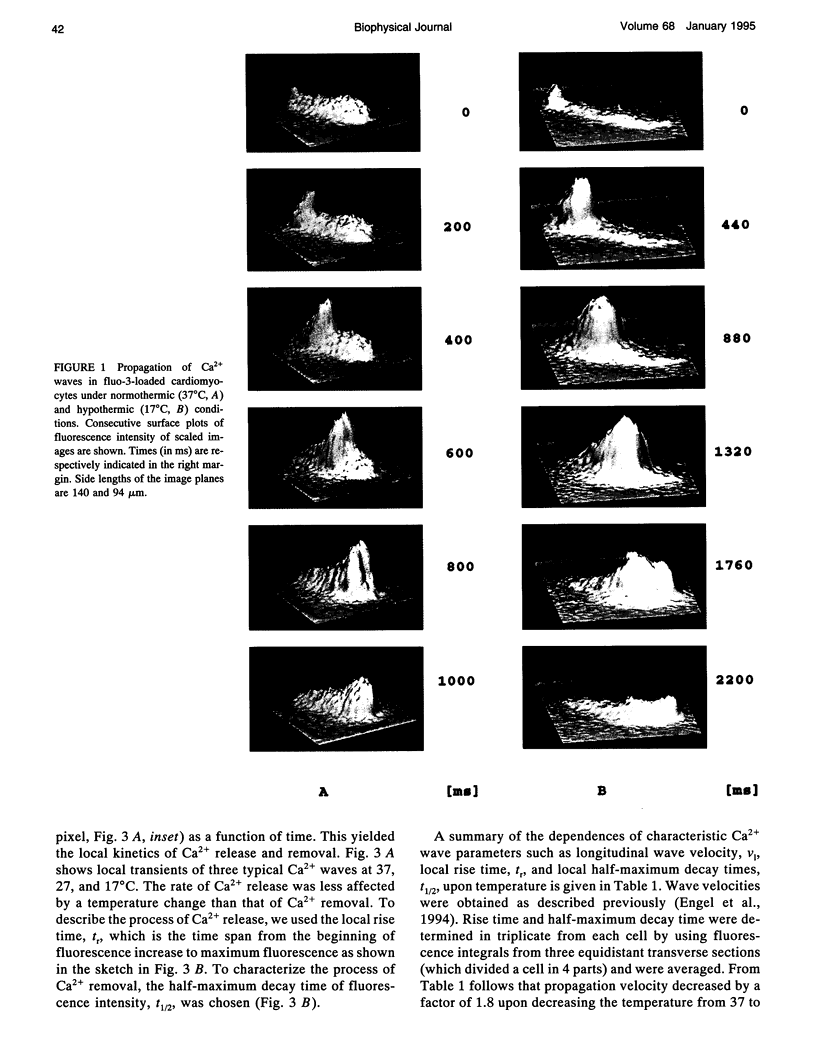
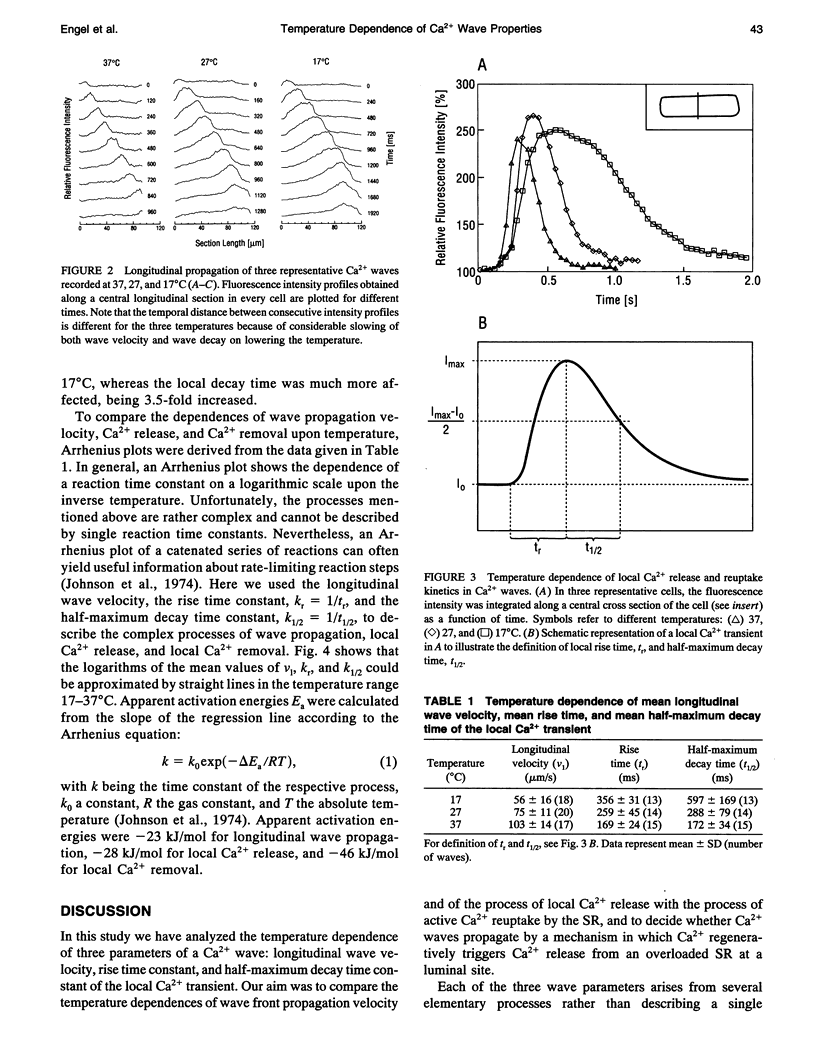
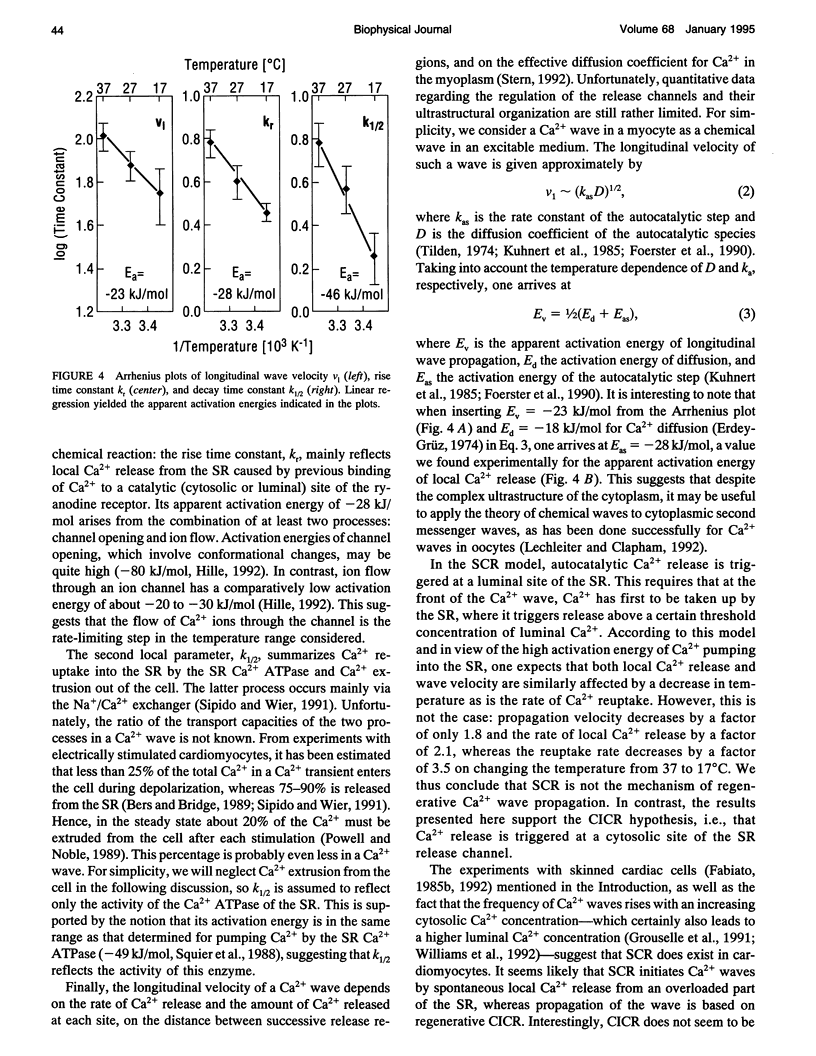
Digital imaging microscopy of fluo-3 fluorescence was used to study the velocity and shape of intracellular Ca2+ waves in isolated rat cardiomyocytes as a function of temperature. Decreasing the temperature from 37 to 17 degrees C reduced the longitudinal wave velocity by a factor of 1.8 and remarkably slowed the decay of [Ca2+]i in the trailing flank of a wave. Using image analysis, rise times, and half-maximum decay times of local Ca2+ transients, which characterize the processes of local Ca2+ release and removal, were determined as a function of temperature. Apparent activation energies for wave front propagation, local Ca2+ release, and local Ca2+ removal were derived from Arrhenius plots and amounted to -23, -28, and -46 kJ/mol, respectively. The high activation energy of Ca2+ removal, which arises from the activity of the sarcoplasmic reticulum (SR) Ca2+ ATPase, relative to those of longitudinal wave propagation and local Ca2+ release excludes the hypothetical mechanism of regenerative "spontaneous Ca2+ release," in which Ca2+ that has been taken up from the approaching wavefront triggers Ca2+ release at a luminal site of the SR. It is consistent, however, with the hypothesis that Ca2+ wave propagation is based on Ca(2+)-induced Ca2+ release where Ca2+ triggers release on the cytosolic face of the SR.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bers D. M., Bridge J. H. Relaxation of rabbit ventricular muscle by Na-Ca exchange and sarcoplasmic reticulum calcium pump. Ryanodine and voltage sensitivity. Circ Res. 1989 Aug;65(2):334–342. doi: 10.1161/01.res.65.2.334. [DOI] [PubMed] [Google Scholar]
- Engel J., Fechner M., Sowerby A. J., Finch S. A., Stier A. Anisotropic propagation of Ca2+ waves in isolated cardiomyocytes. Biophys J. 1994 Jun;66(6):1756–1762. doi: 10.1016/S0006-3495(94)80997-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Two kinds of calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cardiac cells. Adv Exp Med Biol. 1992;311:245–262. doi: 10.1007/978-1-4615-3362-7_18. [DOI] [PubMed] [Google Scholar]
- Grouselle M., Stuyvers B., Bonoron-Adele S., Besse P., Georgescauld D. Digital-imaging microscopy analysis of calcium release from sarcoplasmic reticulum in single rat cardiac myocytes. Pflugers Arch. 1991 Mar;418(1-2):109–119. doi: 10.1007/BF00370459. [DOI] [PubMed] [Google Scholar]
- Ishide N., Miura M., Sakurai M., Takishima T. Initiation and development of calcium waves in rat myocytes. Am J Physiol. 1992 Aug;263(2 Pt 2):H327–H332. doi: 10.1152/ajpheart.1992.263.2.H327. [DOI] [PubMed] [Google Scholar]
- Ishide N., Urayama T., Inoue K., Komaru T., Takishima T. Propagation and collision characteristics of calcium waves in rat myocytes. Am J Physiol. 1990 Sep;259(3 Pt 2):H940–H950. doi: 10.1152/ajpheart.1990.259.3.H940. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F. Classes and mechanisms of calcium waves. Cell Calcium. 1993 Nov;14(10):736–745. doi: 10.1016/0143-4160(93)90099-r. [DOI] [PubMed] [Google Scholar]
- Lakatta E. G. Functional implications of spontaneous sarcoplasmic reticulum Ca2+ release in the heart. Cardiovasc Res. 1992 Mar;26(3):193–214. doi: 10.1093/cvr/26.3.193. [DOI] [PubMed] [Google Scholar]
- Lechleiter J. D., Clapham D. E. Molecular mechanisms of intracellular calcium excitability in X. laevis oocytes. Cell. 1992 Apr 17;69(2):283–294. doi: 10.1016/0092-8674(92)90409-6. [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Microscopic spiral waves reveal positive feedback in subcellular calcium signaling. Biophys J. 1993 Dec;65(6):2272–2276. doi: 10.1016/S0006-3495(93)81316-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minta A., Kao J. P., Tsien R. Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989 May 15;264(14):8171–8178. [PubMed] [Google Scholar]
- Powell T., Noble D. Calcium movements during each heart beat. Mol Cell Biochem. 1989 Sep 7;89(2):103–108. doi: 10.1007/BF00220760. [DOI] [PubMed] [Google Scholar]
- Sipido K. R., Wier W. G. Flux of Ca2+ across the sarcoplasmic reticulum of guinea-pig cardiac cells during excitation-contraction coupling. J Physiol. 1991 Apr;435:605–630. doi: 10.1113/jphysiol.1991.sp018528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squier T. C., Bigelow D. J., Thomas D. D. Lipid fluidity directly modulates the overall protein rotational mobility of the Ca-ATPase in sarcoplasmic reticulum. J Biol Chem. 1988 Jul 5;263(19):9178–9186. [PubMed] [Google Scholar]
- Stern M. D., Capogrossi M. C., Lakatta E. G. Spontaneous calcium release from the sarcoplasmic reticulum in myocardial cells: mechanisms and consequences. Cell Calcium. 1988 Dec;9(5-6):247–256. doi: 10.1016/0143-4160(88)90005-x. [DOI] [PubMed] [Google Scholar]
- Stern M. D. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992 Aug;63(2):497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu T., Wier W. G. Calcium waves in mammalian heart: quantification of origin, magnitude, waveform, and velocity. FASEB J. 1990 Mar;4(5):1519–1525. doi: 10.1096/fasebj.4.5.2307330. [DOI] [PubMed] [Google Scholar]
- Trafford A. W., O'Neill S. C., Eisner D. A. Factors affecting the propagation of locally activated systolic Ca transients in rat ventricular myocytes. Pflugers Arch. 1993 Oct;425(1-2):181–183. doi: 10.1007/BF00374521. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Blatter L. A. Ca(2+)-oscillations and Ca(2+)-waves in mammalian cardiac and vascular smooth muscle cells. Cell Calcium. 1991 Feb-Mar;12(2-3):241–254. doi: 10.1016/0143-4160(91)90024-9. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Delbridge L. M., Cody S. H., Harris P. J., Morgan T. O. Spontaneous and propagated calcium release in isolated cardiac myocytes viewed by confocal microscopy. Am J Physiol. 1992 Mar;262(3 Pt 1):C731–C742. doi: 10.1152/ajpcell.1992.262.3.C731. [DOI] [PubMed] [Google Scholar]
- Williams D. A. Mechanisms of calcium release and propagation in cardiac cells. Do studies with confocal microscopy add to our understanding? Cell Calcium. 1993 Nov;14(10):724–735. doi: 10.1016/0143-4160(93)90098-q. [DOI] [PubMed] [Google Scholar]